Antitumoral Drug: Loaded Hybrid Nanocapsules Based on Chitosan with Potential Effects in Breast Cancer Therapy
Abstract
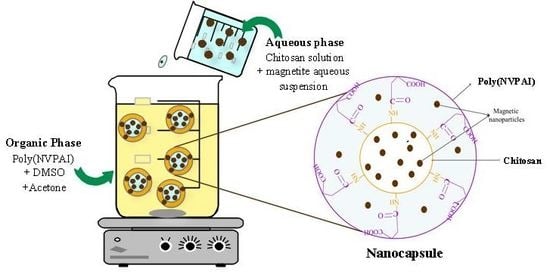
:1. Introduction
2. Results and Discussions
2.1. FTIR Spectroscopy
2.2. Mean Diameter Determination
2.3. Aqueous Suspensions Stability
2.4. Nanocapsule Morphology
2.5. Thermal Behavior
2.6. Magnetic Properties
2.7. Swelling Degree of Magnetic Nanocapsules
2.8. Loading Efficiency of 5-Fluorouracil (5-FU)
2.9. 5-FU Release from Magnetic Nanocapsules
2.10. Theoretical Analysis of Drug Release
2.11. Viability Assay
3. Materials and Methods
3.1. Materials
3.2. Magnetic Nanoparticle Preparation
3.3. Preparation of Magnetic Nanocapsules
3.4. Characterization
3.4.1. Structural Characterization
3.4.2. Morphological Characterization
3.4.3. Thermal Properties
3.4.4. Magnetic Properties
3.4.5. Swelling Behavior in Aqueous Solutions
3.4.6. Drug Loading Studies
3.4.7. Drug Release Kinetics
3.4.8. Theoretical Analysis of Drug Release
3.4.9. In Vitro Cytotoxicity Assay
Cell Culture
Viability Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rata, D.M.; Chailan, J.F.; Peptu, C.A.; Costuleanu, M.; Popa, M. Chitosan: Poly(N-vinylpyrrolidone-alt-itaconic anhydride) nanocapsules—A promising alternative for the lung cancer treatment. J. Nanopart. Res. 2015, 17, 316–327. [Google Scholar] [CrossRef]
- Kalantarian, P.; Najafabadi, A.R.; Haririan, I.; Vatanara, A.; Yamini, Y.; Darabi, M.; Gilani, K. Preparation of 5-fluorouracil nanoparticles by supercritical antisolvents for pulmonary delivery. Int. J. Nanomed. 2010, 5, 763–770. [Google Scholar] [CrossRef]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.M.; Ghergheld, D.; Popa, M. In vitro behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers 2019, 11, 1515. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.K. Advances in the field of nanooncology. BMC Med. 2010, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Ambekar, R.S.; Choudhary, M.; Kandasubramanian, B. Recent Advances in Dendrimer-based Nanoplatform for Cancer Treatment: A Review. Eur. Polym. J. 2020, 126, 109546. [Google Scholar] [CrossRef]
- Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization. Polymers 2020, 12, 1018. [Google Scholar] [CrossRef]
- Wang, Z.; Ho, P.C. A nanocapsular combinatorial sequential drug delivery system for antiangiogenesis and anticancer activities. Biomaterials 2010, 31, 7115–7123. [Google Scholar] [CrossRef]
- Wang, Z.; Ho, P.C. Self-assembled core-shell vascular targeted nanocapsules for temporal antivasculature and anticancer activities. Small 2010, 6, 2576–2583. [Google Scholar] [CrossRef]
- Yurgel, V.; Collares, T.; Seixas, F. Developments in the use of nanocapsules in oncology. Braz. J. Med. Boil. Res. 2013, 46, 486–501. [Google Scholar] [CrossRef]
- Babu, A.; Templeton, A.K.; Munshi, A.; Ramesh, R. Nanoparticle-Based Drug Delivery for Therapy of Lung Cancer: Progress and Challenges. J. Nanomater. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.M.; Kang, Y.J.; Sohn, Y.; Kim, D.K. Dual targeting strategy of magnetic nanoparticle-loaded and RGD peptide-activated stimuli-sensitive polymeric micelles for delivery of paclitaxel. J. Nanomater. 2015, 17, 248. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, P.; Zhou, N.; Su, Y.; Shao, M.; Chi, C. PH-Sensitive N-doped carbon dots–heparin and doxorubicin drug delivery system: Preparation and anticancer research. RSC Adv. 2017, 7, 9347–9356. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Q.; Zhang, A.; Pan, S.; Cheng, J.; Zhi, X.; Ding, X.; Hong, L.; Zi, M.; Cui, D.; et al. Multifunctional co-loaded magnetic nanocapsules for enhancing targeted MR imaging and in vivo photodynamic therapy. Nanomed. Nanotechnol. Boil. Med. 2019, 21, 102047. [Google Scholar] [CrossRef]
- Elumalai, R.; Patil, S.; Maliyakkal, N.; Rangarajan, A.; Kondaiah, P.; Raichur, A.M. Protamine-carboxymethyl cellulose magnetic nanocapsules for enhanced delivery of anticancer drugs against drug resistant cancers. Nanomedicine 2015, 11, 969–981. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; He, J.; Wang, H.; Luo, Y.; Gong, W.; Li, Y.; Huang, Y.; Zhong, L.; Zhao, Y. A Dual Targeting Magnetic Nanoparticle for Human Cancer Detection. Nanoscale Res. Lett. 2019, 14, 228. [Google Scholar] [CrossRef]
- Goya, G.F.; Grazú, V.; Ibarra, M.R. Magnetic Nanoparticles for Cancer Therapy. Curr. Nanosci. 2008, 4, 11–16. [Google Scholar] [CrossRef]
- Zhao, C.; Song, X.; Jin, W.; Wu, F.; Zhang, Q.; Zhang, M.; Zhou, N.; Shen, J. Image-guided cancer therapy using aptamer-functionalized cross-linked magnetic-responsive Fe3O4@carbon nanoparticles. Anal. Chim. Acta 2019, 1056, 108–116. [Google Scholar] [CrossRef]
- Mohan, N.; Mohanan, P.V.; Sabareeswaran, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int. J. Boil. Macromol. 2017, 104, 1936–1945. [Google Scholar] [CrossRef]
- Raţă, D.M.; Popa, M.; Chailan, J.F.; Zamfir, C.L.; Peptu, C.A. Biomaterial properties evaluation of poly(vinyl acetate-alt-maleic anhydride)/chitosan nanocapsules. J. Nanopart. Res. 2014, 16, 2569. [Google Scholar] [CrossRef]
- Balaita, L.; Cadinoiu, A.N.; Postolache, P.; Safarikova, M.; Popa, M. Magnetic polymer particles prepared by double crosslinking in reverse emulsion with potential biomedical applications. J. Optoelectron. Adv. Mater. 2015, 17, 1198–1209. [Google Scholar]
- Marquez, F.; Campo, T.; Cotto, M.; Polanco, R.; Roque, R.; Fierro, P.; Sanz, J.M.; Elizalde, E.; Morant, C. Synthesis and characterization of monodisperse magnetite hollow microspheres. Soft Nanosci. Lett. 2011, 1, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleicacid coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley-VCH Verlag: Weinheim, Germany, 2003. [Google Scholar]
- Khmara, I.; Strbak, O.; Zavisova, V.; Koneracka, M.; Kubovcikova, M.; Antal, I.; Kavecansky, V.; Lucanska, D.; Dobrota, D.; Kopcansky, P. Chitosan-stabilized iron oxide nanoparticles for magnetic resonance imaging. J. Magn. Magn. Mater. 2019, 474, 319–325. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Lundy, D.J.; Chen, K.H.; Toh, E.K.W.; Hsieh, P.C.H. Distribution of Systemically Administered Nanoparticles Reveals a Size-Dependent Effect Immediately following Cardiac Ischaemia-Reperfusion Injury. Sci. Rep. 2016, 6, 25613. [Google Scholar] [CrossRef] [Green Version]
- Hubbs, A.F.; Mercer, R.R.; Benkovic, S.A.; Harkema, J.; Sriram, K.; Schwegler-Berry, D.; Goravanahally, M.P.; Nurkiewicz, T.R.; Castranova, V.; Sargent, L.M. Nanotoxicology—A pathologist’s perspective. Toxicol. Pathol. 2011, 39, 301–324. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Balaita, L.; Chailan, J.F.; Nguyen, X.H.; Bacaita, S.; Popa, M. Hybrid chitosan-gelatine magnetic polymer particles for drug release. J. Optoelectron. Adv. Mater. 2014, 16, 1463–1471. [Google Scholar]
- Alupei, L.; Peptu, C.A.; Lungan, A.M.; Desbrieres, J.; Chiscan, O.; Radji, S.; Popa, M. New hybrid magnetic nanoparticles based on chitosan-maltose derivative for antitumor drug delivery. Int. J. Boil. Macromol. 2016, 92, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Arsalani, N.; Fattahi, H.; Nazarpoor, M. Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. Express Polym. Lett. 2010, 4, 329–338. [Google Scholar] [CrossRef]
- Iurea, D.M.; Peptu, C.A.; Chailan, J.F.; Carriere, P.; Popa, M. Sub-Micronic Capsules Based on Gelatin and Poly(maleic anhydride-alt-vinylacetate) Obtained by Interfacial Condensation with Potential Biomedical Applications. J. Nanosci. Nanotechnol. 2013, 13, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Bacaita, E.S.; Ciobanu, B.C.; Popa, M.; Agop, M.; Desbrieres, J. Phases in the temporal multiscale evolution of the drug release mechanism in IPN-type chitosan based hydrogels. Phys. Chem. Chem. Phys. 2014, 16, 25896–25905. [Google Scholar] [CrossRef] [PubMed]
- Leiter, J.; Abbott, D.J.; Schepartz, S.A. Screening data from the Cancer Chemotherapy National Service Center Screening Laboratories, XXVIII. Cancer Res. 1965, 25, 1626–1769. [Google Scholar] [PubMed]
- Dold, U. Criteria for the evaluation of cytostatic chemotherapy. Int. J. Clin. Pharmacol. Biopharm. 1978, 16, 68–71. [Google Scholar]
- De Vita, V.T., Jr. Cancer: Principles and Practice of Oncology, 7th ed.; De Vita, V.T., Jr., Hellman, S., Rosenberg, S.A., Eds.; Lippincott Williams & Wilkins Publishers: Philadelphia, PA, USA, 2004. [Google Scholar]
- Cismaru, L.; Hamaide, T.; Popa, M. Itaconic anhydride based amphiphilic copolymers: Synthesis, characterization and stabilization of carboxyl functionalized, PEGylated nanoparticles. Eur. Polym. J. 2007, 43, 4843–4851. [Google Scholar] [CrossRef]
- Rodrigues, G.R.; López-Abarrategui, C.; Gómez, I.D.L.S.; Dias, S.C.; Otero-González, A.J.; Franco, O.L. Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. Int. J. Pharm. 2019, 555, 356–367. [Google Scholar] [CrossRef]
- Hritcu, D.; Popa, M.I.; Popa, N.; Badescu, V.; Balan, V. Preparation and characterization of magnetic chitosan nanospheres. Turk. J. Chem. 2009, 33, 785–796. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Laville, N.; Aït-Aïssa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockert, J.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Cann, A.J. Maths from Scratch for Biologists; Jon Willey & Sons Ltd.: New York, NY, USA, 2003. [Google Scholar]











| Sample Code | Molar Ratio (Moles of CS NH2Groups/Moles of Anhydride Cycles of Poly(NVPAI) | % CS (w/v) | Fe3O4/CS (%) | Aqueous Phase/Organic Phase Ratio (v/v) | Yield (%) |
|---|---|---|---|---|---|
| CN | 0.3/1 | 0.75 | - | 1:2 | 45 |
| CNM-1 | 20 | 38 | |||
| CNM-2 | 30 | 40 | |||
| CNM-3 | 40 | 44 | |||
| CNM-4 | 50 | 53 | |||
| CNM-5 | 80 | 57 | |||
| CNM-6 | 50 | 1:2.5 | 68 | ||
| CNM-7 | 1:3 | 78 | |||
| CNM-8 | 1:3.5 | 81 |
| Sample | Zeta-Potential (mV) | Conductivity (mS/cm) |
|---|---|---|
| CNM-1 | −17.7 | 12.5 |
| CNM-2 | −18.5 | 14.1 |
| CNM-3 | −19.6 | 15.4 |
| CNM-4 | −19.8 | 18.7 |
| CNM-5 | −20.9 | 17.6 |
| CNM-6 | −17.9 | 18.2 |
| CNM-7 | −19.8 | 12.2 |
| CNM-8 | −20.1 | 16.9 |
| Sample code | CNM-1 | CNM-4 | CNM-5 | CNM-6 | CNM-7 | CNM-8 |
|---|---|---|---|---|---|---|
| Loading efficiency (%) | 29 | 26 | 22 | 27 | 29 | 32 |
| Sample | CNM-1 | CNM-4 | CNM-5 | CNM-6 | CNM-7 | CNM-8 |
|---|---|---|---|---|---|---|
| k | 0.167 | 0.195 | 0.112 | 0.157 | 0.090 | 0.094 |
| n | 0.184 | 0.187 | 0.273 | 0.220 | 0.308 | 0.303 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dellali, K.Z.; Rata, D.M.; Popa, M.; Djennad, M.; Ouagued, A.; Gherghel, D. Antitumoral Drug: Loaded Hybrid Nanocapsules Based on Chitosan with Potential Effects in Breast Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 5659. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21165659
Dellali KZ, Rata DM, Popa M, Djennad M, Ouagued A, Gherghel D. Antitumoral Drug: Loaded Hybrid Nanocapsules Based on Chitosan with Potential Effects in Breast Cancer Therapy. International Journal of Molecular Sciences. 2020; 21(16):5659. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21165659
Chicago/Turabian StyleDellali, Kheira Zanoune, Delia Mihaela Rata, Marcel Popa, M’hamed Djennad, Abdallah Ouagued, and Daniela Gherghel. 2020. "Antitumoral Drug: Loaded Hybrid Nanocapsules Based on Chitosan with Potential Effects in Breast Cancer Therapy" International Journal of Molecular Sciences 21, no. 16: 5659. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21165659