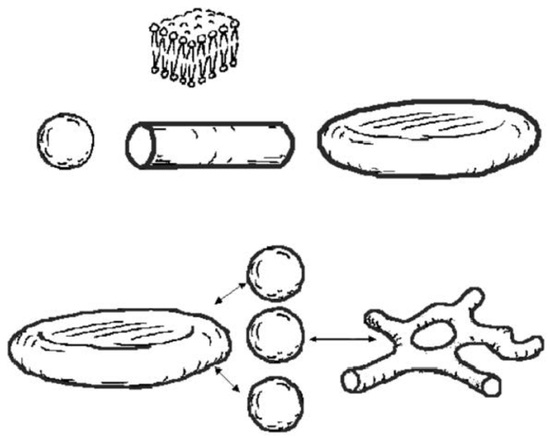
Membrane Curvature, Trans-Membrane Area Asymmetry, Budding, Fission and Organelle Geometry
Abstract
:1. Introduction
2. Factors Determining the Membrane Curvature
3. The Golgi Complex and the Role of TAA in the Sorting Functions of this Organelle
4. Membrane Geometry and Organelle Dynamics
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| COP | coatomer |
| EC | endothelial cell |
| IMM | inner mitochondrial membrane |
| GC | Golgi complex |
| NE | nuclear envelope |
| OMM | outer mitochondria membrane |
| PM | plasma membrane |
| TAA | trans-membrane area asymmetry |
References
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Derganc, J. Curvature-driven lateral segregation of membrane constituents in Golgi cisternae. Phys. Biol. 2007, 4, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Derganc, J.; Mironov, A.A.; Svetina, S. The geometry of organelles of the secretory pathway. In The Golgi Apparatus; Mironov, A.A., Pavelka, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 314–330. [Google Scholar]
- Beznoussenko, G.V.; Pilyugin, S.S.; Geerts, W.J.; Kozlov, M.M.; Burger, K.N.; Luini, A.; Derganc, J.; Mironov, A.A. Trans-membrane area asymmetry controls the shape of cellular organelles. Int. J. Mol. Sci. 2015, 16, 5299–5333. [Google Scholar] [CrossRef] [Green Version]
- Zimmerberg, J.; Kozlov, M.M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 2006, 7, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Arya, P.V.; Singh, S. Lipids and membrane structure. In Introduction to Biomolecular Structure and Biophysics; Misra, G., Ed.; eBook; Springer: Berlin/Heidelberg, Germany, 2017; pp. 139–182. [Google Scholar]
- Ziherl, P.; Svetina, S. Nonaxisymmetric phospholipid vesicles: Rackets, boomerangs, and starfish. Europhys. Lett. 2005, 70, 690–696. [Google Scholar] [CrossRef]
- Mui, B.L.; Dobereiner, H.G.; Madden, T.D.; Cullis, P.R. Influence of transbilayer area asymmetry on the morphology of large unilamellar vesicles. Biophys. J. 1995, 69, 930–941. [Google Scholar] [CrossRef] [Green Version]
- Siegel, D.P.; Kozlov, M.M. The Gaussian curvature elastic modulus of N-monomethylated dioleoylphosphatidylethanolamine: Relevance to membrane fusion and lipid phase behavior. Biophys. J. 2004, 87, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Siegel, D.P.; Cherezov, V.; Greathouse, D.V.; Koeppe, R.E.; Killian, J.A.; Caffrey, M. Transmembrane peptides stabilize inverted cubic phases in a biphasic length-dependent manner: Implications for protein-induced membrane fusion. Biophys. J. 2006, 90, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Tenchov, B.G.; MacDonald, R.C.; Siegel, D.P. Cubic phases in phosphatidylcholine-cholesterol mixtures: Cholesterol as membrane “fusogen’’. Biophys. J. 2006, 91, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Van Helvoort, A.; van Meer, G. Intracellular lipid heterogeneity caused by topology of synthesis and specificity in transport. Example: Sphingolipids. FEBS Lett. 1995, 369, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.A. Fast flip-flop of cholesterol and fatty acids in membranes: Implications for membrane transport proteins. Curr. Opin. Lipidol. 2003, 14, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, T.; Menon, A.K. Lipid flippases and their biological functions. Cell. Mol. Life Sci. 2006, 63, 2908–2921. [Google Scholar] [CrossRef] [PubMed]
- Daleke, D.L. Phospholipid flippases. J. Biol. Chem. 2007, 282, 821–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, U.; Lassmann, G.; Holzhütter, H.G.; Wüstnerk, D.; Müller, P.; Höhlig, A.; Kubelt, J.; Herrmann, A. Rapid flip-flop of phospholipids in endoplasmic reticulum membranes studied by a stopped-flow approach. Biophys. J. 2000, 78, 2628–2640. [Google Scholar] [CrossRef] [Green Version]
- Buton, X.; Herve, P.; Kubelt, J.; Tannert, A.; Burger, K.N.; Fellmann, P.; Muller, P.; Herrmann, A.; Seigneuret, M.; Devaux, P.F. Transbilayer movement of monohexosylsphingolipids in endoplasmic reticulum and Golgi membranes. Biochemistry 2002, 41, 13106–13115. [Google Scholar] [CrossRef]
- Hankins, H.M.; Baldridge, R.D.; Xu, P.; Graham, T.R. Role of flippases, scramblases and transfer proteins in phosphatidylserine subcellular distribution. Traffic 2015, 16, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Roelants, F.M.; Su, B.M.; von Wulffen, J.; Ramachandran, S.; Sartorel, E.; Trott, A.E.; Thorner, J. Protein kinase Gin4 negatively regulates flippase function and controls plasma membrane asymmetry. J. Cell Biol. 2015, 208, 299–311. [Google Scholar] [CrossRef] [Green Version]
- Cullis, P.R.; Fenske, D.B.; Hope, M.J. Physical properties and functional roles of lipids in membranes. In Biochemistry of Lipids, Lipoproteins, and Membranes; Vance, D.E., Vance, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 1–33. [Google Scholar]
- Kearns, B.G.; McGee, T.P.; Mayinger, P.; Gedvilaite, A.; Phillips, S.E.; Kagiwada, S.; Bankaitis, V.A. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature 1997, 387, 21–22. [Google Scholar] [CrossRef]
- Fang, M.; Rivas, M.P.; Bankaitis, V.A. The contribution of lipids and lipid metabolism to cellular functions of the Golgi complex. Biochim. Biophys. Acta 1998, 1404, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, K.W. Phospholipid transfer proteins in perspective. FEBS Lett. 2006, 580, 5436–5441. [Google Scholar] [CrossRef] [Green Version]
- Imran, A.; Popescu, D.; Movileanu, L. Cyclic Activity of an Osmotically Stressed Liposome in a Finite Hypotonic Environment. Langmuir 2020, 36, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Murk, J.L.; Posthuma, G.; Koster, A.J.; Geuze, H.J.; Verkleij, A.J.; Kleijmeer, M.J.; Humbel, B.M. Influence of aldehyde fixation on the morphology of endosomes and lysosomes: Quantitative analysis and electron tomography. J. Microsc. 2003, 212, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. Protein Amphipathic Helix Insertion: A Mechanism to Induce Membrane Fission. Front. Cell Dev. Biol. 2019, 7, 291. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg-Bord, M.; Shai, N.; Schuldiner, M.; Bohnert, M. A Tether Is a Tether Is a Tether: Tethering at Membrane Contact Sites. Dev. Cell 2016, 39, 395–409. [Google Scholar] [CrossRef]
- Ungermann, C.; Kümmel, D. Structure of membrane tethers and their role in fusion. Traffic 2019, 20, 479–490. [Google Scholar] [CrossRef]
- Božič, B.; Guven, J.; Vázquez-Montejo, P.; Svetina, S. Direct and remote constriction of membrane necks. Phys. Rev. 2014, 89, 052701. [Google Scholar] [CrossRef]
- Božič, B.; Das, S.L.; Svetina, S. Sorting of integral membrane proteins mediated by curvature-dependent protein-lipid bilayer interaction. Soft Matter 2015, 11, 2479–2487. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Rost, B.R.; Camacho-Pérez, M.; Davis, M.W.; Söhl-Kielczynski, B.; Rosenmund, C.; Jorgensen, E.M. Ultrafast endocytosis at mouse hippocampal synapses. Nature 2013, 504, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Grønborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brügger, B.; Ringler, P.; et al. Molecular anatomy of a trafficking organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef] [Green Version]
- Fusella, A.; Micaroni, M.; Di Giandomenico, D.; Mironov, A.A.; Beznoussenko, G.V. Segregation of the Qb-SNAREs GS27 and GS28 into Golgi vesicles regulates intra-Golgi transport. Traffic 2013, 14, 568–584. [Google Scholar] [CrossRef]
- Mironov, A.A.; Beznoussenko, G.V. Models of Intracellular Transport: Pros and Cons. Front. Cell Dev. Biol. 2019, 7, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beznoussenko, G.V.; Ragnini-Wilson, A.; Wilson, C.; Mironov, A.A. Three-dimensional and immune electron microscopic analysis of the secretory pathway in Saccharomyces cerevisiae. Histochem. Cell Biol. 2016, 146, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Blood, P.D.; Voth, G.A. Direct observation of Bin/amphiphysin/Rvs (BAR) domain-induced membrane curvature by means of molecular dynamics simulations. Proc. Natl. Acad. Sci. USA 2006, 103, 15068–15072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killian, J.A. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta 1998, 1376, 401–415. [Google Scholar] [CrossRef]
- Kozlov, M.M.; Campelo, F.; Liska, N.; Chernomordik, L.V.; Marrink, S.J.; McMahon, H.T. Mechanisms shaping cell membranes. Curr. Opin. Cell Biol. 2014, 29, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Derganc, J.; Antonny, B.; Copič, A. Membrane bending: The power of protein imbalance. Trends Biochem. Sci. 2013, 38, 576–584. [Google Scholar] [CrossRef]
- Sako, Y.; Kusumi, A. Barriers for lateral diffusion of transferrin receptor in the plasma membrane as characterized by receptor dragging by laser tweezers: Fence versus tether. J. Cell Biol. 1995, 129, 1559–1574. [Google Scholar] [CrossRef] [Green Version]
- Shurer, C.R.; Kuo, J.C.H.; Roberts, L.M.; Gandhi, J.G.; Colville, M.J.; Enoki, T.A.; Pan, H.; Su, J.; Noble, J.M.; Hollander, M.J.; et al. Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell 2019, 177, 1757–1770. [Google Scholar] [CrossRef]
- Rabouille, C.; Levine, T.P.; Peters, J.-M.; Warren, G. An NSF-like ATPase, p97, and NSF mediate costernal regrowth from mitotic Golgi fragments. Cell 1995, 82, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Upadhyaya, A.; Sheetz, M.P. Tension in tubulovesicular networks of Golgi and endoplasmic reticulum membranes. Biophys. J. 2004, 86, 2923–2928. [Google Scholar] [CrossRef] [Green Version]
- Kweon, H.S.; Beznoussenko, G.V.; Micaroni, M.; Polishchuk, R.S.; Trucco, A.; Martella, O.; Di Giandomenico, D.; Marra, P.; Fusella, A.; Di Pentima, A.; et al. Golgi enzymes are enriched in perforated zones of golgi cisternae but are depleted in COPI vesicles. Mol. Biol. Cell 2004, 15, 4710–4724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironov, A.A.; Mironov, A.A., Jr.; Beznoussenko, G.V.; Trucco, A.; Lupetti, P.; Smith, J.D.; Geerts, W.J.; Koster, A.J.; Burger, K.N.; Martone, M.E.; et al. ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev. Cell 2003, 5, 583–594. [Google Scholar] [CrossRef]
- Trucco, A.; Polishchuk, R.S.; Martella, O.; Di Pentima, A.; Fusella, A.; Di Giandomenico, D.; San Pietro, E.; Beznoussenko, G.V.; Polishchuk, E.V.; Baldassarre, M. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat. Cell Biol. 2004, 6, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Sciaky, N.; Presley, J.; Smith, C.; Zaal, K.J.; Cole, N.; Moreira, J.E.; Terasaki, M.; Siggia, E. Lippincott-Schwartz, J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J. Cell Biol. 1997, 139, 1137–1155. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.; Tagaya, M.; Amherdt, M.; Perrelet, A.; Donaldson, J.G.; Lippincott-Schwartz, J.; Klausner, R.D.; Rothman, J.E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell 1991, 64, 1183–1195. [Google Scholar] [PubMed]
- Misteli, T.; Warren, G. COP-coated vesicles are involved in the mitotic fragmentation of Golgi stacks in a cell-free system. J. Cell Biol. 1994, 125, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Happe, S.; Cairns, M.; Roth, R.; Heuser, J.; Weidman, P. Coatomer vesicles are not required for inhibition of Golgi transport by G-protein activators. Traffic 2000, 1, 342–353. [Google Scholar] [CrossRef]
- Misteli, T.; Warren, G. Mitotic disassembly of the Golgi apparatus in vivo. J. Cell Sci. 1995, 108, 2715–2727. [Google Scholar]
- Yang, J.S.; Gad, H.; Lee, S.Y.; Mironov, A.; Zhang, L.; Beznoussenko, G.V.; Valente, C.; Turacchio, G.; Bonsra, A.N.; Du, G.; et al. A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance. Nat. Cell Biol. 2008, 10, 1146–1153. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, V.; Serafini, T.; Orci, L.; Shepherd, J.C.; Rothman, J.E. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell 1989, 8, 329–336. [Google Scholar] [CrossRef]
- Gomez, M.; Scales, S.J.; Kreis, T.E.; Perez, F. Membrane recruitment of coatomer and binding to dilysine signals are separate events. J. Biol. Chem. 2000, 275, 29162–29169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepperkok, R.; Scheel, J.; Horstmann, H.; Hauri, H.P.; Griffiths, G.; Kreis, T.E. Beta-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell 1993, 74, 71–82. [Google Scholar] [CrossRef]
- Ramachandran, R. Mitochondrial dynamics: The dynamin superfamily and execution by collusion. Semin. Cell Dev. Biol. 2018, 76, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.A.; Sesorova, I.S.; Seliverstova, E.V.; Beznoussenko, G.V. Different Golgi ultrastructure across species and tissues: Implications under functional and pathological conditions, and an attempt at classification. Tissue Cell. 2017, 49, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, J.C.; Schmid, E.M.; Ryan, C.J.; Ann, H.S.; Sasaki, D.Y.; Sherman, M.B.; Geissler, P.L.; Fletcher, D.A.; Hayden, C.C. Membrane bending by protein-protein crowding. Nat. Cell Biol. 2012, 14, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Ktistakis, N.T.; Brown, H.A.; Waters, M.G.; Sternweis, P.C.; Roth, M.G. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J. Cell Biol. 1996, 134, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Stamnes, M.; Schiavo, G.; Stenbeck, G.; Söllner, T.H.; Rothman, J.E. ADP-ribosylation factor and phosphatidic acid levels in Golgi membranes during budding of coatomer-coated vesicles. Proc. Natl. Acad. Sci. USA 1998, 95, 13676–13680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothman, J.E. Mechanisms of intracellular protein transport. Nature 1994, 372, 55–63. [Google Scholar] [CrossRef]
- Renard, H.-F.; Johannes, L.; Morsomme, P. Increasing diversity of biological membrane fission mechanisms. Trends Cell Biol. 2018, 28, 274–286. [Google Scholar] [CrossRef]
- Kukulski, W.; Schorb, M.; Kaksonen, M.; Briggs, J.A. Plasma membrane reshaping during endocytosis is revealed by time-resolved electron tomography. Cell 2012, 150, 508–520. [Google Scholar] [CrossRef] [Green Version]
- Liebl, D.; Griffiths, G. Transient assembly of F-actin by phagosomes delays phagosome fusion with lysosomes in cargo-overloaded macrophages. J. Cell Sci. 2009, 122, 2935–2945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacia, K.; Futai, E.; Prinz, S.; Meister, A.; Daum, S.; Glatte, D.; Briggs, J.A.; Schekman, R. Multibudded tubules formed by COPII on artificial liposomes. Sci. Rep. 2011, 1, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeuschner, D.; Geerts, W.J.; van Donselaar, E.; Humbel, B.M.; Slot, J.W.; Koster, A.J.; Klumperman, J. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat. Cell Biol. 2006, 8, 377–383. [Google Scholar] [CrossRef]
- Barlowe, C.; Orci, L.; Yeung, T.; Hosobuchi, M.; Hamamoto, S.; Salama, N.; Rexach, M.F.; Ravazzola, M.; Amherdt, M.; Schekman, R. COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 1994, 77, 895–907. [Google Scholar] [CrossRef]
- Heinrich, V.; Bozic, B.; Svetina, S.; Zeks, B. Vesicle deformation by an axial load: From elongated shapes to tethered vesicles. Biophys. J. 1999, 76, 2056–2071. [Google Scholar] [CrossRef] [Green Version]
- Sauvanet, C.; Wayt, J.; Pelaseyed, T.; Bretscher, A. Structure, regulation, and functional diversity of microvilli on the apical domain of epithelial cells. Annu. Rev. Cell Dev. Biol. 2015, 31, 593–621. [Google Scholar] [CrossRef]
- Mironov, A.A.; Mironov, V.A.; Rekhter, M.D. Changes in the surface and cytoskeletal apparatus of the endotheliocytes of the rat aorta during division (based on scanning electron microscopic data). Tsitologiia 1987, 29, 426–431. [Google Scholar]
- Frémont, S.; Echard, A. Membrane Traffic in the Late Steps of Cytokinesis. Curr. Biol. 2018, 28, R458–R470. [Google Scholar] [CrossRef] [Green Version]
- Lammerding, J.; Wolf, K. Nuclear envelope rupture: Actin fibers are putting the squeeze on the nucleus. J. Cell Biol. 2016, 215, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Yoon, Y. Mitochondrial fission and fusion. Biochem. Soc. Trans. 2016, 44, 1725–1735. [Google Scholar] [CrossRef]
- Cogliati, S.; Enriquez, J.A.; Scorrano, L. Mitochondrial Cristae: Where Beauty Meets Functionality. Trends Biochem. Sci. 2016, 41, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Bioenergetic role of mitochondrial fusion and fission. Biochim. Biophys. Acta 2012, 1817, 1833–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Lee, H.C.; Chen, K.C.; Suhan, J.; Qiu, M.; Ba, Q.; Yang, G. Inner membrane fusion mediates spatial distribution of axonal mitochondria. Sci. Rep. 2016, 6, 18981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironov, A.A.; Beznoussenko, G.V.; Morelli, E.; Vaccari, T. Early autophagosomes are formed from myelin-like structures derived from outer membranes of mitochondria. Ultrast. Pathol. 2017, 41, 73–74. [Google Scholar] [CrossRef]
- McLelland, G.L.; Lee, S.A.; McBride, H.M.; Fon, E.A. Syntaxin-17 delivers PINK1/parkin-dependent mitochondrial vesicles to the endolysosomal system. J. Cell Biol. 2016, 214, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.P.; Shtengel, G.; Xu, C.S.; Campbell, K.R.; Freeman, M.; Wang, L.; Milkie, D.E.; Pasolli, H.A.; Iyer, N.; Bogovic, J.A.; et al. Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells. Science 2020, 367, eaaz5357. [Google Scholar] [CrossRef]
- Rambold, A.S.; Cohen, S.; Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 2015, 32, 678–992. [Google Scholar] [CrossRef] [Green Version]
- Stefan, C.J.; Trimble, W.S.; Grinstein, S.; Drin, G.; Reinisch, K.; De Camilli, P.; Cohen, S.; Valm, A.M.; Lippincott-Schwartz, J.; Levine, T.P.; et al. Membrane dynamics and organelle biogenesis-lipid pipelines and vesicular carriers. BMC Biol. 2017, 15, 102. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Rambold, A.S.; Lippincott-Schwartz, J. Mitochondrial and Lipid Droplet Dynamics Regulate Intra- and IntercellularFatty Acid Trafficking. Mol. Cell Oncol. 2018, 5, e1043038. [Google Scholar] [CrossRef] [Green Version]
- Rambold, A.S.; Kostelecky, B.; Elia, N.; Lippincott-Schwartz, J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl. Acad. Sci. USA 2011, 108, 10190–10195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironov, A.A.; Sesorova, I.S.; Dimov, I.D.; Karelina, N.R.; Beznoussenko, G.V. Intracellular transports and atherogenesis. Front. Biosci. 2020, 25, 1230–1258. [Google Scholar] [CrossRef]
- Volkova, O.V.; Shakhlamov, V.A.; Mironov, A.A. Atlas of scanning electron microscopy of cells, tissues and organs. In Moscow: Meditsina; W.H. Freeman and Company: New York, NY, USA, 1987; 464p. [Google Scholar]
- Sinha, B.; Koster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011, 144, 402–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.P.X.; Nichols, B.J. Caveolae: One function or many? Trends Cell Biol. 2016, 26, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.P.; Nixon, S.J.; Hall, T.E.; Cowling, B.S.; Ferguson, C.; Morgan, G.P.; Schieber, N.L.; Fernandez-Rojo, M.A.; Bastiani, M.; Floetenmeyer, M.; et al. The caveolin-cavin system plays a conserved and critical role in me- chanoprotection of skeletal muscle. J. Cell Biol. 2015, 210, 833–849. [Google Scholar] [CrossRef] [Green Version]
- Parton, R.G.; Joggerst, B.; Simons, K. Regulated internalization of caveolae. J. Cell Biol. 1994, 127, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Golani, G.; Ariotti, N.; Parton, R.G.; Kozlov, M.M. Membrane Curvature and Tension Control the Formation and Collapse of Caveolar Superstructures. Dev. Cell 2019, 48, 523–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, T.; Floetenmeyer, M.; Ferguson, C.; Galea, J.; Goh, J.; Lindsay, M.R.; Morgan, G.P.; Marsh, B.J.; Parton, R.G. High-resolution 3D quantitative analysis of caveolar ultrastructure and Caveola-cytoskeleton interac- tions. Traffic 2008, 9, 893–909. [Google Scholar] [CrossRef]
- Karelina, N.R.; Sesorova, I.S.; Beznusenko, G.V.; Shishlo, V.K.; Kazakova, T.E.; Mironov, A.A. Ultrastructural basis for the process of lymph formation. Morfologiia 2017, 151, 7–19. (In Russian) [Google Scholar]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Wu, H.; Carvalho, P.; Voeltz, G.K. Here, there, and everywhere: The importance of ER membrane contact sites. Science 2018, 361, eaan5835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Simoes, I.C.M.; Morciano, G.; Lebiedzinska-Arciszewska, M.; Aguiari, G.; Pinton, P.; Potes, Y.; Wieckowski, M.R. The mystery of mitochondria-ER contact sites in physiology and pathology: A cancer perspective. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165834. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironov, A.A.; Mironov, A.; Derganc, J.; Beznoussenko, G.V. Membrane Curvature, Trans-Membrane Area Asymmetry, Budding, Fission and Organelle Geometry. Int. J. Mol. Sci. 2020, 21, 7594. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207594
Mironov AA, Mironov A, Derganc J, Beznoussenko GV. Membrane Curvature, Trans-Membrane Area Asymmetry, Budding, Fission and Organelle Geometry. International Journal of Molecular Sciences. 2020; 21(20):7594. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207594
Chicago/Turabian StyleMironov, Alexander A., Anna Mironov, Jure Derganc, and Galina V. Beznoussenko. 2020. "Membrane Curvature, Trans-Membrane Area Asymmetry, Budding, Fission and Organelle Geometry" International Journal of Molecular Sciences 21, no. 20: 7594. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207594