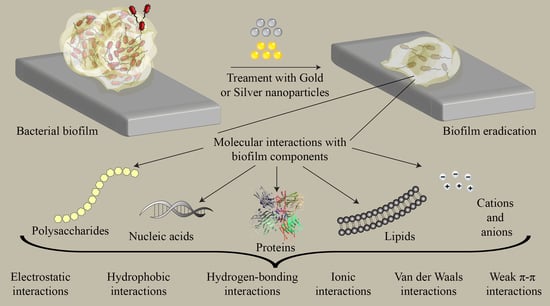
Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance
Abstract
:1. Introduction
1.1. Bacterial Biofilms and the Components of Biofilms
1.2. Current Status of Biofilm Inhibition
2. Gold and Silver Nanoparticles as Antimicrobial Agents
3. Interaction of Gold and Silver Nanoparticles with Biofilm Components
3.1. Interactions with Biofilm Nucleic Acids
3.2. Interactions of Nanoparticles with Biofilm Proteins
3.3. Interactions of Nanoparticles with Biofilm Polysaccharides
3.4. Interactions of Nanoparticles with Biofilm Lipids
3.5. Effect of Physicochemical Properties of Nanoparticles and Biofilms on Interactions
4. Interactions Involved in Biofilm Resistance to the Nanoparticles
5. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| Ag+ | Silver ions |
| AgNDs | Silver nanodiscs |
| AgNPs | Silver nanoparticles |
| AgNS | Silver nanospheres |
| AgNtr | Silver nanotriangular plates |
| AIP-I | Autoinducing peptide-I |
| AMPs | Antimicrobial peptides |
| ATP | Adenosine triphosphate |
| Au+ | Gold ions |
| Au-Ag-NPs | Bimetallic hybrid gold–silver nanoparticles |
| AuNFs | Gold nanoflowers |
| AuNPs | Gold nanoparticles |
| AuNRs | Gold nanorods |
| AuNS | Gold nanospheres |
| AuNSts | Gold nanostars |
| BAP | Biofilm-associated protein |
| bPEI | Branched polyethylenimine |
| bPEI-AgNPs | Branched polyethylenimine coated silver nanoparticles |
| bPEI-AuNPs | Branched polyethylenimine coated gold nanoparticles |
| BslA | Biofilm surface layer protein A |
| Chitosan-AgNPs | Chitosan-coated silver nanoparticles |
| copA | Copper transporter gene |
| cpsB | Capsular polysaccharide regulon gene |
| CTAB | Cetyl trimethylammonium bromide |
| CTAB-AuNPs | Cetyl trimethylammonium bromide coated gold nanoparticles |
| Cyclic-di-GMP | Bis-(3′,5′)-cyclic dimeric guanosine monophosphate |
| DLS | Dynamic light scattering |
| DLVO theory | Derjaguin–Landau–Verwey–Overbeek theory |
| DSPE | 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine |
| DSPE-AuNPs | 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine coated gold nanoparticles |
| EbpA | Enterococcus faecalis pilus tip |
| eDNA | Extracellular deoxyribonucleic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| EPS | Extracellular polymeric substances |
| FapC | Amyloid-like fimbriae protein from Pseudomonas species |
| FnBPs | Fibronectin binding proteins |
| HSP-18 | Heat shock protein-18 |
| LasI | Acyl-homoserine-lactone (AHL) synthase |
| LasR | Transcriptional activator protein required for activation of elastase structural gene (LasB) |
| LPS | Lipopolysaccharides |
| LpxC | UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase (encoded by lpxC gene) |
| MexAb-OprM | Outer membrane efflux protein from P. aeruginosa |
| MreB | Cell shape determining protein of E. coli |
| MvfR | Multiple virulence factor regulator protein from P. aeruginosa |
| NIR | Near infrared |
| NMR | Nuclear magnetic resonance |
| NRs | Nanorods |
| PAA | Poly(acrylic acid) |
| PAA-AuNPs | Poly(acrylic acid)-coated gold nanoparticles |
| PAH-AuNPs | Poly(allylamine hydrochloride)-coated gold nanoparticles |
| PEG | Poly(ethyleneglycol) |
| PEG-AuNPs | Poly(ethyleneglycol)-coated gold nanoparticles |
| PEG-PSB-PALA-AgNAs | Poly(ethyleneglycol)-poly(aminopropyl imidazole aspartate)-polyalanine-coated silver nanoassemblies |
| POPS-AgNPs | 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine-coated silver nanoparticles |
| PS-AuNPs | Polystyrene-coated gold nanoparticles |
| PT | Phosphorothioation |
| PUFAs | Polyunsaturated fatty acids |
| PVP | Polyvinylpyrrolidone |
| QS | Quorum sensing |
| QscR | Quorum sensing control repressor protein |
| RhlR | Regulatory protein required for transcriptional activation of gene associated with rhamnosyltransferase |
| RIP | RNA-III inhibiting peptide |
| ROS | Reactive oxygen species |
| silCFBA | Active efflux transporter complex of proteins (ATPase and silver binding protein SilE) responsible for efflux of silver ions |
| silE | Silver binding/sequestering protein |
| TEGOH | Tetraethylene glycol |
| TTMA | Thioalkyl tetra(ethylene glycol)ated trimethylammonium |
| Vfr | cAMP-activated global transcriptional regulator which controls virulence factor gene expression |
References
- Kassinger, S.J.; van Hoek, M.L. Biofilm architecture: An emerging synthetic biology target. Synth. Syst. Biotechnol. 2020, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hug, J.J.; Krug, D.; Müller, R. Bacteria as genetically programmable producers of bioactive natural products. Nat. Rev. Chem. 2020, 4, 172–193. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Teirlinck, E.; Samal, S.K.; Coenye, T.; Braeckmans, K. Penetrating the bacterial biofilm: Challenges for antimicrobial treatment. In Functionalized Nanomaterials for the Management of Microbial Infection; Chapter 3; Boukherroub, R., Szunerits, S., Drider, D., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 49–76. [Google Scholar]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlén, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 2020, 18, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial biofilms. J. Cell. Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Wang, L.-S.; Gupta, A.; Rotello, V.M. Nanomaterials for the Treatment of Bacterial Biofilms. ACS Infect. Dis. 2016, 2, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikuma, K.; Decho, A.W.; Lau, B.L.T. When nanoparticles meet biofilms—Interactions guiding the environmental fate and accumulation of nanoparticles. Front. Microbiol. 2015, 6, 591. [Google Scholar] [CrossRef]
- Qayyum, S.; Khan, A.U. Nanoparticles vs. biofilms: A battle against another paradigm of antibiotic resistance. MedChemComm 2016, 7, 1479–1498. [Google Scholar] [CrossRef]
- Pinto, R.M.; Lopes-de-Campos, D.; Martins, M.C.L.; Van Dijck, P.; Nunes, C.; Reis, S. Impact of nanosystems in Staphylococcus aureus biofilms treatment. FEMS Microbiol. Rev. 2019, 43, 622–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo-zeid, Y.; Williams, G.R. The potential anti-infective applications of metal oxide nanoparticles: A systematic review. Wires Nanomed. Nanobiotechnol. 2020, 12, e1592. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomim. 2020, 5, 27. [Google Scholar] [CrossRef]
- Seabra, A.B.; Durán, N. Nanotoxicology of Metal Oxide Nanoparticles. Metals 2015, 5, 934–975. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Liu, J.; Yin, Y.; Shen, M. Interactions between engineered nanoparticles and dissolved organic matter: A review on mechanisms and environmental effects. J. Environ. Sci. 2018, 63, 198–217. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Aggarwal, S. Factors impacting the interactions of engineered nanoparticles with bacterial cells and biofilms: Mechanistic insights and state of knowledge. J. Environ. Manag. 2018, 225, 62–74. [Google Scholar] [CrossRef]
- Zhang, X.; Servos, M.R.; Liu, J. Surface Science of DNA Adsorption onto Citrate-Capped Gold Nanoparticles. Langmuir 2012, 28, 3896–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasian, S.; Moshaii, A.; Nikkhah, M.; Farkhari, N. Adsorption of DNA on colloidal Ag nanoparticles: Effects of nanoparticle surface charge, base content and length of DNA. Colloids Surf. B Biointerfaces 2014, 116, 439–445. [Google Scholar] [CrossRef]
- Carnerero, J.M.; Jimenez-Ruiz, A.; Castillo, P.M.; Prado-Gotor, R. Covalent and Non-Covalent DNA–Gold-Nanoparticle Interactions: New Avenues of Research. ChemPhysChem 2017, 18, 17–33. [Google Scholar] [CrossRef]
- Koo, K.M.; Sina, A.A.I.; Carrascosa, L.G.; Shiddiky, M.J.A.; Trau, M. DNA–bare gold affinity interactions: Mechanism and applications in biosensing. Anal. Methods 2015, 7, 7042–7054. [Google Scholar] [CrossRef]
- Jiang, W.-Y.; Ran, S.-Y. Two-stage DNA compaction induced by silver ions suggests a cooperative binding mechanism. J. Chem. Phys. 2018, 148, 205102. [Google Scholar] [CrossRef] [PubMed]
- Radzig, M.; Koksharova, O.; Khmel, I.; Ivanov, V.; Yorov, K.; Kiwi, J.; Rtimi, S.; Tastekova, E.; Aybush, A.; Nadtochenko, V. Femtosecond Spectroscopy of Au Hot-Electron Injection into TiO2: Evidence for Au/TiO2 Plasmon Photocatalysis by Bactericidal Au Ions and Related Phenomena. Nanomaterials 2019, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Howard, S.T.; Newman, K.L.; McNulty, S.; Brown-Elliott, B.A.; Vasireddy, R.; Bridge, L.; Wallace, R.J. Insertion site and distribution of a genomic island conferring DNA phosphorothioation in the Mycobacterium abscessus complex. Microbiology 2013, 159, 2323–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Liao, Q.; Liu, M.; Hou, J.; Zhang, Y.; Liu, J. Antibacterial activity of silver nanoparticles target sara through srna-teg49, a key mediator of hfq, in Staphylococcus aureus. Int. J. Clin. Exp. Med. 2015, 8, 5794–5799. [Google Scholar]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Marino, T.; Russo, N.; Toscano, M.; Pavelka, M. Theoretical investigation on DNA/RNA base pairs mediated by copper, silver, and gold cations. Dalton Trans. 2012, 41, 1816–1823. [Google Scholar] [CrossRef]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyshnava, S.S.; Kanderi, D.K.; Panjala, S.P.; Pandian, K.; Bontha, R.R.; Goukanapalle, P.K.R.; Banaganapalli, B. Effect of Silver Nanoparticles Against the Formation of Biofilm by Pseudomonas aeruginosa an in silico Approach. Appl. Biochem. Biotechnol. 2016, 180, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Huma, Z.-E.; Javed, I.; Zhang, Z.; Bilal, H.; Sun, Y.; Hussain, S.Z.; Davis, T.P.; Otzen, D.E.; Landersdorfer, C.B.; Ding, F.; et al. Nanosilver Mitigates Biofilm Formation via FapC Amyloidosis Inhibition. Small 2020, 16, 1906674. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Seabrook, S.A.; Nedumpully-Govindan, P.; Chen, P.; Yin, H.; Waddington, L.; Epa, V.C.; Winkler, D.A.; Kirby, J.K.; Ding, F.; et al. Thermostability and reversibility of silver nanoparticle–protein binding. Phys. Chem. Chem. Phys. 2015, 17, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, X.; Liao, S.; Jiang, C.; Wang, L.; Tang, Y.; Wu, G.; Dai, G.; Chen, L. Quantitative Proteomics Reveals the Mechanism of Silver Nanoparticles against Multidrug-Resistant Pseudomonas aeruginosa Biofilms. J. Proteome Res. 2020, 19, 3109–3122. [Google Scholar] [CrossRef]
- Gupta, D.; Singh, A.; Khan, A.U. Nanoparticles as Efflux Pump and Biofilm Inhibitor to Rejuvenate Bactericidal Effect of Conventional Antibiotics. Nanoscale Res. Lett. 2017, 12, 454. [Google Scholar] [CrossRef]
- Chakraborty, A.; Biswas, A. Structure, stability and chaperone function of Mycobacterium leprae Heat Shock Protein 18 are differentially affected upon interaction with gold and silver nanoparticles. Int. J. Biol. Macromol. 2020, 152, 250–260. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.-F. Proteomics analysis of the mode of antibacterial action of nanoparticles and their interactions with proteins. TrAC Trends Anal. Chem. 2015, 65, 30–46. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Duan, S.-S.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. BioMetals 2011, 24, 135–141. [Google Scholar] [CrossRef]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Schober, Y.; Römpp, A.; Ghassempour, A.; Spengler, B. Proteomics study of silver nanoparticles toxicity on Bacillus thuringiensis. Ecotoxicol. Environ. Saf. 2014, 100, 122–130. [Google Scholar] [CrossRef]
- Tedesco, S.; Doyle, H.; Blasco, J.; Redmond, G.; Sheehan, D. Exposure of the blue mussel, Mytilus edulis, to gold nanoparticles and the pro-oxidant menadione. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 167–174. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small Molecule-Capped Gold Nanoparticles as Potent Antibacterial Agents That Target Gram-Negative Bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Jena, P.; Bhattacharya, M.; Bhattacharjee, G.; Satpati, B.; Mukherjee, P.; Senapati, D.; Srinivasan, R. Bimetallic gold–silver nanoparticles mediate bacterial killing by disrupting the actin cytoskeleton MreB. Nanoscale 2020, 12, 3731–3749. [Google Scholar] [CrossRef]
- Ortiz-Benítez, E.A.; Velázquez-Guadarrama, N.; Durán Figueroa, N.V.; Quezada, H.; Olivares-Trejo, J.D.J. Antibacterial mechanism of gold nanoparticles on Streptococcus pneumoniae. Metallomics 2019, 11, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Masanam, E.; Ramkumar, V.S.; Baskaraligam, V.; Selvaraj, G. Influence of N-acylhomoserine lactonase silver nanoparticles on the quorum sensing system of Helicobacter pylori: A potential strategy to combat biofilm formation. J. Basic Microbiol. 2020, 60, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Sharma, V.; Srivastava, A.K. Bacterial Polysaccharides: An Overview. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 81–108. [Google Scholar]
- Abadeer, N.S.; Fülöp, G.; Chen, S.; Käll, M.; Murphy, C.J. Interactions of Bacterial Lipopolysaccharides with Gold Nanorod Surfaces Investigated by Refractometric Sensing. ACS Appl. Mater. Interfaces 2015, 7, 24915–24925. [Google Scholar] [CrossRef] [PubMed]
- Pajerski, W.; Ochonska, D.; Brzychczy-Wloch, M.; Indyka, P.; Jarosz, M.; Golda-Cepa, M.; Sojka, Z.; Kotarba, A. Attachment efficiency of gold nanoparticles by Gram-positive and Gram-negative bacterial strains governed by surface charges. J. Nanopart. Res. 2019, 21, 186. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, K.H.; Gunsolus, I.L.; Kuech, T.R.; Troiano, J.M.; Melby, E.S.; Lohse, S.E.; Hu, D.; Chrisler, W.B.; Murphy, C.J.; Orr, G.; et al. Lipopolysaccharide Density and Structure Govern the Extent and Distance of Nanoparticle Interaction with Actual and Model Bacterial Outer Membranes. Environ. Sci. Technol. 2015, 49, 10642–10650. [Google Scholar] [CrossRef] [PubMed]
- Caudill, E.R.; Hernandez, R.T.; Johnson, K.P.; O’Rourke, J.T.; Zhu, L.; Haynes, C.L.; Feng, Z.V.; Pedersen, J.A. Wall teichoic acids govern cationic gold nanoparticle interaction with Gram-positive bacterial cell walls. Chem. Sci. 2020, 11, 4106–4118. [Google Scholar] [CrossRef] [Green Version]
- Bankier, C.; Matharu, R.K.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 16074. [Google Scholar] [CrossRef] [Green Version]
- Ivask, A.; ElBadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity Mechanisms in Escherichia coli Vary for Silver Nanoparticles and Differ from Ionic Silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ehrhardt, C.J.; Bertino, M.F.; Shah, M.R.; Yadavalli, V.K. Chitosan Stabilized Silver Nanoparticles for the Electrochemical Detection of Lipopolysaccharide: A Facile Biosensing Approach for Gram-Negative Bacteria. Micromachines 2020, 11, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitzel, M.R.; Tufenkji, N. Transport of Industrial PVP-Stabilized Silver Nanoparticles in Saturated Quartz Sand Coated with Pseudomonas aeruginosa PAO1 Biofilm of Variable Age. Environ. Sci. Technol. 2014, 48, 2715–2723. [Google Scholar] [CrossRef]
- Dunsing, V.; Irmscher, T.; Barbirz, S.; Chiantia, S. Purely Polysaccharide-Based Biofilm Matrix Provides Size-Selective Diffusion Barriers for Nanoparticles and Bacteriophages. Biomacromolecules 2019, 20, 3842–3854. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef]
- Morales, E.; Ferro-Flores, G.; Ocampo-García, B.; López-Téllez, G.; López-Ortega, J.; Rogel-Ayala, D.; Sánchez-Padilla, D. Antibacterial Efficacy of Gold and Silver Nanoparticles Functionalized with the Ubiquicidin (29–41) Antimicrobial Peptide. J. Nanomater. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Ramesh, S.; Grijalva, M.; Debut, A.; de la Torre, B.G.; Albericio, F.; Cumbal, L.H. Peptides conjugated to silver nanoparticles in biomedicine—A “value-added” phenomenon. Biomater. Sci. 2016, 4, 1713–1725. [Google Scholar] [CrossRef]
- Rajchakit, U.; Sarojini, V. Recent Developments in Antimicrobial-Peptide-Conjugated Gold Nanoparticles. Bioconjugate Chem. 2017, 28, 2673–2686. [Google Scholar] [CrossRef]
- Pal, I.; Bhattacharyya, D.; Kar, R.K.; Zarena, D.; Bhunia, A.; Atreya, H.S. A Peptide-Nanoparticle System with Improved Efficacy against Multidrug Resistant Bacteria. Sci. Rep. 2019, 9, 4485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benincasa, M.; Mattiuzzo, M.; Herasimenka, Y.; Cescutti, P.; Rizzo, R.; Gennaro, R. Activity of antimicrobial peptides in the presence of polysaccharides produced by pulmonary pathogens. J. Pept. Sci. 2009, 15, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.Y.; Hobby, C.R.; Siv, A.W.; Bible, W.C.; Glennon, M.S.; Anderson, D.M.; Symes, S.J.; Giles, D.K. Pseudomonas aeruginosa responds to exogenous polyunsaturated fatty acids (PUFAs) by modifying phospholipid composition, membrane permeability, and phenotypes associated with virulence. BMC Microbiol. 2018, 18, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, N.N.; Alhusban, A.A.; Ali, J.I.; Al-Bakri, A.G.; Hamed, R.; Khalil, E.A. Preferential Accumulation of Phospholipid-PEG and Cholesterol-PEG Decorated Gold Nanorods into Human Skin Layers and Their Photothermal-Based Antibacterial Activity. Sci. Rep. 2019, 9, 5796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri, S.; Cavallaro, A.; Christo, S.N.; Majewski, P.; Barton, M.; Hayball, J.D.; Vasilev, K. Antibacterial Plasma Polymer Films Conjugated with Phospholipid Encapsulated Silver Nanoparticles. ACS Biomater. Sci. Eng. 2015, 1, 1278–1286. [Google Scholar] [CrossRef]
- Khalid, H.F.; Tehseen, B.; Sarwar, Y.; Hussain, S.Z.; Khan, W.S.; Raza, Z.A.; Bajwa, S.Z.; Kanaras, A.G.; Hussain, I.; Rehman, A. Biosurfactant coated silver and iron oxide nanoparticles with enhanced anti-biofilm and anti-adhesive properties. J. Hazard. Mater. 2019, 364, 441–448. [Google Scholar] [CrossRef]
- Tanvir, F.; Yaqub, A.; Tanvir, S.; Anderson, W.A. Colorimetric enumeration of bacterial contamination in water based on β-galactosidase gold nanoshell activity. Enzym. Microb. Technol. 2017, 99, 49–56. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hikmat, S.; Abu Ghith, D.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Int. J. Pharm. 2019, 565, 174–186. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Nano-Photothermal ablation effect of Hydrophilic and Hydrophobic Functionalized Gold Nanorods on Staphylococcus aureus and Propionibacterium acnes. Sci. Rep. 2018, 8, 6881. [Google Scholar] [CrossRef] [Green Version]
- Giri, K.; Rivas Yepes, L.; Duncan, B.; Kolumam Parameswaran, P.; Yan, B.; Jiang, Y.; Bilska, M.; Moyano, D.F.; Thompson, M.A.; Rotello, V.M.; et al. Targeting bacterial biofilms via surface engineering of gold nanoparticles. RSC Adv. 2015, 5, 105551–105559. [Google Scholar] [CrossRef] [Green Version]
- Nallathamby, P.D.; Lee, K.J.; Desai, T.; Xu, X.-H.N. Study of the Multidrug Membrane Transporter of Single Living Pseudomonas aeruginosa Cells Using Size-Dependent Plasmonic Nanoparticle Optical Probes. Biochemistry 2010, 49, 5942–5953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peulen, T.-O.; Wilkinson, K.J. Diffusion of Nanoparticles in a Biofilm. Environ. Sci. Technol. 2011, 45, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef] [Green Version]
- Mitzel, M.R.; Sand, S.; Whalen, J.K.; Tufenkji, N. Hydrophobicity of biofilm coatings influences the transport dynamics of polystyrene nanoparticles in biofilm-coated sand. Water Res. 2016, 92, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Li, H.; Wang, B.; Ye, Z.; Lei, W.; Jia, F.; Jin, Q.; Ren, K.-F.; Ji, J. Surface-Adaptive Gold Nanoparticles with Effective Adherence and Enhanced Photothermal Ablation of Methicillin-Resistant Staphylococcus aureus Biofilm. ACS Nano 2017, 11, 9330–9339. [Google Scholar] [CrossRef]
- Wu, J.; Li, F.; Hu, X.; Lu, J.; Sun, X.; Gao, J.; Ling, D. Responsive Assembly of Silver Nanoclusters with a Biofilm Locally Amplified Bactericidal Effect to Enhance Treatments against Multi-Drug-Resistant Bacterial Infections. ACS Cent. Sci. 2019, 5, 1366–1376. [Google Scholar] [CrossRef] [Green Version]
- Niño-Martínez, N.; Salas Orozco, M.F.; Martínez-Castañón, G.-A.; Torres Méndez, F.; Ruiz, F. Molecular Mechanisms of Bacterial Resistance to Metal and Metal Oxide Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar] [CrossRef] [Green Version]
- Faghihzadeh, F.; Anaya, N.M.; Astudillo-Castro, C.; Oyanedel-Craver, V. Kinetic, metabolic and macromolecular response of bacteria to chronic nanoparticle exposure in continuous culture. Environ. Sci. Nano 2018, 5, 1386–1396. [Google Scholar] [CrossRef]
- Randall, C.P.; Gupta, A.; Jackson, N.; Busse, D.; O’Neill, A.J. Silver resistance in Gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015, 70, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Siemer, S.; Westmeier, D.; Barz, M.; Eckrich, J.; Wünsch, D.; Seckert, C.; Thyssen, C.; Schilling, O.; Hasenberg, M.; Pang, C.; et al. Biomolecule-corona formation confers resistance of bacteria to nanoparticle-induced killing: Implications for the design of improved nanoantibiotics. Biomaterials 2019, 192, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Shang, E.; Xia, X.; Niu, J.; Crittenden, J. Effects of Chloride Ions on Dissolution, ROS Generation, and Toxicity of Silver Nanoparticles under UV Irradiation. Environ. Sci. Technol. 2018, 52, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, J.; Hassinen, J.; Honkanen, R.; Kumar, A.A.; Viskari, H.; Kettunen, A.; Pahimanolis, N.; Pradeep, T.; Rojas, O.J.; Ikkala, O. Effects of Chloride Concentration on the Water Disinfection Performance of Silver Containing Nanocellulose-based Composites. Sci. Rep. 2019, 9, 19505. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, M.; Wang, J.; Marambio-Jones, C.; Peng, F.; Huang, X.; Damoiseaux, R.; Hoek, E.M.V. High-Throughput Screening of Silver Nanoparticle Stability and Bacterial Inactivation in Aquatic Media: Influence of Specific Ions. Environ. Sci. Technol. 2010, 44, 7321–7328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Oyanedel-Craver, V. Evaluation of the Disinfectant Performance of Silver Nanoparticles in Different Water Chemistry Conditions. J. Environ. Eng. 2012, 138, 58–66. [Google Scholar] [CrossRef]
- Kang, F.; Alvarez, P.J.; Zhu, D. Microbial Extracellular Polymeric Substances Reduce Ag+ to Silver Nanoparticles and Antagonize Bactericidal Activity. Environ. Sci. Technol. 2014, 48, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kang, F.; Gao, Y.; Mao, X.; Hu, X. Sequestration of nanoparticles by an EPS matrix reduces the particle-specific bactericidal activity. Sci. Rep. 2016, 6, 21379. [Google Scholar] [CrossRef] [Green Version]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Moussa, I. Antibacterial effects and resistance induction of silver and gold nanoparticles against Staphylococcus aureus-induced mastitis and the potential toxicity in rats. MicrobiologyOpen 2019, 8, e00698. [Google Scholar] [CrossRef] [Green Version]
- Contreras, F.; Vargas, E.; Jiménez, K.; Muñoz-Villagrán, C.; Figueroa, M.; Vásquez, C.; Arenas, F. Reduction of Gold (III) and Tellurium (IV) by Enterobacter cloacae MF01 Results in Nanostructure Formation Both in Aerobic and Anaerobic Conditions. Front. Microbiol. 2018, 9, 3118. [Google Scholar] [CrossRef] [Green Version]
- Ellis, D.H.; Maurer-Gardner, E.I.; Sulentic, C.E.W.; Hussain, S.M. Silver nanoparticle antibacterial efficacy and resistance development in key bacterial species. Biomed. Phys. Eng. Express 2018, 5, 015013. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Choo, Q.; Ashtikar, M.; Rocha, T.C.; Bremer-Hoffmann, S.; Wacker, M.G. Nanomedicines—Tiny particles and big challenges. Adv. Drug Deliv. Rev. 2019, 151–152, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar]
- U.S. Food and Drug Administration. FDA’s Approach to Regulation of Nanotechnology Products; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2015.
- U.S. Food and Drug Administration. Nanotechnology Task Force Report 2007. Available online: https://www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/ucm2006659.htm (accessed on 8 October 2020).
- Halamoda-Kenzaoui, B.; Box, H.; van Elk, M.; Gaitan, S.; Geertsma, R.; Lafuente, E.G.; Owen, A.; Del Pozo, A.; Roesslein, M.; Bremer-Hoffmann, S. Anticipation of Regulatory Needs for Nanotechnology-Enabled Health Products; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- De Vlieger, J.S.; Crommelin, D.J.; Tyner, K.; Drummond, D.C.; Jiang, W.; McNeil, S.E.; Neervannan, S.; Crist, R.M.; Shah, V.P. Report of the AAPS Guidance Forum on the FDA Draft Guidance for Industry: Drug Products, Including Biological Products, that Contain Nanomaterials; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]





| Interactions between Nanoparticles and Biofilm Components during Antimicrobial Action of AuNPs and AgNPs | |
| Component | Type of Interaction |
| Nucleic acids | Electrostatic interactions, Van der Waals interactions, Hydrophobic interactions, Gold–Sulphur (Au-S) chemistry |
| Proteins | Electrostatic interactions, Hydrophobic interactions, Hydrogen-bonding interactions, Van der Waals interactions, π–π interactions, Gold–Sulphur (Au-S) chemistry |
| Polysaccharides | Electrostatic interactions, Hydrophobic interactions, Hydrogen-bonding interactions, Electrosteric repulsion interactions |
| Lipids | Hydrophobic interactions, Electrostatic interactions |
| Interactions between Nanoparticles and Biofilm/Planktonic Bacterial Cells during Resistance to the Antimicrobial Action of AuNPs and AgNPs | |
| Component | Type of Interaction |
| Proteins | Electrostatic interactions, Van der Waals interactions, Hydrophobic interactions, Hydrogen-bonding interactions |
| Polysaccharides | Electrostatic interactions, Physical barrier for nanoparticles (no interactions involved) |
| Cations | Ionic interactions |
| Anions | Electrostatic interactions, ionic interactions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance. Int. J. Mol. Sci. 2020, 21, 7658. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207658
Joshi AS, Singh P, Mijakovic I. Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance. International Journal of Molecular Sciences. 2020; 21(20):7658. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207658
Chicago/Turabian StyleJoshi, Abhayraj S., Priyanka Singh, and Ivan Mijakovic. 2020. "Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance" International Journal of Molecular Sciences 21, no. 20: 7658. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21207658