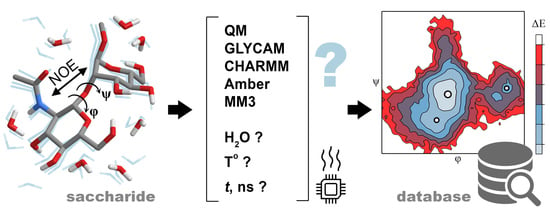
Comparison of Methods for Bulk Automated Simulation of Glycosidic Bond Conformations
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Selection of Objects and Starting Geometries
3.2. Force Fields
3.3. Solvation Models
3.4. Simulation Parameters
3.5. DFT Calculations
3.6. NOE Simulation and Comparison
3.7. Ranking of NOE Simulation Results
3.8. Presentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CSDB | Carbohydrate Structure Database |
| DFT | density functional theory |
| GB | general born |
| HCT | Hawkins, Cramer and Truhlar |
| MD | molecular dynamics |
| NMR | nuclear magnetic resonance |
| NOE | nuclear Overhauser effect |
| NPT | isothermal-isobaric (substance (N), pressure, and temperature are conserved) |
| QM | quantum mechanics |
| PBE | Perdew, Burke, and Ernzerhof |
| RESP | restrained electrostatic potential |
| RMSD | root mean square deviation |
| SMD | solvation model based on density |
| TIP3P | transferable intermolecular potential 3 point |
| TZVP | triple-zeta valence polarized |
References
- Lin, T.L.; Shu, C.C.; Chen, Y.M.; Lu, J.J.; Wu, T.S.; Lai, W.F.; Tzeng, C.M.; Lai, H.C.; Lu, C.C. Like Cures Like: Pharmacological activity of anti-inflammatory lipopolysaccharides from gut microbiome. Front. Pharmacol. 2020, 11, 554. [Google Scholar] [PubMed]
- Rodriguez, E.; Schetters, S.T.T.; van Kooyk, Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat. Rev. Immunol. 2018, 18, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, S.A.; Dror, R.O. Molecular dynamics simulation for all. Neuron 2018, 99, 1129–1143. [Google Scholar] [PubMed] [Green Version]
- Copoiu, L.; Malhotra, S. The current structural glycome landscape and emerging technologies. Curr. Opin. Struct. Biol. 2020, 62, 132–139. [Google Scholar]
- Hernandez-Rodriguez, M.; Rosales-Hernandez, M.C.; Mendieta-Wejebe, J.E.; Martinez-Archundia, M.; Basurto, J.C. Current tools and methods in molecular dynamics (MD) simulations for drug design. Curr. Med. Chem. 2016, 23, 3909–3924. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; Gonzalez-Outeirino, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar]
- Toukach, P.V.; Egorova, K.S. Carbohydrate structure database merged from bacterial, archaeal, plant and fungal parts. Nucleic Acids Res. 2016, 44, D1229–D1236. [Google Scholar] [CrossRef]
- Hricovini, M. Structural aspects of carbohydrates and the relation with their biological properties. Curr. Med. Chem. 2004, 11, 2565–2583. [Google Scholar]
- Marianski, M.; Supady, A.; Ingram, T.; Schneider, M.; Baldauf, C. Assessing the Accuracy of Across-the-Scale Methods for Predicting Carbohydrate Conformational Energies for the Examples of Glucose and alpha-Maltose. J. Chem. Theory Comput. 2016, 12, 6157–6168. [Google Scholar]
- Egorova, K.S.; Kondakova, A.N.; Toukach, P.V. Carbohydrate Structure Database: Tools for statistical analysis of bacterial, plant and fungal glycomes. Database 2015, 2015, bav073. [Google Scholar]
- Torda, A.E.; Scheek, R.M.; van Gunsteren, W.F. Time-averaged nuclear overhauser effect distance restraints applied to tendamistat. J. Mol. Biol. 1990, 214, 223–235. [Google Scholar] [CrossRef]
- Toukach, F.V.; Ananikov, V.P. Recent advances in computational predictions of NMR parameters for the structure elucidation of carbohydrates: Methods and limitations. Chem. Soc. Rev. 2013, 42, 8376–8415. [Google Scholar] [PubMed]
- Allinger, N.L.; Yuh, Y.H.; Lii, J.H. Molecular Mechanics. The MM3 Force Field for Hydrocarbons. J. Am. Chem. Soc. 1989, 1, 8551–8566. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Damm, W.; Frontera, A.; Tirado-Rives, J.; Jorgensen, W.L. OPLS all-atom force field for carbohydrates. J. Comput. Chem. 1997, 18, 1955–1970. [Google Scholar] [CrossRef]
- Ponder, J.W.; Case, D.A. Force fields for protein simulations. Adv. Protein Chem. 2003, 66, 27–85. [Google Scholar]
- Weiner, S.J.; Kollman, P.A.; Nguyen, D.T.; Case, D.A. An all atom force field for simulations of proteins and nucleic acids. J. Comput. Chem. 1986, 7, 230–252. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.R.R.; Brooks, C.L.L.; Mackerell, A.D.D.; Nilsson, L.; Petrella, R.J.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- MacKerell, A.D.; Banavali, J.N.; Foloppe, N. Development and current status of the CHARMM force field for nucleic acids. Biopolymers 2001, 56, 257–265. [Google Scholar] [CrossRef]
- Young, D.C. Computational Chemistry: A Practical Guide for Applying Techniques to Real World Problems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Frank, M. Conformational analysis of oligosaccharides and polysaccharides using molecular dynamics simulations. In Glycoinformatics; Lütteke, T., Frank, M., Eds.; Springer: New York, NY, USA, 2015; Volume 1273, pp. 359–377. [Google Scholar]
- Stortz, C.A. Comparative performance of MM3(92) and two TINKER MM3 versions for the modeling of carbohydrates. J. Comput. Chem. 2005, 26, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Darden, T.A.; Cheatham, I.T.E.; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Walker, R.C.; Zhang, W. AMBER 12; University of California: San Francisco, CA, USA, 2012. [Google Scholar]
- Hess, B.; Kutzner, C.; Van Der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Toukach, P.V.; Egorova, K.S. New Features of Carbohydrate Structure Database Notation (CSDB Linear), As Compared to Other Carbohydrate Notations. J. Chem. Inf. Model. 2020, 60, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- French, A.D.; Johnson, G.P. Computerized molecular modeling of carbohydrates. In The Plant Cell Wall; Popper, Z.A., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 715, pp. 21–42. [Google Scholar]
- Forster, M.J.; Mulloy, B. Rationalizing nuclear overhauser effect data for compounds adopting multiple-solution conformations. J. Comput. Chem. 1994, 15, 155–161. [Google Scholar] [CrossRef]
- Chalmers, G.; Glushka, J.N.; Foley, B.L.; Woods, R.J.; Prestegard, J.H. Direct NOE simulation from long MD trajectories. J. Magn. Reson. 2016, 265, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Galindo-Murillo, R.; Roe, D.R.; Cheatham, T.E., III. Convergence and reproducibility in molecular dynamics simulations of the DNA duplex d(GCACGAACGAACGAACGC). Biochim. Biophys. Acta 2015, 1850, 1041–1058. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.S.; Pendrill, R.; Mallajosyula, S.S.; Widmalm, G.; MacKerell, A.D., Jr. Conformational properties of alpha- or beta-(1-->6)-linked oligosaccharides: Hamiltonian replica exchange MD simulations and NMR experiments. J. Phys. Chem. B 2014, 118, 2851–2871. [Google Scholar] [CrossRef]
- Peric-Hassler, L.; Hansen, H.S.; Baron, R.; Hunenberger, P.H. Conformational properties of glucose-based disaccharides investigated using molecular dynamics simulations with local elevation umbrella sampling. Carbohydr. Res. 2010, 345, 1781–1801. [Google Scholar] [CrossRef]
- Woods, R.J. Predicting the Structures of Glycans, Glycoproteins, and Their Complexes. Chem. Rev. 2018, 118, 8005–8024. [Google Scholar] [CrossRef]
- Stortz, C.A.; Johnson, G.P.; French, A.D.; Csonka, G.I. Comparison of different force fields for the study of disaccharides. Carbohydr. Res. 2009, 344, 2217–2228. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., III; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef]
- du Penhoat, C.H.; Imberty, A.; Roques, N.; Michon, V.; Mentech, J.; Descotes, G.; Perez, S. Conformational behavior of sucrose and its deoxy analog in water as determined by NMR and molecular modeling. J. Am. Chem. Soc. 1991, 113, 3720–3727. [Google Scholar] [CrossRef]
- Brisson, J.R.; Carver, J.P. Solution conformation of alpha D(1-3)- and alpha D(1-6)-linked oligomannosides using proton nuclear magnetic resonance. Biochemistry 1983, 22, 1362–1368. [Google Scholar] [CrossRef]
- Cumming, D.A.; Carver, J.P. Virtual and solution conformations of oligosaccharides. Biochemistry 1987, 26, 6664–6676. [Google Scholar] [CrossRef]
- Peters, T.; Brisson, J.-R.; Bundle, D.R. Conformational analysis of key disaccharide components of Brucella A and M antigens. Can. J. Chem. 1990, 68, 979–988. [Google Scholar] [CrossRef]
- Bock, K.; Lönn, H.; Peters, T. Conformational analysis of a disaccharide fragment of the polysaccharide antigen of Streptococcus pneumoniae type 1 using n.m.r. spectroscopy and HSEA calculations. Carbohydr. Res. 1990, 198, 375–380. [Google Scholar] [CrossRef]
- Mamyan, S.S.; Lipkind, G.M.; Shashkov, A.S.; Bairamova, N.E.; Nikolaev, A.V.; Kochetkov, N.K. Nuclear Overhauser Effect and Conformational States of Glycosyl-(1→2)- and -(1→3)-Rhamnosides in Aqueous Solution. Russ. J. Bioorg. Chem. 1988, 14, 205–215. [Google Scholar]
- Haselhorst, T.; Espinosa, J.F.; Jimenez-Barbero, J.; Sokolowski, T.; Kosma, P.; Brade, H.; Brade, L.; Peters, T. NMR experiments reveal distinct antibody-bound conformations of a synthetic disaccharide representing a general structural element of bacterial lipopolysaccharide epitopes. Biochemistry 1999, 38, 6449–6459. [Google Scholar] [CrossRef]
- Xia, J.; Daly, R.P.; Chuang, F.C.; Parker, L.; Jensen, J.H.; Margulis, C.J. Sugar folding: A novel structural prediction tool for oligosaccharides and polysaccharides 2. J. Chem. Theory Comput. 2007, 3, 1629–1643. [Google Scholar] [CrossRef]
- Chernyshov, I.Y.; Toukach, P.V. REStLESS: Automated translation of glycan sequences from residue-based notation to SMILES and atomic coordinates. Bioinformatics 2018, 34, 2679–2681. [Google Scholar] [CrossRef]
- Rackers, J.A.; Wang, Z.; Lu, C.; Laury, M.L.; Lagardere, L.; Schnieders, M.J.; Piquemal, J.P.; Ren, P.; Ponder, J.W. Tinker 8: Software Tools for Molecular Design. J. Chem. Theory Comput. 2018, 14, 5273–5289. [Google Scholar] [CrossRef]
- Dodda, L.S.; de Vaca, I.C.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Modell. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- da Silva, A.W.S.; Vranken, W.F. ACPYPE-AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, A.; Faller, R.; Reith, D.; Kirschner, K.N. ACPYPE update for nonuniform 1–4 scale factors: Conversion of the GLYCAM06 force field from AMBER to GROMACS. SoftwareX 2019, 10. [Google Scholar] [CrossRef]
- Hawkins, G.D.; Cramer, C.J.; Truhlar, D.G. Parametrized Models of Aqueous Free Energies of Solvation Based on Pairwise Descreening of Solute Atomic Charges from a Dielectric Medium. J. Phys. Chem. 1996, 100, 19824–19839. [Google Scholar] [CrossRef]
- Still, W.C.; Tempczyk, A.; Hawley, R.C.; Hendrickson, T. Semianalytical treatment of solvation for molecular mechanics and dynamics. J. Am. Chem. Soc. 1990, 112, 6127–6129. [Google Scholar] [CrossRef]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An overview of the Amber biomolecular simulation package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Jayaram, B.; Sprous, D.; Beveridge, D.L. Solvation Free Energy of Biomacromolecules: Parameters for a Modified Generalized Born Model Consistent with the AMBER Force Field. J. Phys. Chem. B 1998, 102, 9571–9576. [Google Scholar] [CrossRef]
- Hess, B. P-LINCS: A Parallel Linear Constraint Solver for Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. NbClust: AnRPackage for Determining the Relevant Number of Clusters in a Data Set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Csonka, G.I.; French, A.D.; Johnson, G.P.; Stortz, C.A. Evaluation of Density Functionals and Basis Sets for Carbohydrates. J. Chem. Theory Comput. 2009, 5, 679–692. [Google Scholar] [CrossRef]
- Marjewski, A.A.; Medvedev, M.G.; Gerasimov, I.S.; Panova, M.V.; Perdew, J.P.; Lyssenko, K.A.; Dmitrienko, A.O. Interplay between test sets and statistical procedures in ranking DFT methods: The case of electron density studies. Mendeleev Commun. 2018, 28, 225–235. [Google Scholar] [CrossRef]
- Medvedev, M.G.; Bushmarinov, I.S.; Sun, J.; Perdew, J.P.; Lyssenko, K.A. Density functional theory is straying from the path toward the exact functional. Science 2017, 355, 49–52. [Google Scholar] [CrossRef]
- Grimme, S. Supramolecular binding thermodynamics by dispersion-corrected density functional theory. Chemistry 2012, 18, 9955–9964. [Google Scholar] [CrossRef]
- Li, Y.-P.; Gomes, J.; Mallikarjun Sharada, S.; Bell, A.T.; Head-Gordon, M. Improved Force-Field Parameters for QM/MM Simulations of the Energies of Adsorption for Molecules in Zeolites and a Free Rotor Correction to the Rigid Rotor Harmonic Oscillator Model for Adsorption Enthalpies. J. Phys. Chem. C 2015, 119, 1840–1850. [Google Scholar] [CrossRef]
- Luchini, G.; Alegre-Requena, J.V.; Funes-Ardoiz, I.; Paton, R.S. GoodVibes: Automated thermochemistry for heterogeneous computational chemistry data. F1000Research 2020, 9, 291. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Thøgersen, H.; Lemieux, R.U.; Bock, K.; Meyer, B. Further justification for the exo-anomeric effect. Conformational analysis based on nuclear magnetic resonance spectroscopy of oligosaccharides. Can. J. Chem. 1982, 60, 44–57. [Google Scholar] [CrossRef]
- Fluschnik, T.; Skowron, P.; Triphaus, M.; Wilker, K. Fair knapsack. Proc. AAAI Conf. Artif. Intell. 2019, 33, 1941–1948. [Google Scholar] [CrossRef]
- Kibirige, H.; Lamp, G.; Katins, J.; Austin, O.; Funnell, T.; Finkernagel, F.; Arnfred, J.; Blanchard, D.; Astanin, S.; Chiang, E.; et al. has2k1/plotnine. Available online: https://zenodo.org/record/3878645 (accessed on 14 July 2020).
- Seifert, E. OriginPro 9.1: Scientific data analysis and graphing software-software review. J. Chem. Inf. Model. 2014, 54, 1552. [Google Scholar] [CrossRef]
- Herget, S.; Ranzinger, R.; Maass, K.; Lieth, C.W. GlycoCT-a unifying sequence format for carbohydrates. Carbohydr. Res. 2008, 343, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Sadovnichy, V.; Tikhonravov, A.; Opanasenko, V.; Voevodin, V. “Lomonosov”: Supercomputing at Moscow State University. In Contemporary High Performance Computing: From Petascale toward Exascale; CRC Press: Boca Raton, FL, USA, 2013; pp. 283–307. [Google Scholar]






| # | Force Field | MM3 2000 | MMFF94 | OPLS aa | GLYCAM 06 | CHARMM 36 | Amber ff14SB | DFT a | NOE Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| solvation model b | HCT | TIP3P c | HCT | HCT | GB | TIP3P d | GB | TIP3P d | GB | TIP3P d | SMD | ||
| Temperature, K e | 300 | 300 | 1000 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | ||
| 1 | α-D-Glcp-(1-2)-β-D-Fruf | 0.17 | 0.22 | 0.23 | 0.23 | 0.18 | 0.20 | 0.15 | 0.28 | 0.17 | 0.15 | 0.18 | [39] |
| 2 | α-D-Manp-(1-3)-α-D-Manp-OMe | 0.09 | 0.13 | 0.30 | 0.30 | 0.05 | 0.03 | 0.24 | 0.37 | 0.11 | 0.18 | 0.05 | [40] |
| 3 | α-D-Manp-(1-6)-α-D-Manp-OMe | 0.14 | 0.14 | 0.22 | 0.22 | 0.17 | 0.18 | 0.15 | 0.03 | 0.15 | 0.15 | 0.17 | [40] |
| 4 | α-D-Manp-(1-6)-β-D-Manp-OMe | 0.03 | 0.02 | 0.10 | 0.10 | 0.06 | 0.07 | 0.04 | 0.51 | 0.04 | 0.06 | 0.07 | [41] |
| 5 | β-D-GlcpNAc-(1-6)-α-D-Manp-OMe | 0.04 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.12 | 0.23 | 0.05 | 0.04 | 0.03 | [41] |
| 6 | β-D-GlcpNAc-(1-2)-α-D-Manp-OMe | 0.09 | 0.08 | 0.07 | 0.07 | 0.13 | 0.13 | 0.08 | 0.20 | 0.13 | 0.15 | 0.08 | [41] |
| 7 | α-D-Rhap4N-(1-2)-α-D-Rhap4N-OMe | 0.10 | 0.07 | 0.29 | 0.29 | 0.28 f | 0.15 | 0.41 | 0.13 | 0.09 | 0.15 | 0.04 | [42] |
| 8 | α-D-Rhap4N-(1-3)-α-D-Rhap4N-OMe | 0.16 | 0.10 | 0.32 | 0.32 | 0.16 f | 0.13 | 0.17 | 0.26 | 0.18 | 0.18 | 0.07 | [42] |
| 9 | α-D-GalpA-(1-3)-α-D-FucpNAc4N-OMe | 0.16 | 0.16 | 0.18 | 0.18 | 0.29 f | 0.20 | 0.31 | 0.31 | 0.32 | 0.17 | 0.18 | [43] |
| 10 | α-L-Rhap-(1-2)-α-L-Rhap-OMe | 0.10 | 0.10 | 0.16 | 0.16 | 0.14 | 0.14 | 0.21 | 0.38 | 0.16 | 0.14 | 0.13 | [44] |
| 11 | α-KDO-(2-4)-α-KDO-OAllyl | 0.15 | 0.16 | 0.25 | 0.25 | 0.20 f | 0.08 | 0.25 | 0.18 | 0.20 | 0.12 | 0.10 | [45] |
| average (all NOEs) g | 0.11 (0.33) | 0.11 (0.33) | 0.20 (0.60) | 0.13 (0.41) | 0.15 (0.47) | 0.12 (0.40) | 0.19 (0.64) | 0.26 (0.92) | 0.15 (0.53) | 0.13 (0.42) | 0.10 (0.36) | ||
| average (transglycosidic NOEs) | 0.10 | 0.10 | 0.22 | 0.10 | 0.14 | 0.11 | 0.18 | 0.21 | 0.14 | 0.13 | 0.10 | ||
| overall rank h | 4 | 1 | 5 | 11 | 8 | 3 | 10 | 7 | 12 | 6 | 2 | ||
| software i | Tinker | Tinker | Tinker | Amber, Gromacs | Gromacs | Gromacs | Gaussian | ||||||
| supplementary table | S1 | S1 | S2 | S3 | S4-1 | S4-1 | S5 | S5 | S6 | S6 | |||
| trajectory length, ns | 50 | 50 | 50 | 50 | 100 | 100 | 50 | 50 | 50 | 50 | |||
| Ranking Scheme | 1st Rank | 2nd Rank | 3rd Rank |
|---|---|---|---|
| Absolute RMSD, quadratic scale | MM3, explicit | DFT | Glycam, explicit |
| Absolute RMSD, linear scale | MM3, explicit | DFT | MM3, implicit |
| Relative RMSD, quadratic scale | MM3, implicit | DFT | MM3, explicit |
| Relative RMSD, linear scale | MM3, implicit | MM3, explicit | DFT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroylov, V.; Panova, M.; Toukach, P. Comparison of Methods for Bulk Automated Simulation of Glycosidic Bond Conformations. Int. J. Mol. Sci. 2020, 21, 7626. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207626
Stroylov V, Panova M, Toukach P. Comparison of Methods for Bulk Automated Simulation of Glycosidic Bond Conformations. International Journal of Molecular Sciences. 2020; 21(20):7626. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207626
Chicago/Turabian StyleStroylov, Victor, Maria Panova, and Philip Toukach. 2020. "Comparison of Methods for Bulk Automated Simulation of Glycosidic Bond Conformations" International Journal of Molecular Sciences 21, no. 20: 7626. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207626