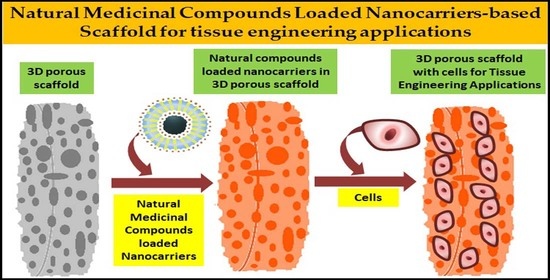
Advancement of Nanobiomaterials to Deliver Natural Compounds for Tissue Engineering Applications
Abstract
:1. Introduction
2. Biomaterials for Tissue Engineering
2.1. Natural Polymer-Based Biomaterials
2.2. Synthetic Polymer-Based Biomaterials
3. The Potential Role of Nanocarriers in Tissue Engineering
4. Effects of Natural Compounds Incorporated Scaffold in Tissue Engineering
5. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ALP | Alkaline phosphatase |
| ECM | Extracellular matrix |
| FDA | Food and Drug Administration |
| G1 phase | Gap 1 phase cell cycle |
| HA | Hyaluronic acid |
| HAp | Hydroxyapatite |
| HEK293 | Human Embryonic Kidney cells |
| HepG2 | Hepatocellular carcinoma cells |
| hMSCs | Human mesenchymal stem cells |
| L-929 | Fibroblast cells |
| MC3T3-E1 | Pre-osteoblast cells |
| MG-63 | Osteoblast like fibroblast cells |
| MSN or MSNPs | Mesoporous silica nanoparticles |
| NGF | Nerve growth factor |
| NIH3T3 | Fibroblast cells |
| nZnO | Nano Zinc Oxide |
| PC12 | Pheochromocytoma cells |
| PCL | Polycaprolactone |
| PGA | Polyglycolic acid |
| PHBV | Polyhydroxybutyrate-co-(3-hydroxyvalerate) |
| PLA | Polylactic acid |
| PLGA | Poly (lactic-co-glycolic acid) |
| PLL | Poly-L-lysine |
| PLLA | Poly (L-lactic acid) |
| RT-PCR | Real time—Polymerase chain reaction |
| Saos-2 | Osteosarcoma epithelial cells |
| Sca-1 | Stem cells antigen 1 |
| SF | Silk fibroin |
| TCP | Tricalcium phosphate |
| TiO2 | Titanium Oxide |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
| Zn | Zinc |
| ZrO2 | Zirconia |
References
- Rezaei, R.; Safaeikatouli, M.; Mozaffari, H.R.; Moradpoor, H.; Karami, S.; Golshah, A.; Salimi, B.; Karami, H. The Role of Nanomaterials in the Treatment of Diseases and Their Effects on the Immune System. Open Access Maced. J. Med. Sci. 2019, 7, 1884–1890. [Google Scholar] [CrossRef] [Green Version]
- Patil, M.; Mehta, D.S.; Guvva, S. Future impact of nanotechnology on medicine and dentistry. J. Indian Soc. Periodontol. 2008, 12, 34–40. [Google Scholar] [CrossRef]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [Green Version]
- Sahai, N.; Ahmad, N.; Gogoi, M. Nanoparticles Based Drug Delivery for Tissue Regeneration Using Biodegradable Scaffolds: A Review. Curr. Pathobiol. Rep. 2018, 6, 219–224. [Google Scholar] [CrossRef]
- Olson, J.L.; Atala, A.; Yoo, J.J. Tissue Engineering: Current Strategies and Future Directions. Chonnam. Med. J. 2011, 47, 1–13. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Mahomoodally, M. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evid. Based Complement. Altern. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Sarkar, N. Natural Medicinal Compounds in Bone Tissue Engineering. Trends Biotechnol. 2020, 38, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, S.; Mitchell, G. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, W.; He, J.; Sang, F.; Ding, B.; Chen, L.; Cui, S.; Li, K.; Han, Q.; Tan, W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite–tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2016, 58, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.-Q.; Peng, S.; Song, Z.-Y.; Lin, S. Collagen biomaterial for the treatment of myocardial infarction: An update on cardiac tissue engineering and myocardial regeneration. Drug Deliv. Transl. Res. 2019, 9, 920–934. [Google Scholar] [CrossRef]
- Shi, C.; Li, Q.; Zhao, Y.; Chen, W.; Chen, B.; Xiao, Z.; Lin, H.; Nie, L.; Wang, N.; Dai, J. Stem-cell-capturing collagen scaffold promotes cardiac tissue regeneration. Biomaterials 2011, 32, 2508–2515. [Google Scholar] [CrossRef]
- Yoon, D.; Cho, Y.S.; Joo, S.Y.; Seo, C.H.; Cho, Y.-S. A clinical trial with a novel collagen dermal substitute for wound healing in burn patients. Biomater. Sci. 2019, 8, 823–829. [Google Scholar] [CrossRef]
- Boccafoschi, F.; Habermehl, J.; Vesentini, S.; Mantovani, D. Biological performances of collagen-based scaffolds for vascular tissue engineering. Biomaterials 2005, 26, 7410–7417. [Google Scholar] [CrossRef]
- Minardi, S.; Taraballi, F.; Wang, X.; Cabrera, F.J.; Van Eps, J.L.; Robbins, A.B.; Sandri, M.; Moreno, M.R.; Weiner, B.K.; Tasciotti, E. Biomimetic collagen/elastin meshes for ventral hernia repair in a rat model. Acta Biomater. 2017, 50, 165–177. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Silk Fibroin in Tissue Engineering. Adv. Healthc. Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Wang, Y.; Dai, W. Silk fibroin-based biomaterials for musculoskeletal tissue engineering. Mater. Sci. Eng. C 2018, 89, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, X.; Shi, J.; Zhu, R.; Zhang, J.; Zhang, Z.; Ma, D.; Hou, Y.; Lin, F.; Yang, J.; et al. A Biomimetic Silk Fibroin/Sodium Alginate Composite Scaffold for Soft Tissue Engineering. Sci. Rep. 2016, 6, 39477. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.N.; Pramanik, K. Fabrication and evaluation of non-mulberry silk fibroin fiber reinforced chitosan based porous composite scaffold for cartilage tissue engineering. Tissue Cell 2018, 55, 83–90. [Google Scholar] [CrossRef]
- Yan, C.; Ren, Y.; Sun, X.; Jin, L.; Liu, X.; Chen, H.; Wang, K.; Yu, M.; Zhao, Y. Photoluminescent functionalized carbon quantum dots loaded electroactive Silk fibroin/PLA nanofibrous bioactive scaffolds for cardiac tissue engineering. J. Photochem. Photobiol. B Biol. 2020, 202, 111680. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [Green Version]
- Mallepally, R.R.; Marin, M.A.; Surampudi, V.; Subia, B.; Rao, R.R.; Kundu, S.C.; McHugh, M.A. Silk fibroin aerogels: Potential scaffolds for tissue engineering applications. Biomed. Mater. 2015, 10, 035002. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Nguyen, Q.T.; Chen, A.C.; Kaplan, D.L.; Sah, R.L.; Kundu, S.C. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials 2011, 32, 5773–5781. [Google Scholar] [CrossRef] [Green Version]
- Mobika, J.; Rajkumar, M.; Priya, V.N.; Sibi, S.L. Substantial effect of silk fibroin reinforcement on properties of hydroxyapatite/silk fibroin nanocomposite for bone tissue engineering application. J. Mol. Struct. 2020, 1206, 127739. [Google Scholar] [CrossRef]
- Reizabal, A.; Brito-Pereira, R.; Fernandes, M.M.; Castro, N.; Correia, V.; Ribeiro, C.; Costa, C.M.; Perez, L.; Vilas, J.; Lanceros-Méndez, S. Silk fibroin magnetoactive nanocomposite films and membranes for dynamic bone tissue engineering strategies. Materials 2020, 12, 100709. [Google Scholar] [CrossRef]
- Wöltje, M.; Böbel, M.; Bienert, M.; Neuss, S.; Aibibu, D.; Cherif, C. Functionalized silk fibers from transgenic silkworms for wound healing applications: Surface presentation of bioactive epidermal growth factor. J. Biomed. Mater. Res. Part A 2018, 106, 2643–2652. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Burgo, L.S.; Pedraz, J.L.; Orive, G.; Echave, M.C. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Li, Z.; Qu, T.; Ding, C.; Ma, C.; Sun, H.; Li, S.; Liu, X. Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomater. 2014, 13, 88–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, A.; Tonda-Turo, C.; De Pasquale, D.; Ruini, F.; Genchi, G.; Nitti, S.; Cappello, V.; Gemmi, M.; Mattoli, V.; Ciardelli, G.; et al. Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 386–395. [Google Scholar] [CrossRef]
- Ali, M.G.; Mousa, H.M.; Blaudez, F.; El-Sadek, M.A.; Mohamed, M.; Abdel-Jaber, G.; Abdal-Hay, A.; Ivanovski, S. Dual nanofiber scaffolds composed of polyurethane-gelatin/nylon 6- gelatin for bone tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124817. [Google Scholar] [CrossRef]
- Nooeaid, P.; Chuysinuan, P.; Pengsuk, C.; Dechtrirat, D.; Lirdprapamongkol, K.; Techasakul, S.; Svasti, J. Polylactic Acid Microparticles Embedded Porous Gelatin Scaffolds with Multifunctional properties for Soft Tissue Engineering. J. Sci. Adv. Mater. Devices 2020. [Google Scholar] [CrossRef]
- Sharifi, F.; Irani, S.; Azadegan, G.; Pezeshki-Modaress, M.; Zandi, M.; Saeed, M. Co-electrospun gelatin-chondroitin sulfate/polycaprolactone nanofibrous scaffolds for cartilage tissue engineering. Bioact. Carbohydr. Diet. Fibre 2020, 22, 100215. [Google Scholar] [CrossRef]
- Govindan, R.; Gu, F.; Karthi, S.; Girija, E. Effect of phosphate glass reinforcement on the mechanical and biological properties of freeze-dried gelatin composite scaffolds for bone tissue engineering applications. Mater. Today Commun. 2020, 22, 100765. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.-Y.; Huang, W.-C.; Chen, Y.; Baskaran, N.; Yu, J.; Wei, Y.; Kumar, B.N. Effect of Varied Hair Protein Fractions on the Gel Properties of Keratin/Chitosan Hydrogels for the Use in Tissue Engineering. Colloids Surf. B Biointerfaces 2020, 195, 111258. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Kumar, R.; Sripriya, R.; Kakkar, P.; Ramesh, D.V.; Reddy, P.N.K.; Sehgal, P. Preparation and comparative characterization of keratin–chitosan and keratin-gelatin composite scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2012, 32, 975–982. [Google Scholar] [CrossRef]
- Lv, X.; Li, Z.; Zhang, M.; Xie, M.; Huang, J.; Peng, X.; Yang, R.; Wang, H.; Xu, Y.-M.; Feng, C. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 2016, 84, 99–110. [Google Scholar] [CrossRef]
- Dou, J.; Wang, Y.; Jin, X.; Li, P.; Wang, L.; Yuan, J.; Shen, J. PCL/sulfonated keratin mats for vascular tissue engineering scaffold with potential of catalytic nitric oxide generation. Mater. Sci. Eng. C 2020, 107, 110246. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydr. Polym. 2011, 83, 1433–1445. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [Green Version]
- Kiuchi, H.; Kai, W.; Inoue, Y. Preparation and characterization of poly(ethylene glycol) crosslinked chitosan films. J. Appl. Polym. Sci. 2007, 107, 3823–3830. [Google Scholar] [CrossRef]
- Gümüşderelioğlu, M.; Aday, S. Heparin-functionalized chitosan scaffolds for bone tissue engineering. Carbohydr. Res. 2011, 346, 606–613. [Google Scholar] [CrossRef]
- Li, G.; Xiao, Q.; Zhang, L.; Zhao, Y.; Yang, Y. Nerve growth factor loaded heparin/chitosan scaffolds for accelerating peripheral nerve regeneration. Carbohydr. Polym. 2017, 171, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Wang, H.; Zhao, W.; Li, N.; Kong, D.; Yang, J.; Zhang, Y. Gradient nanofibrous chitosan/poly ε-caprolactone scaffolds as extracellular microenvironments for vascular tissue engineering. Biomaterials 2012, 33, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Rodrigues, G.; Martins, G.G.; Henriques, C.; Silva, J.C. Evaluation of nanofibrous scaffolds obtained from blends of chitosan, gelatin and polycaprolactone for skin tissue engineering. Int. J. Biol. Macromol. 2017, 102, 1174–1185. [Google Scholar] [CrossRef]
- Wang, S.; Sun, C.; Guan, S.; Li, W.; Xu, J.; Ge, D.; Zhuang, M.; Liu, T.; Ma, X. Chitosan/gelatin porous scaffolds assembled with conductive poly(3,4-ethylenedioxythiophene) nanoparticles for neural tissue engineering. J. Mater. Chem. B 2017, 5, 4774–4788. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, Y.-J.; Ren, L.; Wu, G.; Caridade, S.G.; Fan, J.-B.; Wang, L.-Y.; Ji, P.-H.; Oliveira, J.; Oliveira, J.T.; et al. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J. Biomed. Mater. Res. Part A 2010, 95, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Li, F.; Ducker, M.; Sun, B.; Szele, F.; Czernuszka, J.T. Interpenetrating polymer networks of collagen, hyaluronic acid, and chondroitin sulfate as scaffolds for brain tissue engineering. Acta Biomater. 2020, 112, 122–135. [Google Scholar] [CrossRef]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef]
- Suner, S.S.; Demirci, S.; Yetiskin, B.; Fakhrullin, R.; Naumenko, E.; Okay, O.; Ayyala, R.S.; Sahiner, N. Cryogel composites based on hyaluronic acid and halloysite nanotubes as scaffold for tissue engineering. Int. J. Biol. Macromol. 2019, 130, 627–635. [Google Scholar] [CrossRef]
- Kenar, H.; Ozdogan, C.Y.; Dumlu, C.; Doger, E.; Kose, G.T.; Hasirci, V. Microfibrous scaffolds from poly(l-lactide-co-ε-caprolactone) blended with xeno-free collagen/hyaluronic acid for improvement of vascularization in tissue engineering applications. Mater. Sci. Eng. C 2019, 97, 31–44. [Google Scholar] [CrossRef]
- Florczyk, S.J.; Wang, K.; Jana, S.; Wood, D.L.; Sytsma, S.K.; Sham, J.G.; Kievit, F.M.; Zhang, M. Porous chitosan-hyaluronic acid scaffolds as a mimic of glioblastoma microenvironment ECM. Biomaterials 2013, 34, 10143–10150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, C.R.; Moreira-Teixeira, L.S.; Moroni, L.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; Mano, J.F. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng. Part C Methods 2011, 17, 717–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [Green Version]
- Coimbra, P.; Alves, P.; Valente, T.; Santos, R.; Correia, I.J.; Ferreira, P. Sodium hyaluronate/chitosan polyelectrolyte complex scaffolds for dental pulp regeneration: Synthesis and characterization. Int. J. Biol. Macromol. 2011, 49, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kang, E.; Kang, S.-W.; Huh, K.M. Thermo-irreversible glycol chitosan/hyaluronic acid blend hydrogel for injectable tissue engineering. Carbohydr. Polym. 2020, 244, 116432. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, A.R.; Sasikala, A.R.K.; Kim, C.S.; Kim, C.S. A unique scaffold for bone tissue engineering: An osteogenic combination of graphene oxide–hyaluronic acid–chitosan with simvastatin. J. Ind. Eng. Chem. 2017, 46, 182–191. [Google Scholar] [CrossRef]
- Lee, S.J.; Nah, H.; Heo, D.N.; Kim, K.-H.; Seok, J.M.; Heo, M.; Moon, H.-J.; Lee, D.; Lee, J.S.; An, S.Y.; et al. Induction of osteogenic differentiation in a rat calvarial bone defect model using an In situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon 2020, 168, 264–277. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Lancucka, J.; Guzdek-Zajac, K.; Karewicz, A.; Horak, W.; Lach, R.; Wójcik, K.; Nowakowska, M. Bioactive yet antimicrobial structurally stable collagen/chitosan/lysine functionalized hyaluronic acid-based injectable hydrogels for potential bone tissue engineering applications. Int. J. Biol. Macromol. 2020, 155, 938–950. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462. [Google Scholar] [CrossRef]
- Bae, H.; Ahari, A.F.; Shin, H.; Nichol, J.W.; Hutson, C.B.; Masaeli, M.; Kim, S.-H.; Aubin, H.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered pullulan methacrylate hydrogels promote cell proliferation and 3D cluster formation. Soft Matter 2011, 7, 1903–1911. [Google Scholar] [CrossRef]
- Arora, A.; Sharma, P.; Katti, D.S. Pullulan-based composite scaffolds for bone tissue engineering: Improved osteoconductivity by pore wall mineralization. Carbohydr. Polym. 2015, 123, 180–189. [Google Scholar] [CrossRef]
- Han, Y.; Lv, S. Synthesis of chemically crosslinked pullulan/gelatin-based extracellular matrix-mimetic gels. Int. J. Biol. Macromol. 2019, 122, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Dalgic, A.D.; Atila, D.; Karatas, A.; Tezcaner, A.; Keskin, D. Diatom shell incorporated PHBV/PCL-pullulan co-electrospun scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Schlaubitz, S.; Derkaoui, S.M.; Marosa, L.; Miraux, S.; Renard, M.; Catros, S.; Le Visage, C.; Letourneur, D.; Amédée, J.; Fricain, J.-C. Pullulan/dextran/nHA Macroporous Composite Beads for Bone Repair in a Femoral Condyle Defect in Rats. PLoS ONE 2014, 9, e110251. [Google Scholar] [CrossRef] [PubMed]
- Della Giustina, G.; Gandin, A.; Brigo, L.; Panciera, T.; Giulitti, S.; Sgarbossa, P.; D’Alessandro, D.; Trombi, L.; Danti, S.; Brusatin, G. Polysaccharide hydrogels for multiscale 3D printing of pullulan scaffolds. Mater. Des. 2019, 165, 107566. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Joupari, M.D. Bioactive and biostable hyaluronic acid-pullulan dermal hydrogels incorporated with biomimetic hydroxyapatite spheres. Mater. Sci. Eng. C 2020, 112, 110906. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Tezcaner, A. Crosslinked pullulan/cellulose acetate fibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2016, 69, 1103–1115. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate. Carbohydr. Polym. 2019, 230, 115650. [Google Scholar] [CrossRef]
- Kwon, H.J.; Han, Y. Chondroitin sulfate-based biomaterials for tissue engineering. Turk. J. Biol. 2016, 40, 290–299. [Google Scholar] [CrossRef]
- Sadeghi, A.; Zandi, M.; Pezeshki-Modaress, M.; Rajabi, S. Tough, hybrid chondroitin sulfate nanofibers as a promising scaffold for skin tissue engineering. Int. J. Biol. Macromol. 2019, 132, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Pallela, R.; Bhatnagar, I.; Kim, S.-K. Chitosan-amylopectin/hydroxyapatite and chitosan-chondroitin sulphate/hydroxyapatite composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 51, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Piai, J.F.; Da Silva, M.L.A.; Martins, A.; Torres, A.B.; Faria, S.; Reis, R.L.; Muniz, E.C.; Neves, N. Chondroitin sulfate immobilization at the surface of electrospun nanofiber meshes for cartilage tissue regeneration approaches. Appl. Surf. Sci. 2017, 403, 112–125. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, X.; Cai, D.; Li, J.; Mu, Q.; Zhang, W.; Zhu, S.; Jiang, Y.Z.; Shen, W.L.; Zhang, S.; et al. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017, 63, 64–75. [Google Scholar] [CrossRef]
- Bhowmick, S.; Rother, S.; Zimmermann, H.; Lee, P.S.; Moeller, S.; Schnabelrauch, M.; Koul, V.; Jordan, R.; Hintze, V.; Scharnweber, D. Biomimetic electrospun scaffolds from main extracellular matrix components for skin tissue engineering application—The role of chondroitin sulfate and sulfated hyaluronan. Mater. Sci. Eng. C 2017, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Saporito, F.; Sandri, G.; Bonferoni, M.; Rossi, S.; Malavasi, L.; Del Fante, C.; Vigani, B.; Black, L.D.; Ferrari, F. Electrospun Gelatin–Chondroitin Sulfate Scaffolds Loaded with Platelet Lysate Promote Immature Cardiomyocyte Proliferation. Polymers 2018, 10, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zărnescu, O.; Craciunescu, O.; Seciu, A.-M.; Stanciuc, A.M.; Moldovan, L. Collagen-chondroitin 4-sulfate-fibronectin scaffold: Characterization and in vitro biocompatibility. J. Biotechnol. 2018, 280, S91. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, S.; Guan, Y.; Deng, J.; Lou, S.; Feng, D.; Kong, D.; Li, C. Pancreatic islet surface engineering with a starPEG-chondroitin sulfate nanocoating. Biomater. Sci. 2019, 7, 2308–2316. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef]
- Fenbo, M.; Sijing, L.; Ruiz-Ortega, L.; Yuanjun, Z.; Lei, X.; Kui, W.; Lijun, L.; Bin, T. Effects of alginate/chondroitin sulfate-based hydrogels on bone defects healing. Mater. Sci. Eng. C 2020, 116, 111217. [Google Scholar] [CrossRef]
- Ma, F.B.; Xia, X.Y.; Tang, B. Strontium chondroitin sulfate/silk fibroin blend membrane containing microporous structure modulates macrophage responses for guided bone regeneration. Carbohydr. Polym. 2019, 213, 266–275. [Google Scholar]
- Kudryavtseva, V.; Stankevich, K.S.; Gudima, A.; Kibler, E.; Zvereva, I.A.; Bolbasov, E.; Malashicheva, A.; Zhuravlev, M.; Riabov, V.; Liu, T.; et al. Atmospheric pressure plasma assisted immobilization of hyaluronic acid on tissue engineering PLA-based scaffolds and its effect on primary human macrophages. Mater. Des. 2017, 127, 261–271. [Google Scholar] [CrossRef]
- Hassanajili, S.; Pour, A.K.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Hassanajili, S.; Sahvieh, S.; Azarpira, N. Effectiveness of mesenchymal stem cell-seeded onto the 3D polylactic acid/polycaprolactone/hydroxyapatite scaffold on the radius bone defect in rat. Life Sci. 2020, 257, 118038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-K.; Wang, H.; Yang, R.; Liu, M.; Ban, Q.; Chen, W.; Zhao, M.; You, R.; Jin, Y.; Guan, Y.-Q. Bone marrow stem cells combined with polycaprolactone-polylactic acid-polypropylene amine scaffolds for the treatment of acute liver failure. Chem. Eng. J. 2019, 360, 1564–1576. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Kerimoglu, O.; Alarcin, E. Poly(Lactic-Co-Glycolic Acid) Based Drug Delivery Devices For Tissue Engineering And Regenerative Medicine. ANKEM Derg. 2012, 26, 86–98. [Google Scholar] [CrossRef]
- Nokhasteh, S.; Sadeghi-Avalshahr, A.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Molavi, A.M. Effect of bioactive glass nanoparticles on biological properties of PLGA/collagen scaffold. Prog. Biomater. 2018, 7, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooney, D.J.; Mazzoni, C.L.; Breuer, C.; McNamara, K.; Hern, D.; Vacanti, J.P.; Langer, R. Stabilized polyglycolic acid fibre-based tubes for tissue engineering. Biomaterials 1996, 17, 115–124. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Yang, X.; Xi, Z.; Zhao, L.; Cen, L.; Lu, E.; Yang, Y. Novel Fabricating Process for Porous Polyglycolic Acid Scaffolds by Melt-Foaming Using Supercritical Carbon Dioxide. ACS Biomater. Sci. Eng. 2018, 4, 694–706. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, X.; Gan, Z.; Wang, F. Modification of porous PLGA microspheres by poly-l-lysine for use as tissue engineering scaffolds. Colloids Surf. B Biointerfaces 2018, 161, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.X.J.; Lee, L.Y.; Davoodi, P.; Wang, C.-H. Production of drug-releasing biodegradable microporous scaffold using a two-step micro-encapsulation/supercritical foaming process. J. Supercrit. Fluids 2018, 133, 263–269. [Google Scholar] [CrossRef]
- Amirthalingam, M.; Kasinathan, N.; Amuthan, A.; Mutalik, S.; Reddy, M.S.; Nayanabhirama, U. Bioactive PLGA–curcumin microparticle-embedded chitosan scaffold: In vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, R.; Zhou, F.; Streubel, P.N.; Chen, S.; Duan, B. Electrospun Thymosin Beta-4 Loaded PLGA/PLA Nanofiber/Microfiber Hybrid Yarns for Tendon Tissue Engineering Application. Mater. Sci. Eng. C 2019, 106, 110268. [Google Scholar] [CrossRef] [PubMed]
- Qodratnama, R.; Serino, L.P.; Cox, H.C.; Qutachi, O.; White, L.J. Formulations for modulation of protein release from large-size PLGA microparticles for tissue engineering. Mater. Sci. Eng. C 2015, 47, 230–236. [Google Scholar] [CrossRef]
- Khojasteh, A.; Fahimipour, F.; Eslaminejad, M.B.; Jafarian, M.; Jahangir, S.; Bastami, F.; Tahriri, M.; Karkhaneh, A.; Tayebi, L. Development of PLGA-coated β-TCP scaffolds containing VEGF for bone tissue engineering. Mater. Sci. Eng. C 2016, 69, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Fahimipour, F.; Rasoulianboroujeni, M.; Dashtimoghadam, E.; Khoshroo, K.; Tahriri, M.; Bastami, F.; Lobner, D.; Tayebi, L. 3D printed TCP-based scaffold incorporating VEGF-loaded PLGA microspheres for craniofacial tissue engineering. Dent. Mater. 2017, 33, 1205–1216. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Harikrishnan, P.; Sivasamy, A. Preparation, characterization of Electrospun Polycaprolactone-nano Zinc oxide composite scaffolds for Osteogenic applications. Nano Struct. Nano Objects 2020, 23, 100518. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.; Wang, S.; Steffen, R.; Ackermann, M.; Haep, N.D.; Schröder, H.C.; Müller, W.E. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017, 64, 377–388. [Google Scholar] [CrossRef]
- Tan, H.-L.; Kai, D.; Pasbakhsh, P.; Teow, S.-Y.; Lim, Y.-Y.; Pushpamalar, J. Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: A potential scaffold for tissue engineering. Colloids Surf. B Biointerfaces 2020, 188, 110713. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Synthesis and characterization of nHA-PEG and nBG-PEG scaffolds for hard tissue engineering applications. Ceram. Int. 2019, 45, 8370–8379. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.; Tamer, T.M.; El-Meligy, M.A.; Eldin, M.S.M. Poly(vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Kanimozhi, K.; Basha, S.K.; Kumari, V.S. Fabrication of chitosan based hybrid porous scaffolds by salt leaching for soft tissue engineering. Surf. Interfaces 2016, 1, 7–12. [Google Scholar] [CrossRef]
- Bi, S.; Pang, J.; Huang, L.; Sun, M.; Cheng, X.J.; Chen, X. The toughness chitosan-PVA double network hydrogel based on alkali solution system and hydrogen bonding for tissue engineering applications. Int. J. Biol. Macromol. 2020, 146, 99–109. [Google Scholar] [CrossRef]
- Thangprasert, A.; Tansakul, C.; Thuaksubun, N.; Meesane, J. Mimicked hybrid hydrogel based on gelatin/PVA for tissue engineering in subchondral bone interface for osteoarthritis surgery. Mater. Des. 2019, 183, 108113. [Google Scholar] [CrossRef]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef]
- Ali, A.; Bano, S.; Priyadarshi, R.; Negi, Y.S. Effect of carbon based fillers on properties of Chitosan/PVA/βTCP based composite scaffold for bone tissue engineering. Mater. Today Proc. 2019, 15, 173–182. [Google Scholar] [CrossRef]
- Kim, H.; Yang, G.H.; Choi, C.H.; Cho, Y.-S.; Kim, G.H. Gelatin/PVA scaffolds fabricated using a 3D-printing process employed with a low-temperature plate for hard tissue regeneration: Fabrication and characterizations. Int. J. Biol. Macromol. 2018, 120, 119–127. [Google Scholar] [CrossRef]
- Lin, B.; Hu, H.; Deng, Z.; Pang, L.; Jiang, H.; Wang, D.; Li, J.; Liu, Z.; Wang, H.; Zeng, X. Novel bioactive glass cross-linked PVA hydrogel with enhanced chondrogenesis properties and application in mice chondrocytes for cartilage repair. J. Non-Cryst. Solids 2020, 529, 119594. [Google Scholar] [CrossRef]
- Koosha, M.; Raoufi, M.; Moravvej, H. One-pot reactive electrospinning of chitosan/PVA hydrogel nanofibers reinforced by halloysite nanotubes with enhanced fibroblast cell attachment for skin tissue regeneration. Colloids Surf. B Biointerfaces 2019, 179, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Salatin, S.; Saadat, Y.R.; Vahed, S.Z.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E.; et al. The Use of Nanomaterials in Tissue Engineering for Cartilage Regeneration; Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaeini-Hesaroeiye, S.; Bagtash, H.R.R.; Boddohi, S.; Vasheghani-F, E.; Jabbari, E. Thermoresponsive Nanogels Based on Different Polymeric Moieties for Biomedical Applications. Gels 2020, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Vicario-De-La-Torre, M.; Forcada, J. The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels 2017, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Nanogels for regenerative medicine. J. Control. Release 2019, 313, 148–160. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Ota, M.; Shimoda, A.; Nakahama, K.-I.; Akiyoshi, K.; Miyamoto, Y.; Iseki, S. Cholesteryl group- and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials 2012, 33, 7613–7620. [Google Scholar] [CrossRef]
- Sato, Y.; Yamamoto, K.; Horiguchi, S.; Tahara, Y.; Nakai, K.; Kotani, S.-I.; Oseko, F.; Pezzotti, G.; Yamamoto, T.; Kishida, T.; et al. Nanogel tectonic porous 3D scaffold for direct reprogramming fibroblasts into osteoblasts and bone regeneration. Sci. Rep. 2018, 8, 15824. [Google Scholar] [CrossRef] [Green Version]
- Mohandesnezhad, S.; Pilehvar-Soltanahmadi, Y.; Alizadeh, E.; Goodarzi, A.; Davaran, S.; Khatamian, M.; Zarghami, N.; Samiei, M.; Aghazadeh, M.; Akbarzadeh, A. In vitro evaluation of Zeolite-nHA blended PCL/PLA nanofibers for dental tissue engineering. Mater. Chem. Phys. 2020, 252, 123152. [Google Scholar] [CrossRef]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Phan, T.T.V.; Kim, H.H.; Nguyen, T.P.; Oh, J. Rapid microwave-assisted synthesis of gold loaded hydroxyapatite collagen nano-bio materials for drug delivery and tissue engineering application. Ceram. Int. 2019, 45, 2977–2988. [Google Scholar] [CrossRef]
- Johari, N.; Hosseini, H.M.; Samadikuchaksaraei, A. Optimized composition of nanocomposite scaffolds formed from silk fibroin and nano-TiO2 for bone tissue engineering. Mater. Sci. Eng. C 2017, 79, 783–792. [Google Scholar] [CrossRef]
- Oliveira, J.; Kotobuki, N.; Tadokoro, M.; Hirose, M.; Mano, J.F.; Reis, R.L.; Ohgushi, H. Ex vivo culturing of stromal cells with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles promotes ectopic bone formation. Bone 2010, 46, 1424–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Fan, C.; Xiao, Z.; Zhao, Y.; Zhang, H.; Sun, J.; Zhuang, Y.; Wu, X.; Shi, J.; Chen, Y.; et al. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/β-catenin signaling for spinal cord injury repair. Biomaterials 2018, 183, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Arranja, A.; Chen, H.; Mytnyk, S.; Wang, Y.; Oldenhof, S.; Van Esch, J.H.; Mendes, E. A nano-fibrous platform of copolymer patterned surfaces for controlled cell alignment. RSC Adv. 2018, 8, 21777–21785. [Google Scholar] [CrossRef] [Green Version]
- Rasoulianboroujeni, M.; Fahimipour, F.; Shah, P.; Khoshroo, K.; Tahriri, M.; Eslami, H.; Yadegari, A.; Dashtimoghadam, E.; Tayebi, L. Development of 3D-printed PLGA/TiO2 nanocomposite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2019, 96, 105–113. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.-H.; Eltohamy, M.; Perez, R.A.; Lee, E.-J.; Kim, H.-W. Collagen gel combined with mesoporous nanoparticles loading nerve growth factor as a feasible therapeutic three-dimensional depot for neural tissue engineering. RSC Adv. 2013, 3, 24202. [Google Scholar] [CrossRef]
- Nabavinia, M.; Khoshfetrat, A.B.; Naderi-Meshkin, H. Nano-hydroxyapatite-alginate-gelatin microcapsule as a potential osteogenic building block for modular bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Fricain, J.C.; Schlaubitz, S.; Visage, C.L.; Arnault, I. A nano-hydroxyapatite—Pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials 2013, 34, 2947–2959. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Bagri, L.P.; Bajpai, A. Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications. Colloids Surf. B Biointerfaces 2019, 177, 211–218. [Google Scholar] [CrossRef]
- Teimour, A.; Ebrahimi, R.; Emadi, R.; Beni, B.H.; Chermahini, A.N. Nano-composite of silk fibroin–chitosan/Nano ZrO2 for tissue engineering applications: Fabrication and morphology. Int. J. Biol. Macromol. 2015, 76, 292–302. [Google Scholar] [CrossRef]
- Paşcu, E.I.; Stokes, J.; McGuinness, G. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 4905–4916. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Lisar, H.A.; Kashi, T.S.J.; Tahriri, M.; Ansari, M.; Rafiei, T.; Bastami, F.; Shahin-Shamsabadi, A.; Abbas, F.M.; Tayebi, L. Poly(lactic-co-glycolic acid)(PLGA)/TiO2 nanotube bioactive composite as a novel scaffold for bone tissue engineering: In vitro and in vivo studies. Biologicals 2018, 53, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Asadollahi, M.A.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol. 2015, 79, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pathak, J.L.; Liang, D.; Zhong, N.; Guan, H.; Wan, M.; Miao, G.; Li, Z.; Ge, L. Fabrication and characterization of strontium-hydroxyapatite/silk fibroin biocomposite nanospheres for bone-tissue engineering applications. Int. J. Biol. Macromol. 2020, 142, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases. Molecules 2018, 23, 835. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.S.D.; Mahadevan, S.; Vijayaraghavan, R.; Mandal, A.B.; MacFarlane, D.R. Cucrumin loaded poly(2-hydroxymethyl methacrylate) nanoparticles from gelled ionic liquid—In vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur. J. Pharm. Sci. 2014, 51, 34–44. [Google Scholar] [CrossRef]
- Ahangari, N.; Kargozar, S.; Ghayour-Mobarhan, M.; Baino, F.; Pasdar, A.; Sahebkar, A.; Ferns, G.A.A.; Kim, H.-W.; Mozafari, M. Curcumin in tissue engineering: A traditional remedy for modern medicine. BioFactors 2018, 45, 135–151. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Mahesh, A.; Mahadevan, S.; Mandal, A.B. Synthesis and characterization of curcumin loaded polymer/lipid based nanoparticles and evaluation of their antitumor effects on MCF-7 cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 1913–1922. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-N.; Hsu, S.-L.; Liao, M.-Y.; Liu, Y.-T.; Lai, C.-H.; Chen, J.-F.; Nguyen, M.-H.T.; Su, Y.-H.; Chen, S.-T.; Wu, L.-C. Ameliorative Effect of Curcumin-Encapsulated Hyaluronic Acid–PLA Nanoparticles on Thioacetamide-Induced Murine Hepatic Fibrosis. Int. J. Environ. Res. Public Health 2016, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Liu, J.; Zheng, H.; Wichmann, J.; Hopfner, U.; Sudhop, S.; Prein, C.; Shen, Y.; Machens, H.-G.; Schilling, A.F. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater. 2017, 9, e368. [Google Scholar] [CrossRef] [Green Version]
- Montalban, M.G.; Coburn, J.M.; Lozano-Pérez, A.A.; Cenis, J.L.; Víllora, G.; Kaplan, D.L. Production of Curcumin-Loaded Silk Fibroin Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganeshkumar, M.; Ponrasu, T.; Subamekala, M.K.; Janani, M.; Suguna, L. Curcumin loaded on pullulan acetate nanoparticles protects the liver from damage induced by DEN. RSC Adv. 2016, 6, 5599–5610. [Google Scholar] [CrossRef]
- Govindaraju, R.; Karki, R.; Chandrashekarappa, J.; Santhanam, M.; Shankar, A.K.; Joshi, H.K.; Divakar, G.; Roopa, G.; Jayanthi, C.; Mukunthan, S.; et al. Enhanced Water Dispersibility of Curcumin Encapsulated in Alginate-polysorbate 80 Nano Particles and Bioavailability in Healthy Human Volunteers. Pharm. Nanotechnol. 2019, 7, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Yanda, Y.L.; Rahma, A.; Mase, N. Curcumin-Loaded PLA Nanoparticles: Formulation and Physical Evaluation. Sci. Pharm. 2016, 84, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Nair, H.B.B.; Sung, B.; Kunnumakkara, A.B.; Yadav, V.R.; Tekmal, R.R.; Aggarwal, B.B. RETRACTED: Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem. Pharmacol. 2010, 79, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Sadeghianmaryan, A.; Yazdanpanah, Z.; Soltani, Y.A.; Sardroud, H.A.; Nasirtabrizi, M.H.; Chen, X.; Nasirtabrizi, M.H. Curcumin-loaded electrospun polycaprolactone/montmorillonite nanocomposite: Wound dressing application with anti-bacterial and low cell toxicity properties. J. Biomater. Sci. Polym. Ed. 2019, 31, 169–187. [Google Scholar] [CrossRef]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; Abrar, M. The therapeutic properties and applications of Aloe vera: A review. J. Herb. Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Eshun, K.; He, Q. Aloe Vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef]
- Salah, F.; El-Ghoul, Y.; Mahdhi, A.; Majdoub, H.; Jarroux, N.; Sakli, F. Effect of the deacetylation degree on the antibacterial and antibiofilm activity of acemannan from Aloe vera. Ind. Crops Prod. 2017, 103, 13–18. [Google Scholar] [CrossRef]
- Nema, J.; Shrivastava, S.K.; Mitra, N.G. Physicochemical study of acemannan polysaccharide in Aloe species under the influence of soil reaction (pH) and moisture application. Afr. J. Pure Appl. Chem. 2012, 6, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.S.; Popa, E.G.; Gomes, M.E.; Cerqueira, M.T.; Marques, A.P.; Caridade, S.G.; Teixeira, P.; Sousa, C.; Mano, J.F.; Reis, R.L. An investigation of the potential application of chitosan/aloe-based membranes for regenerative medicine. Acta Biomater. 2013, 9, 6790–6797. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.S.; Oliveira, M.B.; Mano, J.F.; Reis, R.L. Bio-inspired Aloe vera sponges for biomedical applications. Carbohydr. Polym. 2014, 112, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Jithendra, P.; Rajam, A.M.; Kalaivani, T.; Mandal, A.B.; Rose, C. Preparation and Characterization of Aloe Vera Blended Collagen-Chitosan Composite Scaffold for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2013, 5, 7291–7298. [Google Scholar] [CrossRef]
- Aghamohamadi, N.; Sharifi-Sanjani, N.; Majidi, R.F.; Nasrollahi, S.A. Preparation and characterization of Aloe vera acetate and electrospinning fibers as promising antibacterial properties materials. Mater. Sci. Eng. C 2018, 94, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Abou-Zeid, R.E.; Cruz-Maya, I.; Guarino, V. Soy protein hydrolysate grafted cellulose nanofibrils with bioactive signals for bone repair and regeneration. Carbohydr. Polym. 2019, 229, 115472. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, P.S.W.; Rhee, K.C. Functional properties of proteolytic enzyme modified soy protein isolate. J. Agric. Food Chem. 1990, 38, 651–656. [Google Scholar] [CrossRef]
- Mauri, A.N.; Añón, M.C. Effect of solution pH on solubility and some structural properties of soybean protein isolate films. J. Sci. Food Agric. 2006, 86, 1064–1072. [Google Scholar] [CrossRef]
- Tansaz, S.; Schulte, M.; Kneser, U.; Mohn, D.; Stark, W.; Roether, J.A.; Cicha, I.; Boccaccini, A.R. Soy protein isolate/bioactive glass composite membranes: Processing and properties. Eur. Polym. J. 2018, 106, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Tansaz, S.; Liverani, L.; Vester, L.; Boccaccini, A.R. Soy protein meets bioactive glass: Electrospun composite fibers for tissue engineering applications. Mater. Lett. 2017, 199, 143–146. [Google Scholar] [CrossRef]
- Tansaz, S.; Durmann, A.-K.; Detsch, R.; Boccaccini, A.R. Hydrogel films and microcapsules based on soy protein isolate combined with alginate. J. Appl. Polym. Sci. 2016, 134, 44358. [Google Scholar] [CrossRef]
- Peles, Z.; Zilberman, M. Novel soy protein wound dressings with controlled antibiotic release: Mechanical and physical properties. Acta Biomater. 2012, 8, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Vaz, C.M.; Coutinho, O.P.; Cunha, A.M.; Reis, R.L. In vitro degradation and cytocompatibility evaluation of novel soy and sodium caseinate-based membrane biomaterials. J. Mater. Sci. Mater. Electron. 2003, 14, 1055–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedghi, R.; Sayyari, N.; Shaabani, A.; Niknejad, H.; Tayebi, T. Novel biocompatible zinc-curcumin loaded coaxial nanofibers for bone tissue engineering application. Polymers 2018, 142, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Mokhames, Z.; Rezaie, Z.; Ardeshirylajimi, A.; Basiri, A.; Taheri, M.; Omrani, M.D. Efficient smooth muscle cell differentiation of iPS cells on curcumin-incorporated chitosan/collagen/polyvinyl-alcohol nanofibers. In Vitro Cell. Dev. Biol. Anim. 2020, 56, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, S.E.; Park, J.; Ahn, G.Y.; Yun, T.H.; Choi, I.; Kim, H.; Choi, S.-W. Curcumin-loaded biodegradable polyurethane scaffolds modified with gelatin using 3D printing technology for cartilage tissue engineering. Polym. Adv. Technol. 2019, 30, 3083–3090. [Google Scholar] [CrossRef]
- Ghaee, A.; Bagheri-Khoulenjani, S.; Afshar, H.A.; Bogheiri, H. Biomimetic nanocomposite scaffolds based on surface modified PCL-nanofibers containing curcumin embedded in chitosan/gelatin for skin regeneration. Compos. Part B Eng. 2019, 177, 107339. [Google Scholar] [CrossRef]
- Suganya, S.; Venugopal, J.; Ramakrishna, S.; Lakshmi, B.; Dev, V.R.G. Naturally derived biofunctional nanofibrous scaffold for skin tissue regeneration. Int. J. Biol. Macromol. 2014, 68, 135–143. [Google Scholar] [CrossRef]
- Yin, J.; Xu, L. Batch preparation of electrospun polycaprolactone/chitosan/aloe vera blended nanofiber membranes for novel wound dressing. Int. J. Biol. Macromol. 2020, 160, 352–363. [Google Scholar] [CrossRef]
- Pereira, R.F.B.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bartolo, P.J. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Merolli, A.; Nicolais, L.; Ambrosio, L.; Santin, M. A degradable soybean-based biomaterial used effectively as a bone filler in vivo in a rabbit. Biomed. Mater. 2010, 5, 15008. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Morris, C.; Standen, G.; Nicolais, L.; Ambrosio, L. A New Class of Bioactive and Biodegradable Soybean-Based Bone Fillers. Biomacromolecules 2007, 8, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Bose, S. Controlled release of soy isoflavones from multifunctional 3D printed bone tissue engineering scaffolds. Acta Biomater. 2020, 114, 407–420. [Google Scholar] [CrossRef]




| Name of the Nanocarrier Loaded Biomaterial/Composite/Scaffolds | Fabrication Techniques | Role of Nanocarriers | Tissue Engineering Applications | Outcomes |
|---|---|---|---|---|
| Nano Zinc Oxide (nZnO) and polycaprolactone based nanofiber. | Electrospinning method | Antibacterial properties | Bone tissue regeneration | The scaffold provides a nanoporous environment, which helped to increase cell adhesion and proliferation in MH63 cells [109]. |
| Zeolite-nanoHAp based PCL/PLA nanofibers | Hydrothermal method (nanoHAp and Zeolite) and Electrospinning technique (nanofiber) | nanoHAp—bioactive ceramic in dentistry | Dental tissue regeneration | Plain PCL and PLA nanofibers showed low cell adhesion and migration due to their poor hydrophilic and smooth surface properties. Zeolite- and nHA-based composites overcome the limitations associated with PCL and PLA nanofibers and had positive outcomes on the osteoconductivity and osteoinductivity of scaffold for bone and tooth tissue engineering applications [129]. |
| Gold nanoparticles loaded HAp and collagen-based biomaterial. | Chemical precipitation techniques—HAp nanomaterials. Microwave-assisted rapid heating methods—Gold loaded HAp | Carrier molecule | Tissue engineering | The synthesized biomaterials have shown excellent cytocompatibility against MG-63 osteoblast cells and been suitable as an ECM in tissue engineering. Gold loading concentration was considered an important parameter and it showed little toxicity when it reached 0.5% [130]. |
| Nano TiO2 loaded SF-based nanocomposite | Freeze drying method | It leads mechanical interlocking and induces bone formation | Bone tissue engineering | High TiO2 concentrations (15 wt.%) improved the bioactivity behavior, and cell attachment. The low concentrations of TiO2 (5 wt.%) allowed the cells to spread only on the surface [131]. |
| Dexamethasone-loaded carboxymethyl chitosan/poly(amidoamine) dendrimer nanoparticles | Precipitation method | Regulation of osteogenesis (in vivo) | Bone tissue engineering | An in vivo rat study showed that the synthesized dendrimer-based nanoparticles acted as an excellent intracellular nanocarrier for dexamethasone release and significantly enhanced the ectopic bone formation [132]. |
| Paclitaxel-liposome loaded collagen microchannel scaffolds | Lyophilization method | The bilayer membrane of liposomes can help to improve the solubility issues associated with hydrophobic drugs such as paclitaxel. | Spinal cord injury repair | Sustained release of paclitaxel was achieved. It alleviates myelin inhibition and enhance neuronal differentiation (in vitro). It provides microenvironment support for neural stem cells to differentiate into mature neurons (in vivo) [133]. |
| Nanofibrous micelles | Quenching, self-assembly and soft lithography approaches | It regulates cellular responses | Cellular alignment in tissue engineering | It mimics native fibrous networks surrounded by cells [134]. |
| TiO2 Nanoparticles loaded porous PLGA-based scaffolds. | 3D-printing technique | To improve mechanical properties of the scaffold | Bone tissue engineering | Osteoblast proliferation considerably increased in PLGA/TiO2 compared to pure PLGA [135]. |
| Mesoporous silica nanoparticles (MSN) loaded collagen hydrogel. | Conventional method | Porous morphology to load nerve growth factor (NGF) | Neural tissue engineering | NGF-loaded collagen-MSN scaffolds show significant effects on neurite outgrowth patterns compared to NGF-loaded scaffold without MSN [136]. |
| Nano-hydroxyapatite (HAp)-alginate-gelatin based microcapsule | Electrostatic encapsulation method | Nano-HAp promotes microencapsulated cell osteogenesis | Bone tissue engineering | The composite provided an efficient osteogenic building block. Alginate improves the swelling, stability, and mechanical strength of hydrogels. Further studies related to the composition of the hydrogels are required to improve their performance in static and dynamic cultures [137]. |
| Nano-HAp, pullulan/dextran based composite | Freeze drying | Induced mineralization | Bone tissue engineering | The composite activates early calcification and osteoid tissue formation [138]. |
| Nano silver, HAp, gelatin, alginate, poly (vinyl alcohol) based 3D scaffolds | Freezing thawing approach | Antibacterial activity | Bone tissue engineering | The 3D scaffold showed superior mechanical properties. The release of silver ions from scaffold materials leads to enhanced antibacterial activity against Bacillus and E.coli sps. Although it shows some positive outcomes in in vitro, more in vivo studies are required to find the suitability of the synthesized material for human beings [139]. |
| Nano zirconia (nano ZrO2) loaded chitosan and SF-based nanocomposite | Freeze drying method | Chemical stability, mechanical and biocompatibility property for bone scaffolds | Tissue engineering | The interconnected porous composite material showed better physical, and mechanical properties. Enhanced biocompatibility and proliferation were observed in Human Gingival Fibroblast cells compared to the control [140]. |
| Nano-HAp loaded polyhydroxybutyrate-co-(3-hydroxyvalerate) (PHBV) and SF-based composite. | Electrospinning methods | Nano-sized HAp promote cellular activity and rate of mineralization | Bone tissue engineering | The scaffold supports the attachment and proliferation of human osteoblast cells. The mechanical properties of this matrix show the decreased Young’s modulus when increasing concentration to 5 wt.% [141]. |
| TiO2 Nanotube loaded 3D porous PLGA-based microspheres. | Single emulsion and microsphere sintered techniques | To provide compressive modulus and strength, | Bone tissue engineering | The existence of TiO2 improved the bioactivity of PLGA scaffold, promoting cell attachment (in vitro) and enhanced bone regeneration (in vivo) [142]. |
| Mesoporous silica nanoparticles (MSNPs) loaded PLGA/gelatin nanofibrous scaffolds. | Electrospinning method for scaffold, Template removal method for MSNPs | To increase solution viscosity, conductivity, and hydrophilicity of the scaffolds | Nerve regeneration | The surface morphology, physical and biological properties of the scaffolds made it more suitable for nerve tissue engineering applications [143]. |
| Strontium-doped HAp/SF biocomposite nanospheres | Ultrasonic coprecipitation method | Osteoinductive components | Bone regeneration | The synthesized nanospheres are biocompatible, facilitating osteogenic differentiation and osteoinductive properties (in vitro). The limitation of this study is that the author did not show the in situ bone defect healing potential of strontium-doped HAp/SF biocomposite nanospheres, but their hypothesis strongly recommended the use of this biomaterial as an in situ bone filling material [144]. |
| Detail of the Scaffold Material | Fabrication Type | Active Medicinal Compound Incorporated | Potential Role, Physicochemical Properties, and the Release Profile of Incorporated Active Medicinal Compound from the Nanobiomaterials | Outcomes |
|---|---|---|---|---|
| Novel Graphene oxide (GO) and Zn-Curcumin based composite nanofibers | Electrospinning | Curcumin | Core-shell nanofibers (153 nm diameter). Core (Zinc and curcumin complex) and shell (blend of carboxymethyl chitosan, PVA and GO) part of the nanofiber was confirmed through FTIR and XRD analysis. The presence of GO in the blend aided to improve the mechanical properties of nanofibers. In vitro drug release studies were performed for 25 days and revealed that the curcumin release was slower and more prolonged from nanofibers | The synthesized Zn-curcumin composite nanofibers showed excellent support for cell adhesion, spreading and the proliferation process and enhanced the activity of alkaline phosphatase. It has good antibacterial activity and promising potential for bone tissue engineering [174]. |
| Composite nanofibrous scaffold (Curcumin incorporated chitosan, collagen, and polyvinyl-alcohol polymer-based nanofibers) | Electrospinning | Curcumin | The presence of nanometer sized fibers with interconnected pores were confirmed through scanning electron microscopy (SEM) study. An in vitro curcumin release from nanofibers was observed in phosphate-buffered saline (PBS) at 37 °C, which showed that the 20% of initial burst release in 24 h and sustained cumulative curcumin release was slowly increased by almost 90%, observed over a period of 21 days | A biocompatible scaffold used for tissue engineering applications, with well-interconnected pores helping to achieve optimal curcumin release, and increased cell attachment and cell viability. The nanofiber scaffold with curcumin showed higher α-SMA protein expression than the nanofiber scaffold without curcumin [175]. |
| Bifunctional 3D printed scaffold (Liposome encapsulated curcumin onto 3D printed tricalcium phosphate (TCP)) | Thin-film hydration | Curcumin | Transmission electron microscope (TEM) study revealed that the curcumin-encapsulated liposomes showed homogenous size distribution in the range of 40–50 nm. The properties of liposomes showed more controlled and sustained drug release of curcumin (17% released in 60 days) | It helps prevent bone cancer cells and promotes healthy bone cells, and this liposome-based, curcumin-loaded, bifunctional, 3D-printed scaffold can be used as a potential substitute to bone graft treatments after tumor removal [176]. |
| 3D printed biodegradable scaffolds (Curcumin, polyurethane, and gelatin) | One-step 3D printing process | Curcumin | The surface hydrophilicity, crosslinking density and nanoporous structure of the scaffold facilitated curcumin release. The burst release of curcumin was observed due to the surface hydrophilicity of the synthesized scaffolds. | Hydrophilic biodegradable porous scaffold exhibits excellent cell adhesion and cell proliferation properties. It can be used to regenerate cartilage tissues [177]. |
| Biomimetic nanocomposite scaffolds (Polycaprolactone, Chitosan, Gelatin and Curcumin) | Freeze drying | Curcumin | SEM image revealed that the size of curcumin-loaded nanofibers was 139 nm, whereas the curcumin-free nanofibers were 195 nm. The addition of curcumin significantly reduced the size of the nanofibers. Slow curcumin release was observed in all types of scaffold studied in this work. | It mimics the ECM structure of soft tissues and showed suitable physicochemical and biological properties for skin regeneration [178]. |
| SF-based biofunctional nanofibrous scaffold | Electrospinning method | Aloe vera | The field emission SEM study revealed the average size fiber diameter of Aloe-vera-loaded nanofiber was in the range of 212 ± 27 nm. The successful incorporation of aloe compound in the scaffold was confirmed through FTIR study. | The biological responses of the synthesized nanofibrous scaffolds, such as cell adhesion and migration, have been evaluated, and they provide a stable environment in the growth of human dermal fibroblasts for skin tissue engineering applications [179]. |
| Polycaprolactone, chitosan and Aloe vera (AV) blended nanofiber membranes | Electrospinning method | Aloe vera | 2% of AV plays an important role in the size of the nanofibers diameters, making it not easy to break. The average size diameter of nanofibers was 37.58 ± 3.24 (sloping free surface electrospinning method) and 53.63 ± 12.31 (modified bubble electrospinning method) | It has shown enhanced antibacterial activity against E. coli and S. aureus and Cytocompatibility against human umbilical vein endothelial cells. It is suiSection for treating acute wounds [180]. |
| Alginate based hydrogel | Solvent-casting process | Aloe vera | The chemical composition of AV existence in the hydrogel was confirmed through FTIR study and thermogravimetric analysis results showed that the presence of AV increased the thermal stability of the material. | The synthesized films were evaluated with different physical and mechanical properties and could be applied for skin applications. The loading efficiency of Aloe vera was greatly increased due to the water absorption and swelling behavior of the hydrogel film [181]. |
| Biodegradable soybean-based biomaterial | Thermosetting | Soybean | Genistein isoflavones from soybean could stimulate protein synthesis and osteoblastic functions and it plays a major role in bone regeneration (in vivo). The degradation of soybean granules was observed in the periphery of the defects through polarized light microscopy. | An in vivo rabbit study confirmed the osteogenic potential of the soybean-based biomaterial as a bone filler for bone regeneration [182]. |
| Soybean-based biomaterial granules | Simple thermosetting method | Soybean | Genistein is one of the soy isoflavones present in the soybean. Approximately 0.08 µg/mL genistein release was observed after 100 h of the study in PBS pH 7.4 at 37 °C | An in vitro study has revealed that it reduced the activity of macrophages, differentiates osteoblast and may be functionally used for bone regeneration [183]. |
| Multifunctional 3D printed TCP scaffolds | Binder jetting technique | Soy isoflavones | The multifunctional scaffold was prepared using all the three soy isoflavones in the ratio of 5:4:1 (genistein, daidzein and glycitein) and the release of all three isoflavones were observed in both pH 7.4 and 5.0 for 16 days. It revealed that 72.5% (genistein), 100% (daidzein) and 13.75% (glycitein) release in pH 7.4 and 25.1% (genistein), 23.3% (daidzein) and 2.97% (glycitein) release in acidic pH 5.0 | It may be used in postsurgical applications, which include bone graft substitutes, drug delivery vehicle, localized tumor cell suppression and bone cell proliferation. The scaffolds must be tested with other malignant cell lines to confirm their chemopreventive efficacy and characterizations related to the expression of different bone markers [184]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.S.D.; Abrahamse, H. Advancement of Nanobiomaterials to Deliver Natural Compounds for Tissue Engineering Applications. Int. J. Mol. Sci. 2020, 21, 6752. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186752
Kumar SSD, Abrahamse H. Advancement of Nanobiomaterials to Deliver Natural Compounds for Tissue Engineering Applications. International Journal of Molecular Sciences. 2020; 21(18):6752. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186752
Chicago/Turabian StyleKumar, Sathish Sundar Dhilip, and Heidi Abrahamse. 2020. "Advancement of Nanobiomaterials to Deliver Natural Compounds for Tissue Engineering Applications" International Journal of Molecular Sciences 21, no. 18: 6752. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186752