CCL18 in the Progression of Cancer
Abstract
:1. Introduction
2. The CCL18 Gene and the CCL18 Protein
- -
- macrophage inflammatory protein 4 (MIP-4)
- -
- pulmonary and activation-regulated chemokine (PARC)
- -
- alternative macrophage activation-associated C-C chemokine-1 (AMAC-1)
- -
- dendritic cell-derived C-C chemokine 1 (DCCK1)
3. CCL18 in Tumor Progression
3.1. Effect of CCL18 on Cancer Cells
3.1.1. Influence of CCL18 on Cancer Cell Proliferation
3.1.2. CCL18 as an Inducer of EMT and Migration of Tumor Cells
3.1.3. Influence of microRNA in the Function of CCL18
3.2. Influence of CCL18 on Tumor-Associated Cells and Tumor Microenvironment
3.2.1. Effect of CCL18 on Angiogenesis and Lymphangiogenesis
3.2.2. Tumor-Associated Macrophages and CCL18 in the Neoplastic Tumor
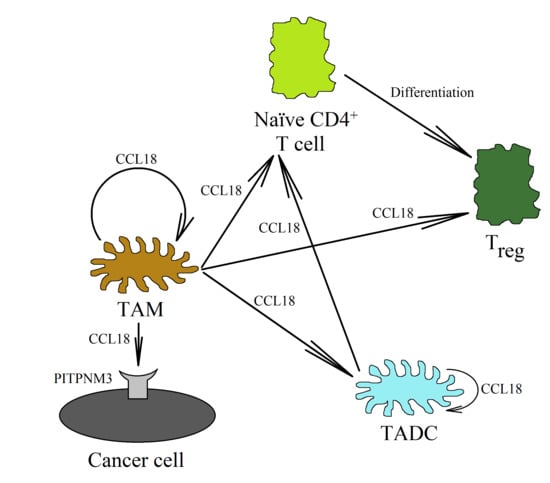
3.2.3. CCL18 and Treg Recruitment into the Tumor Niche
3.2.4. Tumor-Associated Dendritic Cells and CCL18
3.2.5. Significance of the Effects of CCL18 on CAFs in the Neoplastic Tumor
3.2.6. Role of Glycosaminoglycans in the Mechanisms of Action of CCL18
4. CCL18 as a Tumor Marker
5. CCL18 as a Therapeutic Target in Anti-Cancer Therapy
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CAF | Cancer-associated fibroblast |
| CCL | C-C motif chemokine ligand |
| CCR | C-C motif chemokine receptor |
| DC | Dendritic cell |
| EMT | Epithelial-to-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| IL | Interleukin |
| mTOR | Mammalian target of rapamycin |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor κB |
| PITPNM3 | Phosphatidylinositol transfer protein membrane-associated 3 |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PGE2 | Prostaglandin E2 |
| Treg | Regulatory T cell |
| TADC | Tumor-associated dendritic cell |
| TAM | Tumor-associated macrophage |
| TIL | Tumor-infiltrating lymphocyte |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- André, F.E.; Foulkes, M.A. A phased approach to clinical testing: Criteria for progressing from Phase I to Phase II to Phase III studies. Dev. Biol. Stand. 1998, 95, 57–60. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struyf, S.; Schutyser, E.; Gouwy, M.; Gijsbers, K.; Proost, P.; Benoit, Y.; Opdenakker, G.; Van Damme, J.; Laureys, G. PARC/CCL18 is a plasma CC chemokine with increased levels in childhood acute lymphoblastic leukemia. Am. J. Pathol. 2003, 163, 2065–2075. [Google Scholar] [CrossRef] [Green Version]
- Müller-Quernheim, U.C.; Potthast, L.; Müller-Quernheim, J.; Zissel, G. Tumor-cell co-culture induced alternative activation of macrophages is modulated by interferons in vitro. J. Interferon Cytokine Res. 2012, 32, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Qian, Y.; Ye, Z.; Chen, H.; Xie, H.; Zhou, L.; Shen, Y.; Zheng, S. Cancer-associated fibroblasts promote M2 polarization of macrophages in pancreatic ductal adenocarcinoma. Cancer Med. 2017, 6, 463–470. [Google Scholar] [CrossRef]
- Sawa-Wejksza, K.; Dudek, A.; Lemieszek, M.; Kaławaj, K.; Kandefer-Szerszeń, M. Colon cancer-derived conditioned medium induces differentiation of THP-1 monocytes into a mixed population of M1/M2 cells. Tumour Biol. 2018, 40, 1010428318797880. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F.R. The chemokine system and cancer. J. Pathol. 2012, 226, 148–157. [Google Scholar] [CrossRef]
- Sharma, I.; Singh, A.; Sharma, K.C.; Saxena, S. Gene Expression Profiling of Chemokines and Their Receptors in Low and High Grade Astrocytoma. Asian Pac. J. Cancer Prev. 2017, 18, 1307–1313. [Google Scholar] [PubMed]
- Ma, L.; Wang, H.; Li, Z.; Geng, X.; Li, M. Chemokine (C-C motif) ligand 18 is highly expressed in glioma tissues and promotes invasion of glioblastoma cells. J. Cancer Res. Ther. 2019, 15, 358–364. [Google Scholar]
- Hieshima, K.; Imai, T.; Baba, M.; Shoudai, K.; Ishizuka, K.; Nakagawa, T.; Tsuruta, J.; Takeya, M.; Sakaki, Y.; Takatsuki, K.; et al. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 alpha/LD78 alpha and chemotactic for T lymphocytes, but not for monocytes. J. Immunol. 1997, 159, 1140–1149. [Google Scholar]
- Guan, P.; Burghes, A.H.; Cunningham, A.; Lira, P.; Brissette, W.H.; Neote, K.; McColl, S.R. Genomic organization and biological characterization of the novel human CC chemokine DC-CK-1/PARC/MIP-4/SCYA18. Genomics 1999, 56, 296–302. [Google Scholar] [CrossRef]
- Kodelja, V.; Müller, C.; Politz, O.; Hakij, N.; Orfanos, C.E.; Goerdt, S. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 alpha with a Th2-associated expression pattern. J. Immunol. 1998, 160, 1411–1418. [Google Scholar] [PubMed]
- Schutyser, E.; Struyf, S.; Proost, P.; Opdenakker, G.; Laureys, G.; Verhasselt, B.; Peperstraete, L.; Van de Putte, I.; Saccani, A.; Allavena, P.; et al. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J. Biol. Chem. 2002, 277, 24584–24593. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.G.; Ren, M.; Zhao, F.; Tang, W.J. Structures of human CCL18, CCL3, and CCL4 reveal molecular determinants for quaternary structures and sensitivity to insulin-degrading enzyme. J. Mol. Biol. 2015, 427, 1345–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasaki, Y.; Fukuda, S.; Iio, M.; Miura, R.; Imai, T.; Sugano, S.; Yoshie, O.; Hughes, A.L.; Nomiyama, H. Chemokine PARC gene (SCYA18) generated by fusion of two MIP-1alpha/LD78alpha-like genes. Genomics 1999, 55, 353–357. [Google Scholar] [CrossRef]
- de Nadaï, P.; Charbonnier, A.S.; Chenivesse, C.; Sénéchal, S.; Fournier, C.; Gilet, J.; Vorng, H.; Chang, Y.; Gosset, P.; Wallaert, B.; et al. Involvement of CCL18 in allergic asthma. J. Immunol. 2006, 176, 6286–6293. [Google Scholar] [CrossRef]
- Lindhout, E.; Vissers, J.L.; Hartgers, F.C.; Huijbens, R.J.; Scharenborg, N.M.; Figdor, C.G.; Adema, G.J. The dendritic cell-specific CC-chemokine DC-CK1 is expressed by germinal center dendritic cells and attracts CD38-negative mantle zone B lymphocytes. J. Immunol. 2001, 166, 3284–3289. [Google Scholar] [CrossRef] [Green Version]
- Krohn, S.; Garin, A.; Gabay, C.; Proudfoot, A.E. The Activity of CCL18 is Principally Mediated through Interaction with Glycosaminoglycans. Front. Immunol. 2013, 4, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schraufstatter, I.; Takamori, H.; Sikora, L.; Sriramarao, P.; DiScipio, R.G. Eosinophils and monocytes produce pulmonary and activation-regulated chemokine, which activates cultured monocytes/macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L494–L501. [Google Scholar] [CrossRef] [Green Version]
- Adema, G.J.; Hartgers, F.; Verstraten, R.; de Vries, E.; Marland, G.; Menon, S.; Foster, J.; Xu, Y.; Nooyen, P.; McClanahan, T.; et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature 1997, 387, 713–717. [Google Scholar] [CrossRef]
- Porcheray, F.; Viaud, S.; Rimaniol, A.C.; Léone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dormont, D.; Gras, G. Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [Green Version]
- Azzaoui, I.; Yahia, S.A.; Chang, Y.; Vorng, H.; Morales, O.; Fan, Y.; Delhem, N.; Ple, C.; Tonnel, A.B.; Wallaert, B.; et al. CCL18 differentiates dendritic cells in tolerogenic cells able to prime regulatory T cells in healthy subjects. Blood 2011, 118, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Shen, M.H.; Xie, X.H.; Ruan, S.M. Inhibition of breast cancer metastasis via PITPNM3 by pachymic acid. Asian Pac. J. Cancer Prev. 2012, 13, 1877–1880. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Liang, Y.X.; Zhu, J.G.; Fu, X.; Chen, Y.F.; Mo, R.J.; Zhou, L.; Fu, H.; Bi, X.C.; He, H.C.; et al. CC chemokine ligand 18 correlates with malignant progression of prostate cancer. Biomed. Res. Int. 2014, 2014, 230183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Chen, X.; Hong, Y.; Wu, T.; Chen, X.; Xia, J.; Cheng, B. Elevated autocrine chemokine ligand 18 expression promotes oral cancer cell growth and invasion via Akt activation. Oncotarget 2016, 7, 16262–16272. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Zhang, B.; Sun, X.; Zhang, X.; Lv, S.; Li, H.; Wang, X.; Zhao, C.; Zhang, H.; Xie, X.; et al. CC chemokine ligand 18(CCL18) promotes migration and invasion of lung cancer cells by binding to Nir1 through Nir1-ELMO1/DOC180 signaling pathway. Mol. Carcinog. 2016, 55, 2051–2062. [Google Scholar] [CrossRef]
- Islam, S.A.; Ling, M.F.; Leung, J.; Shreffler, W.G.; Luster, A.D. Identification of human CCR8 as a CCL18 receptor. J. Exp. Med. 2013, 210, 1889–1898. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Deng, W.; Huang, M.; Wu, Y.; Zhou, Z.; Zhu, K.; Wang, Y.; Cheng, X.; Zhou, X.; et al. CCL18 enhances migration, invasion and EMT by binding CCR8 in bladder cancer cells. Mol. Med. Rep. 2019, 19, 1678–1686. [Google Scholar] [CrossRef]
- Zissel, G.; Höhne, K.; Kilic, A.; Maier, C.; Goldmann, T.; Prasse, A.; Ploenes, T.; Trepel, M.; Eibel, H.; Müller-Quernheim, J. Identification of the CCL18 Receptor—Effects of CCL18 on Human Lung Fibroblasts in Pulmonary Fibrosis are Mediated via CCR6. Pneumologie 2012, 66, P3_012. [Google Scholar] [CrossRef]
- Catusse, J.; Wollner, S.; Leick, M.; Schröttner, P.; Schraufstätter, I.; Burger, M. Attenuation of CXCR4 responses by CCL18 in acute lymphocytic leukemia B cells. J. Cell. Physiol. 2010, 225, 792–800. [Google Scholar] [CrossRef]
- Günther, C.; Zimmermann, N.; Berndt, N.; Grosser, M.; Stein, A.; Koch, A.; Meurer, M. Up-regulation of the chemokine CCL18 by macrophages is a potential immunomodulatory pathway in cutaneous T-cell lymphoma. Am. J. Pathol. 2011, 179, 1434–1442. [Google Scholar] [CrossRef]
- Nibbs, R.J.; Salcedo, T.W.; Campbell, J.D.; Yao, X.T.; Li, Y.; Nardelli, B.; Olsen, H.S.; Morris, T.S.; Proudfoot, A.E.; Patel, V.P.; et al. C-C chemokine receptor 3 antagonism by the beta-chemokine macrophage inflammatory protein 4, a property strongly enhanced by an amino-terminal alanine-methionine swap. J. Immunol. 2000, 164, 1488–1497. [Google Scholar] [CrossRef] [Green Version]
- Krohn, S.C.; Bonvin, P.; Proudfoot, A.E. CCL18 exhibits a regulatory role through inhibition of receptor and glycosaminoglycan binding. PLoS ONE 2013, 8, e72321. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, Y.; Wu, X.; Meng, S.; Yu, W.; Zhao, J.; Zhang, H.; Wang, J.; Li, W. CCL18 secreted from M2 macrophages promotes migration and invasion via the PI3K/Akt pathway in gallbladder cancer. Cell. Oncol. 2019, 42, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Chen, J.; Huang, D.; Yao, Y.; Chen, J.; Ding, L.; Zeng, J.; Su, S.; Chao, X.; Su, F.; et al. Tumor-Associated Macrophages Promote Malignant Progression of Breast Phyllodes Tumors by Inducing Myofibroblast Differentiation. Cancer Res. 2017, 77, 3605–3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.Y.; Lee, Y.H.; Leu, S.J.; Wang, C.Y.; Wei, C.P.; Hung, K.S.; Pai, M.H.; Tsai, M.D.; Wu, C.H. CC-chemokine ligand 18/pulmonary activation-regulated chemokine expression in the CNS with special reference to traumatic brain injuries and neoplastic disorders. Neuroscience 2010, 165, 1233–1243. [Google Scholar] [CrossRef]
- Pettersen, J.S.; Fuentes-Duculan, J.; Suárez-Fariñas, M.; Pierson, K.C.; Pitts-Kiefer, A.; Fan, L.; Belkin, D.A.; Wang, C.Q.; Bhuvanendran, S.; Johnson-Huang, L.M.; et al. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J. Investig. Dermatol. 2011, 131, 1322–1330. [Google Scholar] [CrossRef] [Green Version]
- Gabrusiewicz, K.; Rodriguez, B.; Wei, J.; Hashimoto, Y.; Healy, L.M.; Maiti, S.N.; Thomas, G.; Zhou, S.; Wang, Q.; Elakkad, A.; et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight 2016, 1, e85841. [Google Scholar] [CrossRef]
- Yuan, R.; Chen, Y.; He, X.; Wu, X.; Ke, J.; Zou, Y.; Cai, Z.; Zeng, Y.; Wang, L.; Wang, J.; et al. CCL18 as an independent favorable prognostic biomarker in patients with colorectal cancer. J. Surg. Res. 2013, 183, 163–169. [Google Scholar] [CrossRef]
- Leung, S.Y.; Yuen, S.T.; Chu, K.M.; Mathy, J.A.; Li, R.; Chan, A.S.; Law, S.; Wong, J.; Chen, X.; So, S. Expression profiling identifies chemokine (C-C motif) ligand 18 as an independent prognostic indicator in gastric cancer. Gastroenterology 2004, 127, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhao, L.; Hou, Y.; Sun, Y.; Wang, L.; Luo, H.; Peng, H.; Liu, M. Biological characteristics and genetic heterogeneity between carcinoma-associated fibroblasts and their paired normal fibroblasts in human breast cancer. PLoS ONE 2013, 8, e60321. [Google Scholar] [CrossRef]
- Wu, J.; Long, Z.; Cai, H.; Du, C.; Liu, X.; Yu, S.; Wang, Y. High expression of WISP1 in colon cancer is associated with apoptosis, invasion and poor prognosis. Oncotarget 2016, 7, 49834–49847. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, Y.; Zhao, Z.; Chen, Z.; Wang, Z.; Xu, S.; Zhang, X.; Liu, T.; Yu, S. The long non-coding RNA CRNDE competed endogenously with miR-205 to promote proliferation and metastasis of melanoma cells by targeting CCL18. Cell Cycle 2018, 17, 2296–2308. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.M.; Ji, S.; Li, Y.; Fu, L.Y.; Jiang, T.; Meng, F.D. β-Catenin promotes cell proliferation, migration, and invasion but induces apoptosis in renal cell carcinoma. Onco Targets Ther. 2017, 10, 711–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenivesse, C.; Chang, Y.; Azzaoui, I.; Ait Yahia, S.; Morales, O.; Plé, C.; Foussat, A.; Tonnel, A.B.; Delhem, N.; Yssel, H.; et al. Pulmonary CCL18 recruits human regulatory T cells. J. Immunol. 2012, 189, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Du, C.; Xu, P.; Miao, C. Surgical trauma-induced CCL18 promotes recruitment of regulatory T cells and colon cancer progression. J. Cell. Physiol. 2019, 234, 4608–4616. [Google Scholar] [CrossRef] [Green Version]
- Ploenes, T.; Scholtes, B.; Krohn, A.; Burger, M.; Passlick, B.; Müller-Quernheim, J.; Zissel, G. CC-chemokine ligand 18 induces epithelial to mesenchymal transition in lung cancer A549 cells and elevates the invasive potential. PLoS ONE 2013, 8, e53068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, Y.X.; Chen, L.P.; Ji, M.L. Upregulation of microRNA-181b inhibits CCL18-induced breast cancer cell metastasis and invasion via the NF-κB signaling pathway. Oncol. Lett. 2016, 12, 4411–4418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, Y.X.; Zhang, D.Z.; Fang, X.J.; Sun, P.S.; Xue, H.C. Let-7a mimic attenuates CCL18 induced breast cancer cell metastasis through Lin 28 pathway. Biomed. Pharmacother. 2016, 78, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Huang, L.; Gu, Y.; Lu, H.; Feng, Z. The expression of CCL18 in diffuse large B cell lymphoma and its mechanism research. Cancer Biomark. 2018, 21, 925–934. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Yu, H.; Yin, Q.; Li, M.; Shi, L.; Zhang, W.; Li, D.; Li, L. CCL18 from tumor-cells promotes epithelial ovarian cancer metastasis via mTOR signaling pathway. Mol. Carcinog 2016, 55, 1688–1699. [Google Scholar] [CrossRef]
- Su, Y.; Zhou, Y.; Sun, Y.J.; Wang, Y.L.; Yin, J.Y.; Huang, Y.J.; Zhang, J.J.; He, A.N.; Han, K.; Zhang, H.Z.; et al. Macrophage-derived CCL18 promotes osteosarcoma proliferation and migration by upregulating the expression of UCA1. J. Mol. Med. 2019, 97, 49–61. [Google Scholar] [CrossRef]
- Bo, S.; Donghao, S.; Guangqi, K.; Ye, T. CC Chemokine Ligand 18 Promotes Cell Proliferation and Metastasis of Urothelial Carcinoma via Activating PI3K/mTOR Signaling in Patient with Renal Transplantation. Urol. Int. 2018, 101, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, Y.; Qiao, H. CCL18 promotes the invasion and migration of gastric cancer cells via ERK1/2/NF-κB signaling pathway. Tumour Biol. 2016, 37, 641–651. [Google Scholar] [CrossRef]
- Meng, F.; Li, W.; Li, C.; Gao, Z.; Guo, K.; Song, S. CCL18 promotes epithelial-mesenchymal transition, invasion and migration of pancreatic cancer cells in pancreatic ductal adenocarcinoma. Int. J. Oncol. 2015, 46, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liang, X.; Li, M.; Tao, X.; Tai, S.; Fan, Z.; Wang, Z.; Cheng, B.; Xia, J. Chemokine (CC motif) ligand 18 upregulates Slug expression to promote stem-cell like features by activating the mammalian target of rapamycin pathway in oral squamous cell carcinoma. Cancer Sci. 2017, 108, 1584–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, L.; Qin, Y.; Wang, J.; Liu, C.; Zhu, G.; Li, G.; Wei, M.; Chen, C.; Liu, G.; Zhang, D.; et al. Tumor-associated macrophages derived CCL18 promotes metastasis in squamous cell carcinoma of the head and neck. Cancer Cell Int. 2018, 18, 120. [Google Scholar] [CrossRef]
- Lin, Z.; Li, W.; Zhang, H.; Wu, W.; Peng, Y.; Zeng, Y.; Wan, Y.; Wang, J.; Ouyang, N. CCL18/PITPNM3 enhances migration, invasion, and EMT through the NF-κB signaling pathway in hepatocellular carcinoma. Tumour Biol. 2016, 37, 3461–3468. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, C.; Li, H.; Shi, L.; Liu, N.; Sun, Y.; Lu, S.; Liu, Y.; Sun, L.; Li, X.; et al. Nir1 promotes invasion of breast cancer cells by binding to chemokine (C-C motif) ligand 18 through the PI3K/Akt/GSK3β/Snail signalling pathway. Eur. J. Cancer 2013, 49, 3900–3913. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Yu, J.; Liu, H.; Chen, Z.; Zhong, H.; Wan, Y. CCL18-dependent translocation of AMAP1 is critical for epithelial to mesenchymal transition in breast cancer. J. Cell. Physiol. 2018, 233, 3207–3217. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; Li, Q.; Li, S.; Lai, H.; Song, E.; Li, D.; Chen, J. Discovery of CCL18 antagonist blocking breast cancer metastasis. Clin. Exp. Metastasis 2019, 36, 243–255. [Google Scholar] [CrossRef]
- Lane, D.; Matte, I.; Laplante, C.; Garde-Granger, P.; Carignan, A.; Bessette, P.; Rancourt, C.; Piché, A. CCL18 from ascites promotes ovarian cancer cell migration through proline-rich tyrosine kinase 2 signaling. Mol. Cancer 2016, 15, 58. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Zhou, Q.; Zheng, S.; Li, G.; Lin, Q.; Wei, L.; Fu, Z.; Zhang, B.; Liu, Y.; Li, Z.; et al. Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018, 9, 453. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Wang, J.; Zhu, G.; Li, G.; Tan, H.; Chen, C.; Pi, L.; She, L.; Chen, X.; Wei, M.; et al. CCL18 promotes the metastasis of squamous cell carcinoma of the head and neck through MTDH-NF-κB signalling pathway. J. Cell. Mol. Med. 2019, 23, 2689–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yao, Y.; Gong, C.; Yu, F.; Su, S.; Chen, J.; Liu, B.; Deng, H.; Wang, F.; Lin, L.; et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell 2011, 19, 541–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Huang, Z.; Sun, X.; Zheng, X.; Liu, J.; Shen, J.; Jia, B.; Luo, H.; Mai, Z.; Chen, G.; et al. CCL18-NIR1 promotes oral cancer cell growth and metastasis by activating the JAK2/STAT3 signaling pathway. BMC Cancer 2020, 20, 632. [Google Scholar] [CrossRef]
- Shen, T.; Guo, Q. Role of Pyk2 in Human Cancers. Med. Sci. Monit. 2018, 24, 8172–8182. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Eddy, S.F.; Sherr, D.H.; Sonenshein, G.E. NF-kappaB and epithelial to mesenchymal transition of cancer. J. Cell. Biochem. 2008, 104, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Komai, M.; Fujimoto, S.; Itoh, T.; Imano, M.; Sakamoto, K.; Shimaoka, H.; Takeda, T.; Ogawa, N.; Mashimo, K.; et al. Activation of NF-κB by the RANKL/RANK system up-regulates snail and twist expressions and induces epithelial-to-mesenchymal transition in mammary tumor cell lines. J. Exp. Clin. Cancer Res. 2013, 32, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, B.R.; Mencalha, A.L.; Ferreira, G.M.; de Souza, W.F.; Morgado-Díaz, J.A.; Maia, A.M.; Corrêa, S.; Abdelhay, E.S. NF-kappaB Is Involved in the Regulation of EMT Genes in Breast Cancer Cells. PLoS ONE 2017, 12, e0169622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.H.; Bak, Y.; Kwon, T.; Kwon, S.B.; Oh, J.W.; Park, J.H.; Choi, Y.K.; Hong, J.T.; Yoon, D.Y. Interleukin-32θ inhibits tumor-promoting effects of macrophage-secreted CCL18 in breast cancer. Cell Commun. Signal. 2019, 17, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.Y.; Cui, X.Y.; Wu, W.; Yu, F.Y.; Yao, H.R.; Liu, Q.; Song, E.W.; Chen, J.Q. Pyk2 and Src mediate signaling to CCL18-induced breast cancer metastasis. J. Cell. Biochem. 2014, 115, 596–603. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, S.; Yan, Z.; Deng, Z.; Wang, R.; Zhang, B. CCL18 promotes the invasion and metastasis of breast cancer through Annexin A2. Oncol. Rep. 2020, 43, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, L.; Yao, Y.; Zhao, R.; Cui, X.; Chen, J.; Hou, K.; Zhang, M.; Su, F.; Chen, J.; et al. CCL18-mediated down-regulation of miR98 and miR27b promotes breast cancer metastasis. Oncotarget 2015, 6, 20485–20499. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Song, X.; Liu, W.; Yuan, X.; Jiang, J.; Wang, W.; Mullen, M.; Zhao, X.; Zhang, Y.; Liu, F.; Du, S.; et al. Acetylation of ACAP4 regulates CCL18-elicited breast cancer cell migration and invasion. J. Mol. Cell Biol. 2018, 10, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Wang, W.; Wang, H.; Yuan, X.; Yang, F.; Zhao, L.; Mullen, M.; Du, S.; Zohbi, N.; Muthusamy, S.; et al. Acetylation of ezrin regulates membrane-cytoskeletal interaction underlying CCL18-elicited cell migration. J. Mol. Cell Biol. 2020, 12, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.K. mTOR: Role in cancer, metastasis and drug resistance. Semin. Cancer Biol. 2019, 59, 92–111. [Google Scholar] [CrossRef]
- Jing, X.; Peng, J.; Dou, Y.; Sun, J.; Ma, C.; Wang, Q.; Zhang, L.; Luo, X.; Kong, B.; Zhang, Y.; et al. Macrophage ERα promoted invasion of endometrial cancer cell by mTOR/KIF5B-mediated epithelial to mesenchymal transition. Immunol. Cell Biol. 2019, 97, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Tao, Y.; Ni, N.; Zhou, X.; Xiong, J.; Zeng, X.; Xu, X.; Qi, J.; Sun, J. miR-128 targets the CC chemokine ligand 18 gene (CCL18) in cutaneous malignant melanoma progression. J. Dermatol. Sci. 2018, 91, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Sun, X.; Xu, K. The suppressing role of miR-622 in renal cell carcinoma progression by down-regulation of CCL18/MAPK signal pathway. Cell Biosci. 2018, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wu, D.; He, X.; Hu, X.; Hu, C.; Shen, Z.; Lin, J.; Pan, Z.; He, Z.; Lin, H.; et al. CCL18-induced HOTAIR upregulation promotes malignant progression in esophageal squamous cell carcinoma through the miR-130a-5p-ZEB1 axis. Cancer Lett. 2019, 460, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Span, P.N.; Bussink, J. Biology of hypoxia. Semin. Nucl. Med. 2015, 45, 101–109. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef] [Green Version]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Lin, L.; Chen, Y.S.; Yao, Y.D.; Chen, J.Q.; Chen, J.N.; Huang, S.Y.; Zeng, Y.J.; Yao, H.R.; Zeng, S.H.; Fu, Y.S.; et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget 2015, 6, 34758–34773. [Google Scholar] [CrossRef] [Green Version]
- Bosco, M.C.; Puppo, M.; Santangelo, C.; Anfosso, L.; Pfeffer, U.; Fardin, P.; Battaglia, F.; Varesio, L. Hypoxia modifies the transcriptome of primary human monocytes: Modulation of novel immune-related genes and identification of CC-chemokine ligand 20 as a new hypoxia-inducible gene. J. Immunol. 2006, 177, 1941–1955. [Google Scholar] [CrossRef]
- Ricciardi, A.; Elia, A.R.; Cappello, P.; Puppo, M.; Vanni, C.; Fardin, P.; Eva, A.; Munroe, D.; Wu, X.; Giovarelli, M.; et al. Transcriptome of hypoxic immature dendritic cells: Modulation of chemokine/receptor expression. Mol. Cancer Res. 2008, 6, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Blengio, F.; Raggi, F.; Pierobon, D.; Cappello, P.; Eva, A.; Giovarelli, M.; Varesio, L.; Bosco, M.C. The hypoxic environment reprograms the cytokine/chemokine expression profile of human mature dendritic cells. Immunobiology 2013, 218, 76–89. [Google Scholar] [CrossRef]
- Dehne, N.; Brüne, B. Hypoxic inhibition of JMJD3 reduces H3K27me3 demethylation and induction of the STAT6 target gene CCL18. Biochim. Biophys. Acta 2016, 1859, 1490–1501. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhu, Y.; Chen, L.; An, H.; Zhang, W.; Wang, G.; Lin, Z.; Xu, J. Prognostic value of diametrically polarized tumor-associated macrophages in renal cell carcinoma. Ann. Surg. Oncol. 2014, 21, 3142–3150. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Shen, Z.; Xu, J.; Qin, J.; Sun, Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer 2015, 18, 740–750. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Li, D.; Mao, Y.; Mo, F.; Du, W.; Ma, X. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2017, 147, 181–187. [Google Scholar] [CrossRef]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Walens, A.; DiMarco, A.V.; Lupo, R.; Kroger, B.R.; Damrauer, J.S.; Alvarez, J.V. CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. Elife 2019, 8, e43653. [Google Scholar] [CrossRef]
- Chen, X.J.; Deng, Y.R.; Wang, Z.C.; Wei, W.F.; Zhou, C.F.; Zhang, Y.M.; Yan, R.M.; Liang, L.J.; Zhong, M.; Liang, L.; et al. Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment. Cell Death Dis. 2019, 10, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Jain, A.; Syed, S.N.; Snodgrass, R.G.; Pflüger-Müller, B.; Leisegang, M.S.; Weigert, A.; Brandes, R.P.; Ebersberger, I.; Brüne, B.; et al. IL-6 augments IL-4-induced polarization of primary human macrophages through synergy of STAT3, STAT6 and BATF transcription factors. Oncoimmunology 2018, 7, e1494110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzoni, M.; Mauro, G.; Erreni, M.; Romeo, P.; Minna, E.; Vizioli, M.G.; Belgiovine, C.; Rizzetti, M.G.; Pagliardini, S.; Avigni, R.; et al. Senescent thyrocytes and thyroid tumor cells induce M2-like macrophage polarization of human monocytes via a PGE2-dependent mechanism. J. Exp. Clin. Cancer Res. 2019, 38, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiemessen, M.M.; Jagger, A.L.; Evans, H.G.; van Herwijnen, M.J.; John, S.; Taams, L.S. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 19446–19451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kenawi, A.; Gatenbee, C.; Robertson-Tessi, M.; Bravo, R.; Dhillon, J.; Balagurunathan, Y.; Berglund, A.; Vishvakarma, N.; Ibrahim-Hashim, A.; Choi, J.; et al. Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. Br. J. Cancer 2019, 121, 556–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, X.; Shi, W.; Xu, Y.; Xu, C.; Zhao, T.; Geng, B.; Yang, J.; Pan, J.; Hu, S.; Zhang, C.; et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 2018, 17, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.L.; Rios, E.; Silva, A.C.; Neves, S.C.; Caires, H.R.; Pinto, A.T.; Durães, C.; Carvalho, F.A.; Cardoso, A.P.; Santos, N.C.; et al. Decellularized human colorectal cancer matrices polarize macrophages towards an anti-inflammatory phenotype promoting cancer cell invasion via CCL18. Biomaterials 2017, 124, 211–224. [Google Scholar] [CrossRef]
- Su, S.; Liu, Q.; Chen, J.; Chen, J.; Chen, F.; He, C.; Huang, D.; Wu, W.; Lin, L.; Huang, W.; et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 2014, 25, 605–620. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.T.; Yuan, J.H.; Ma, J.Z.; Yang, W.J.; Liu, X.N.; Yin, Y.P.; Liu, Y.; Pan, W.; Sun, S.H. CTGF secreted by mesenchymal-like hepatocellular carcinoma cells plays a role in the polarization of macrophages in hepatocellular carcinoma progression. Biomed. Pharmacother. 2017, 95, 111–119. [Google Scholar] [CrossRef]
- Schraufstatter, I.U.; Zhao, M.; Khaldoyanidi, S.K.; Discipio, R.G. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology 2012, 135, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Wimmer, A.; Khaldoyanidi, S.K.; Judex, M.; Serobyan, N.; Discipio, R.G.; Schraufstatter, I.U. CCL18/PARC stimulates hematopoiesis in long-term bone marrow cultures indirectly through its effect on monocytes. Blood 2006, 108, 3722–3729. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Zhu, H.; Xu, J.; Zheng, Y.; Cao, X.; Liu, Q. Tumor-Derived CXCL1 Promotes Lung Cancer Growth via Recruitment of Tumor-Associated Neutrophils. J. Immunol. Res. 2016, 2016, 6530410. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Han, X.; Sun, Y.; Shang, C.; Wei, M.; Ba, X.; Zeng, X. Chemokine (C-X-C motif) ligand 1 and CXCL2 produced by tumor promote the generation of monocytic myeloid-derived suppressor cells. Cancer Sci. 2018, 109, 3826–3839. [Google Scholar] [CrossRef] [PubMed]
- Soria, G.; Ben-Baruch, A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef]
- Miyake, M.; Goodison, S.; Urquidi, V.; Gomes Giacoia, E.; Rosser, C.J. Expression of CXCL1 in human endothelial cells induces angiogenesis through the CXCR2 receptor and the ERK1/2 and EGF pathways. Lab. Investig. 2013, 93, 768–778. [Google Scholar] [CrossRef] [Green Version]
- Duluc, D.; Corvaisier, M.; Blanchard, S.; Catala, L.; Descamps, P.; Gamelin, E.; Ponsoda, S.; Delneste, Y.; Hebbar, M.; Jeannin, P. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int. J. Cancer 2009, 125, 367–373. [Google Scholar] [CrossRef]
- Furudate, S.; Fujimura, T.; Kakizaki, A.; Hidaka, T.; Asano, M.; Aiba, S. Tumor-associated M2 macrophages in mycosis fungoides acquire immunomodulatory function by interferon alpha and interferon gamma. J. Dermatol. Sci. 2016, 83, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Attias, M.; Al-Aubodah, T.; Piccirillo, C.A. Mechanisms of human FoxP3+ Treg cell development and function in health and disease. Clin. Exp. Immunol. 2019, 197, 36–51. [Google Scholar]
- Iellem, A.; Mariani, M.; Lang, R.; Recalde, H.; Panina-Bordignon, P.; Sinigaglia, F.; D’Ambrosio, D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001, 194, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Plitas, G.; Konopacki, C.; Wu, K.; Bos, P.D.; Morrow, M.; Putintseva, E.V.; Chudakov, D.M.; Rudensky, A.Y. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity 2016, 45, 1122–1134. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Dong, X.; Qi, P.; Ye, Y.; Shen, W.; Leng, L.; Wang, L.; Li, X.; Luo, X.; Chen, Y.; et al. Sox2 Communicates with Tregs Through CCL1 to Promote the Stemness Property of Breast Cancer Cells. Stem Cells 2017, 35, 2351–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehnemuth, B.; Piseddu, I.; Wiedemann, G.M.; Lauseker, M.; Kuhn, C.; Hofmann, S.; Schmoeckel, E.; Endres, S.; Mayr, D.; Jeschke, U.; et al. CCL1 is a major regulatory T cell attracting factor in human breast cancer. BMC Cancer 2018, 18, 1278. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Kono, K.; Kawaguchi, Y.; Akaike, H.; Kamimura, K.; Sugai, H.; Fujii, H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int. J. Cancer 2008, 122, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, G.M.; Röhrle, N.; Makeschin, M.C.; Fesseler, J.; Endres, S.; Mayr, D.; Anz, D. Peritumoural CCL1 and CCL22 expressing cells in hepatocellular carcinomas shape the tumour immune infiltrate. Pathology 2019, 51, 586–592. [Google Scholar] [CrossRef]
- Ren, L.; Yu, Y.; Wang, L.; Zhu, Z.; Lu, R.; Yao, Z. Hypoxia-induced CCL28 promotes recruitment of regulatory T cells and tumor growth in liver cancer. Oncotarget 2016, 7, 75763–75773. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, N.; Li, Q.; Zhang, W.; Ke, F.; Leng, Q.; Wang, H.; Chen, J.; Wang, H. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS ONE 2011, 6, e19495. [Google Scholar] [CrossRef]
- Chen, K.J.; Lin, S.Z.; Zhou, L.; Xie, H.Y.; Zhou, W.H.; Taki-Eldin, A.; Zheng, S.S. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE 2011, 6, e24671. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Qi, Y.; Li, X.N.; Yang, Y.; Liu, D.L.; Zhao, J.; Zhu, D.Y.; Wu, K.; Zhou, X.D.; Zhao, S. The role of CCL20/CCR6 axis in recruiting Treg cells to tumor sites of NSCLC patients. Biomed. Pharmacother. 2015, 69, 242–248. [Google Scholar] [CrossRef]
- Su, S.; Liao, J.; Liu, J.; Huang, D.; He, C.; Chen, F.; Yang, L.; Wu, W.; Chen, J.; Lin, L.; et al. Blocking the recruitment of naive CD4+ T cells reverses immunosuppression in breast cancer. Cell Res. 2017, 27, 461–482. [Google Scholar] [CrossRef]
- Hoves, S.; Krause, S.W.; Schütz, C.; Halbritter, D.; Schölmerich, J.; Herfarth, H.; Fleck, M. Monocyte-derived human macrophages mediate anergy in allogeneic T cells and induce regulatory T cells. J. Immunol. 2006, 177, 2691–2698. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lee, J.H.; Kim, C.H. Optimal population of FoxP3+ T cells in tumors requires an antigen priming-dependent trafficking receptor switch. PLoS ONE 2012, 7, e30793. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; de Nadai, P.; Azzaoui, I.; Morales, O.; Delhem, N.; Vorng, H.; Tomavo, S.; Ait Yahia, S.; Zhang, G.; Wallaert, B.; et al. The chemokine CCL18 generates adaptive regulatory T cells from memory CD4+ T cells of healthy but not allergic subjects. FASEB J. 2010, 24, 5063–5072. [Google Scholar] [PubMed]
- Wang, L.; Simons, D.L.; Lu, X.; Tu, T.Y.; Solomon, S.; Wang, R.; Rosario, A.; Avalos, C.; Schmolze, D.; Yim, J.; et al. Connecting blood and intratumoral Treg cell activity in predicting future relapse in breast cancer. Nat. Immunol. 2019, 20, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Malhotra, R.; Mayer, G.; Gorochov, G.; Miyara, M. Human FOXP3+ T regulatory cell heterogeneity. Clin. Transl. Immunol. 2018, 7, e1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middel, P.; Brauneck, S.; Meyer, W.; Radzun, H.J. Chemokine-mediated distribution of dendritic cell subsets in renal cell carcinoma. BMC Cancer 2010, 10, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, N.C.; Flament, C.; Crépineau, F.; Angevin, E.; Vivier, E.; Zitvogel, L. Dendritic cells (DC) promote natural killer (NK) cell functions: Dynamics of the human DC/NK cell cross talk. Eur. Cytokine Netw. 2002, 13, 17–27. [Google Scholar]
- Sarhan, D.; Palma, M.; Mao, Y.; Adamson, L.; Kiessling, R.; Mellstedt, H.; Österborg, A.; Lundqvist, A. Dendritic cell regulation of NK-cell responses involves lymphotoxin-α, IL-12, and TGF-β. Eur. J. Immunol. 2015, 45, 1783–1793. [Google Scholar] [CrossRef]
- Leone, P.; Berardi, S.; Frassanito, M.A.; Ria, R.; De Re, V.; Cicco, S.; Battaglia, S.; Ditonno, P.; Dammacco, F.; Vacca, A.; et al. Dendritic cells accumulate in the bone marrow of myeloma patients where they protect tumor plasma cells from CD8+ T-cell killing. Blood 2015, 126, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.W.; Housseau, F. The ‘kiss of death’ by dendritic cells to cancer cells. Cell Death Differ. 2008, 15, 58–69. [Google Scholar] [CrossRef]
- Charles, J.; Di Domizio, J.; Salameire, D.; Bendriss-Vermare, N.; Aspord, C.; Muhammad, R.; Lefebvre, C.; Plumas, J.; Leccia, M.T.; Chaperot, L. Characterization of circulating dendritic cells in melanoma: Role of CCR6 in plasmacytoid dendritic cell recruitment to the tumor. J. Investig. Dermatol. 2010, 130, 1646–1656. [Google Scholar] [CrossRef]
- Vulcano, M.; Struyf, S.; Scapini, P.; Cassatella, M.; Bernasconi, S.; Bonecchi, R.; Calleri, A.; Penna, G.; Adorini, L.; Luini, W.; et al. Unique regulation of CCL18 production by maturing dendritic cells. J. Immunol. 2003, 170, 3843–3849. [Google Scholar] [CrossRef]
- Yu, G.; Fang, M.; Gong, M.; Liu, L.; Zhong, J.; Feng, W.; Xiong, P.; Wang, C.Y.; Gong, F. Steady state dendritic cells with forced IDO expression induce skin allograft tolerance by upregulation of regulatory T cells. Transpl. Immunol. 2008, 18, 208–219. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Huang, M.S.; Cheng, D.E.; Hung, J.Y.; Yang, C.J.; Chou, S.H.; Kuo, P.L. Lung tumor-associated dendritic cell-derived amphiregulin increased cancer progression. J. Immunol. 2011, 187, 1733–1744. [Google Scholar] [CrossRef] [Green Version]
- Kuo, P.L.; Huang, M.S.; Cheng, D.E.; Hung, J.Y.; Yang, C.J.; Chou, S.H. Lung cancer-derived galectin-1 enhances tumorigenic potentiation of tumor-associated dendritic cells by expressing heparin-binding EGF-like growth factor. J. Biol. Chem. 2012, 287, 9753–9764. [Google Scholar] [CrossRef] [Green Version]
- Kan, J.Y.; Wu, D.C.; Yu, F.J.; Wu, C.Y.; Ho, Y.W.; Chiu, Y.J.; Jian, S.F.; Hung, J.Y.; Wang, J.Y.; Kuo, P.L. Chemokine (C-C Motif) Ligand 5 is Involved in Tumor-Associated Dendritic Cell-Mediated Colon Cancer Progression Through Non-Coding RNA MALAT-1. J. Cell. Physiol. 2015, 230, 1883–1894. [Google Scholar] [CrossRef]
- von Bergwelt-Baildon, M.S.; Popov, A.; Saric, T.; Chemnitz, J.; Classen, S.; Stoffel, M.S.; Fiore, F.; Roth, U.; Beyer, M.; Debey, S.; et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: Additional mechanisms of T-cell inhibition. Blood 2006, 108, 228–237. [Google Scholar] [CrossRef]
- Cheng, D.E.; Tsai, Y.M.; Hsu, Y.L.; Hou, M.F.; Tsai, E.M.; Wang, J.Y.; Kan, J.Y.; Kuo, P.L. Cluster of differentiation 45 activation is crucial in interleukin-10-dependent tumor-associated dendritic cell differentiation. Oncol. Lett. 2014, 8, 620–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyagaki, T.; Sugaya, M.; Suga, H.; Ohmatsu, H.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. Increased CCL18 expression in patients with cutaneous T-cell lymphoma: Association with disease severity and prognosis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e60–e67. [Google Scholar] [CrossRef] [PubMed]
- Jonuleit, H.; Schmitt, E.; Schuler, G.; Knop, J.; Enk, A.H. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 2000, 192, 1213–1222. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Balyasnikova, I.V.; Chang, A.L.; Ahmed, A.U.; Moon, K.S.; Auffinger, B.; Tobias, A.L.; Han, Y.; Lesniak, M.S. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res. 2012, 18, 6110–6121. [Google Scholar] [CrossRef] [Green Version]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Kempen, L.C.; Rijntjes, J.; Mamor-Cornelissen, I.; Vincent-Naulleau, S.; Gerritsen, M.J.; Ruiter, D.J.; van Dijk, M.C.; Geffrotin, C.; van Muijen, G.N. Type I collagen expression contributes to angiogenesis and the development of deeply invasive cutaneous melanoma. Int. J. Cancer 2008, 122, 1019–1029. [Google Scholar] [CrossRef]
- Wang, K.; Wu, F.; Seo, B.R.; Fischbach, C.; Chen, W.; Hsu, L.; Gourdon, D. Breast cancer cells alter the dynamics of stromal fibronectin-collagen interactions. Matrix Biol. 2017, 60–61, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Nie, Y.; Huang, H.; Guo, M.; Chen, J.; Wu, W.; Li, W.; Xu, X.; Lin, X.; Fu, W.; Yao, Y.; et al. Breast Phyllodes Tumors Recruit and Repolarize Tumor-Associated Macrophages via Secreting CCL5 to Promote Malignant Progression, Which Can Be Inhibited by CCR5 Inhibition Therapy. Clin. Cancer Res. 2019, 25, 3873–3886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atamas, S.P.; Luzina, I.G.; Choi, J.; Tsymbalyuk, N.; Carbonetti, N.H.; Singh, I.S.; Trojanowska, M.; Jimenez, S.A.; White, B. Pulmonary and activation-regulated chemokine stimulates collagen production in lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2003, 29, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Luzina, I.G.; Highsmith, K.; Pochetuhen, K.; Nacu, N.; Rao, J.N.; Atamas, S.P. PKCalpha mediates CCL18-stimulated collagen production in pulmonary fibroblasts. Am. J. Respir. Cell Mol. Biol. 2006, 35, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Prasse, A.; Pechkovsky, D.V.; Toews, G.B.; Jungraithmayr, W.; Kollert, F.; Goldmann, T.; Vollmer, E.; Müller-Quernheim, J.; Zissel, G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am. J. Respir. Crit. Care Med. 2006, 173, 781–792. [Google Scholar] [CrossRef] [Green Version]
- Hanley, C.J.; Noble, F.; Ward, M.; Bullock, M.; Drifka, C.; Mellone, M.; Manousopoulou, A.; Johnston, H.E.; Hayden, A.; Thirdborough, S.; et al. A subset of myofibroblastic cancer-associated fibroblasts regulate collagen fiber elongation, which is prognostic in multiple cancers. Oncotarget 2016, 7, 6159–6174. [Google Scholar] [CrossRef] [Green Version]
- Begum, A.; McMillan, R.H.; Chang, Y.T.; Penchev, V.R.; Rajeshkumar, N.V.; Maitra, A.; Goggins, M.G.; Eshelman, J.R.; Wolfgang, C.L.; Rasheed, Z.A.; et al. Direct Interactions with Cancer-Associated Fibroblasts Lead to Enhanced Pancreatic Cancer Stem Cell Function. Pancreas 2019, 48, 329–334. [Google Scholar] [CrossRef]
- Verkaar, F.; van Offenbeek, J.; van der Lee, M.M.C.; van Lith, L.H.C.J.; Watts, A.O.; Rops, A.L.W.M.M.; Aguilar, D.C.; Ziarek, J.J.; van der Vlag, J.; Handel, T.M.; et al. Chemokine cooperativity is caused by competitive glycosaminoglycan binding. J. Immunol. 2014, 192, 3908–3914. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, A.E.I.; Johnson, Z.; Bonvin, P.; Handel, T.M. Glycosaminoglycan Interactions with Chemokines Add Complexity to a Complex System. Pharmaceuticals 2017, 10, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shellenberger, T.D.; Wang, M.; Gujrati, M.; Jayakumar, A.; Strieter, R.M.; Burdick, M.D.; Ioannides, C.G.; Efferson, C.L.; El-Naggar, A.K.; Roberts, D.; et al. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004, 64, 8262–8270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, R.; Izukuri, K.; Kato, Y.; Sasaki, S.; Mukaida, N.; Maehata, Y.; Miyamoto, C.; Akasaka, T.; Yang, X.; Nagashima, Y.; et al. Suppressed rate of carcinogenesis and decreases in tumour volume and lung metastasis in CXCL14/BRAK transgenic mice. Sci. Rep. 2015, 5, 9083. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chang, Q.; Wu, X.; Yu, Y.; Zhang, H. Effect of chemokine CXCL14 on in vitro angiogenesis of human hepatocellular carcinoma cells. Arch. Physiol. Biochem. 2020, 1–7. [Google Scholar] [CrossRef]
- Simson, L.; Ellyard, J.I.; Dent, L.A.; Matthaei, K.I.; Rothenberg, M.E.; Foster, P.S.; Smyth, M.J.; Parish, C.R. Regulation of carcinogenesis by IL-5 and CCL11: A potential role for eosinophils in tumor immune surveillance. J. Immunol. 2007, 178, 4222–4229. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Tian, Y.; Kurosawa, T.; Matsui, S.; Touma, M.; Yanai, T.; Wu, Q.; Sugimoto, K. CCL11-induced eosinophils inhibit the formation of blood vessels and cause tumor necrosis. Genes Cells 2016, 21, 624–638. [Google Scholar] [CrossRef] [Green Version]
- Berenguer, J.; Lagerweij, T.; Zhao, X.W.; Dusoswa, S.; van der Stoop, P.; Westerman, B.; de Gooijer, M.C.; Zoetemelk, M.; Zomer, A.; Crommentuijn, M.H.W.; et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J. Extracell. Vesicles 2018, 7, 1446660. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Weaver, A.M. Extracellular vesicles: Important collaborators in cancer progression. Essays Biochem. 2018, 62, 149–163. [Google Scholar]
- Omland, S.H.; Wettergren, E.E.; Mollerup, S.; Asplund, M.; Mourier, T.; Hansen, A.J.; Gniadecki, R. Cancer associated fibroblasts (CAFs) are activated in cutaneous basal cell carcinoma and in the peritumoural skin. BMC Cancer 2017, 17, 675. [Google Scholar] [CrossRef]
- Gao, J.; Li, Z.H.; Tang, W.; Wu, Q.N.; Liu, G.H.; Zheng, W.B. Chemokine C-C motif ligand 18 expression correlates with tumor malignancy in breast cancer. Pathol. Biol. 2015, 63, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Nariţa, D.; Seclaman, E.; Ursoniu, S.; Ilina, R.; Cireap, N.; Anghel, A. Expression of CCL18 and interleukin-6 in the plasma of breast cancer patients as compared with benign tumor patients and healthy controls. Rom. J. Morphol. Embryol. 2011, 52, 1261–1267. [Google Scholar]
- Sun, J.H.; Fan, N.; Zhang, Y. Correlation between serum level of chemokine (C-C motif) ligand 18 and poor prognosis in breast cancer. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Wang, J.; Qin, Y.; Zhu, G.; Huang, D.; Wei, M.; Li, G.; She, L.; Zhang, D.; Wang, G.; Chen, X.; et al. High serum CCL18 predicts a poor prognosis in patients with laryngeal squamous cell carcinoma. J. Cancer 2019, 10, 6910–6914. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, J.; Hu, W.J.; Chen, C.; Luo, H.Q.; Tang, X.D.; Zhou, K.Y.; Zhong, W.T.; Li, X.Y. The serum level of CC chemokine ligand 18 correlates with the prognosis of non-small cell lung cancer. Int. J. Biol. Mark. 2019, 34, 156–162. [Google Scholar] [CrossRef]
- Plönes, T.; Krohn, A.; Burger, M.; Veelken, H.; Passlick, B.; Müller-Quernheim, J.; Zissel, G. Serum level of CC-chemokine ligand 18 is increased in patients with non-small-cell lung cancer and correlates with survival time in adenocarcinomas. PLoS ONE 2012, 7, e41746. [Google Scholar] [CrossRef]
- Yuan, L.; Wan, J.; Huang, C.; Liang, J.; Liu, M.; Yue, C.; Li, L. Evaluation of serum CCL18 as a potential biomarker for ovarian cancer. Cancer Biomark. 2017, 21, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Sun, S.K.; Zhang, X. CC chemokine ligand 18 and IGF-binding protein 6 as potential serum biomarkers for prostate cancer. Tohoku J. Exp. Med. 2014, 233, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Miyake, M.; Ross, S.; Lawton, A.; Chang, M.; Dai, Y.; Mengual, L.; Alcaraz, A.; Giacoia, E.G.; Goodison, S.; Rosser, C.J. Investigation of CCL18 and A1AT as potential urinary biomarkers for bladder cancer detection. BMC Urol. 2013, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Schmid, S.; Le, U.T.; Haager, B.; Mayer, O.; Dietrich, I.; Elze, M.; Kemna, L.J.; Zissel, G.; Passlick, B. Local Concentrations of CC-Chemokine-Ligand 18 Correlate with Tumor Size in Non-small Cell Lung Cancer and Are Elevated in Lymph Node-positive Disease. Anticancer Res. 2016, 36, 4667–4671. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Li, J.; Hu, W.J.; Chen, M.H.; Chen, S.S.; Chen, C.; Luo, H.Q.; Zhou, K.Y.; Liu, X.G.; Li, X.Y. Positive expression of chemokine (C-C Motif) ligand 18 and prognosis in cancer: A meta-analysis. J. BUON 2018, 23, 1185–1194. [Google Scholar] [PubMed]
- Mao, L.; Zhuang, R.; Qin, L.; Han, Z.; Huang, X.; Chen, R.; Su, Y.; Ge, L.; Yang, J.; Li, J.; et al. CCL18 overexpression predicts a worse prognosis in oral squamous cell carcinoma (OSCC). Neoplasma 2020, 67, 700–706. [Google Scholar] [CrossRef] [Green Version]
- Schmid, S.; Csanadi, A.; Kozhuharov, N.; Tchudjin, M.; Kayser, C.; Rawluk, J.; Passlick, B.; Werner, M.; Prasse, A.; Kayser, G. CC-Chemokine Ligand 18 Is an Independent Prognostic Marker in Lymph Node-positive Non-small Cell Lung Cancer. Anticancer Res. 2018, 38, 3913–3918. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [Green Version]
- Pio, R.; Ajona, D.; Ortiz-Espinosa, S.; Mantovani, A.; Lambris, J.D. Complementing the Cancer-Immunity Cycle. Front. Immunol. 2019, 10, 774. [Google Scholar] [CrossRef] [Green Version]
- Litviakov, N.; Tsyganov, M.; Larionova, I.; Ibragimova, M.; Deryusheva, I.; Kazantseva, P.; Slonimskaya, E.; Frolova, I.; Choinzonov, E.; Cherdyntseva, N.; et al. Expression of M2 macrophage markers YKL-39 and CCL18 in breast cancer is associated with the effect of neoadjuvant chemotherapy. Cancer Chemother. Pharmacol. 2018, 82, 99–109. [Google Scholar] [CrossRef]




| Type of Cancer | Number of Patients Studied | Prognosis with Increased CCL18 Levels in the Tumor | Comments | Reference |
|---|---|---|---|---|
| Breast cancer (primary ductal carcinoma) | 562 | ↓ | CCL18+ TAM count | [69] |
| Breast cancer | 1,017 | ↓ | Serum level of CCL18 and expression in tumor | [108] |
| Breast cancer | 207 | ↓ | Serum level of CCL18 | [173] |
| Breast phyllodes tumor | 268 | ↓ | [39] | |
| Colorectal cancer | 371 | ↑ | [43] | |
| Cutaneous T-cell lymphoma | 38 | ↓ | Serum level of CCL18 | [148] |
| Gastric adenocarcinoma | 90 | ↑ | [44] | |
| Laryngeal squamous cell carcinoma | 146 | ↓ | Serum level of CCL18 | [174] |
| Lung cancer (adenocarcinoma) | 170 | ↓ | Serum level of CCL18 | [176] |
| Lung cancer (non-small cell lung cancer) | 80 | ↓ | Serum level of CCL18 | [175] |
| Lung cancer (non-small cell lung cancer) | 243 | ↑ | CCL18+ TAM count | [183] |
| Oral squamous cell carcinoma | 102 | ↓ | [182] | |
| Ovarian cancer | 59 | ↓ | [55] | |
| Ovarian cancer | 187 | ↓ | [177] | |
| Osteosarcoma | 102 | ↓ | CCL18+ TAM count | [56] |
| Pancreatic ductal adenocarcinoma | 62 | ↓ | CCL18+ cells count | [59] |
| Pancreatic ductal adenocarcinoma | 134 | ↓ | [67] | |
| Esophageal squamous cell carcinoma | 25 | ↓ | [86] |
| Type of Cancer | Prognosis with an Increased Expression of CCL18 in the Tumor | Prognosis with an Increased Expression of CCR6 in the Tumor | Prognosis with an Increased Expression of CCR8 in the Tumor | Prognosis with an Increased Expression of PITPNM3 in the Tumor | Prognosis with an Increased Expression of GPER1/GPR30 in the Tumor | Prognosis with an Increased Expression of GPER1/GPR30 in the Tumor (In Men) |
|---|---|---|---|---|---|---|
| Glioma | ↓ | ↑ p = 0.076 | ↓ | ↓ | -- | -- |
| Thyroid cancer | -- | -- | -- | -- | ↓ p = 0.073 | ↓ |
| Lung cancer | -- | ↑ | ↑ | ↑ p = 0.069 | ↑ p = 0.064 | -- |
| Colorectal cancer | ↓ p = 0.057 | ↑ | -- | ↑ | ↓ | ↓ p = 0.064 |
| Head and neck cancer | ↑ | ↑ | ↑ | ↑ | ↑ p = 0.062 | ↑ p = 0.093 |
| Stomach cancer | -- | ↓ | ↑ p = 0.080 | ↑ p = 0.096 | ↓ | ↓ |
| Liver cancer | -- | -- | -- | ↑ | -- | -- |
| Pancreatic cancer | ↓ | ↑ | ↑ p = 0.074 | ↓ | ↑ | ↑ p = 0.057 |
| Renal cancer | ↑ | ↓ | ↓ | -- | ↑ | ↑ |
| Urothelial cancer | ↓ | -- | -- | -- | ↓ p = 0.078 | ↓ p = 0.091 |
| Prostate cancer | ↓ | ↑ | -- | ↓ | -- | -- |
| Testis cancer | -- | ↓ p = 0.080 | ↓ | -- | -- | -- |
| Breast cancer | ↑ p = 0.089 | ↑ | -- | ↓ | ↑ | N/A |
| Cervical cancer | ↑ | ↑ | ↑ | ↑ | -- | N/A |
| Endometrial cancer | ↑ p = 0.096 | -- | ↑ p = 0.10 | -- | ↑ | N/A |
| Ovarian cancer | ↑ | -- | ↑ | ↓ | -- | N/A |
| Melanoma | -- | -- | ↑ | ↓ | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbecki, J.; Olbromski, M.; Dzięgiel, P. CCL18 in the Progression of Cancer. Int. J. Mol. Sci. 2020, 21, 7955. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21217955
Korbecki J, Olbromski M, Dzięgiel P. CCL18 in the Progression of Cancer. International Journal of Molecular Sciences. 2020; 21(21):7955. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21217955
Chicago/Turabian StyleKorbecki, Jan, Mateusz Olbromski, and Piotr Dzięgiel. 2020. "CCL18 in the Progression of Cancer" International Journal of Molecular Sciences 21, no. 21: 7955. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21217955