Peripheral Glycolysis in Neurodegenerative Diseases
Abstract
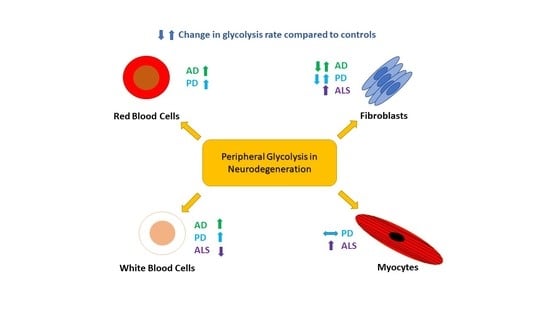
:1. Introduction
2. Glycolysis
3. Glycolytic Changes in Alzheimer’s Disease
4. Glycolytic Changes in Parkinson’s Disease
5. Glycolytic Changes in Amyotrophic Lateral Sclerosis
6. Challenges to Developing Peripheral Biomarkers of Glycolysis in ND Disease
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid Beta |
| AD | Alzheimer ’s disease |
| ALS | Amyotrophic Lateral Sclerosis |
| ALT | Alanine transaminase |
| APP | Amyloid Precursor Protein |
| AMBRoSIA | A Multicenter Biomarker Resource Strategy in ALS |
| AST | Aspartate transaminase |
| ATP | Adenosine Triphosphate |
| CNS | Central Nervous System |
| CRISPR | Clustered regularly interspaced short palindromic repeat |
| DNA | Deoxyribonucleic Acid |
| 2,3-DPG | 2,3-diphosphoglycerate |
| ECAR | extracellular acidification rate |
| ETC | Electron Transport Chain |
| FDG-PET | fluorodeoxyglucose-positron emission tomography |
| GA3PDH | glyceraldehyde-3-phosphate dehydrogenase |
| GFAP | Glial fibrillary acidic protein |
| HIF1 | Hypoxia-inducible factor 1 |
| HK | Hexokinase |
| 6OHDA | 6-hydroxydopamine |
| LCL | Lymphoblastic Cell Lines |
| LDH | Lactate Dehydrogenase |
| MEF | Mouse embryonic fibroblasts |
| MPP+ | Methyl-4-phenylpyridinium |
| NAD | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| ND | Neurodegenerative disease |
| OxPHOS | Oxidative phosphorylation |
| PBMCs | Peripheral blood mononuclear cells |
| PD | Parkinson’s disease |
| PDK | Pyruvate dehydrogenase kinase |
| PDK4 | Pyruvate dehydrogenase lipoamide kinase isozyme 4 |
| PFK | Phosphofructokinase |
| PFKFB3 | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 |
| PGK | Phosphoglycerate kinase |
| PK | Pyruvate Kinase |
| PINK1 | PTEN-induced kinase 1 |
| PPS | Pentose Phosphate Shunt |
| RBC | Red Blood Cells |
| ROS | Reactive Oxygen Species |
| SN | Substantia Nigra Pars Compacta |
| TCA | Tricarboxylic Acid Cycle |
| TIGAR | TP53 Induced Glycolysis Regulatory Phosphatase |
References
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Williams, A. Defining neurodegenerative diseases. BMJ 2002, 324, 1465–1466. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; Santopaolo, M.; Faicchia, D.; Colamatteo, A.; Formisano, L.; De Candia, P.; Galgani, M.; De Rosa, V.; Matarese, G. Role of metabolism in neurodegenerative disorders. Metabolism 2016, 65, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Hames, B.D.; Hooper, N.M. Instant Notes in Biochemistry; Kingston, F., Ed.; Taylor & Francis Ltd.: Abingdon, UK, 2000; pp. 278–288. [Google Scholar]
- Allen, S.P.; Hall, B.; Castelli, L.M.; Francis, L.; Woof, R.; Siskos, A.P.; Kouloura, E.; Gray, E.; Thompson, A.G.; Talbot, K.; et al. Astrocyte adenosine deaminase loss increases motor neuron toxicity in amyotrophic lateral sclerosis. Brain 2019, 142, 586–605. [Google Scholar] [CrossRef] [Green Version]
- Bell, S.M.; De Marco, M.; Barnes, K.; Shaw, P.J.; Ferraiuolo, L.; Blackburn, D.J.; Mortiboys, H.; Venneri, A. Deficits in Mitochondrial Spare Respiratory Capacity Contribute to the Neuropsychological Changes of Alzheimer’s Disease. J. Pers. Med. 2020, 10, 32. [Google Scholar] [CrossRef]
- Konrad, C.; Kawamata, H.; Bredvik, K.; Arreguin, A.; Cajamarca, S.A.; Hupf, J.C.; Ravits, J.; Miller, T.M.; Maragakis, N.J.; Hales, C.M.; et al. Fibroblast bioenergetics to classify amyotrophic lateral sclerosis patients. Mol. Neurodegener. 2017, 12, 76. [Google Scholar] [CrossRef] [Green Version]
- Sonntag, K.-C.; Ryu, W.-I.; Amirault, K.M.; Healy, R.A.; Siegel, A.J.; McPhie, N.L.; Forester, B.; Cohen, B.M. Late-onset Alzheimer’s disease is associated with inherent changes in bioenergetics profiles. Sci. Rep. 2017, 7, 14038. [Google Scholar] [CrossRef] [Green Version]
- Dashty, M. A quick look at biochemistry: Carbohydrate metabolism. Clin. Biochem. 2013, 46, 1339–1352. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Aging, Alzheimer’s Disease and Dysfunctional Glycolysis; Similar Effects of Too Much and Too Little. Aging Dis. 2019, 10, 1328–1331. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-B.; Gu, J.-D.; Zhou, Q.-H. Review of aerobic glycolysis and its key enzymes—New targets for lung cancer therapy. Thorac. Cancer 2015, 6, 17–24. [Google Scholar] [CrossRef]
- Wamelink, M.M.; Struys, E.A.; Jakobs, C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: A review. J. Inherit. Metab. Dis. 2008, 31, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A.; Cruz, N.F. Aerobic glycolysis during brain activation: Adrenergic regulation and influence of norepinephrine on astrocytic metabolism. J. Neurochem. 2016, 138, 14–52. [Google Scholar] [CrossRef] [Green Version]
- Mosconi, L.; Tsui, W.-H.; De Santi, S.; Li, J.; Rusinek, H.; Convit, A.; Li, Y.; Boppana, M.; De Leon, M.J. Reduced hippocampal metabolism in MCI and AD: Automated FDG-PET image analysis. Neurology 2005, 64, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Beiser, A.; Breteler, M.M.B.; Fratiglioni, L.; Helmer, C.; Hendrie, H.C.; Honda, H.; Ikram, M.A.; Langa, K.M.; Lobo, A.; et al. The changing prevalence and incidence of dementia over time—Current evidence. Nat. Rev. Neurol. 2017, 13, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Prince, M.J. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Alzheimer’s Association. What is Dementia. Available online: https://www.alz.org/alzheimers-dementia/what-is-dementia (accessed on 7 May 2020).
- Perl, D.P. Neuropathology of Alzheimer’s disease. Mt. Sinai J. Med. 2010, 77, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Vlassenko, A.G.; Vaishnavi, S.N.; Couture, L.; Sacco, D.; Shannon, B.J.; Mach, R.H.; Morris, J.C.; Raichle, M.E.; Mintun, M.A. Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ) deposition. Proc. Natl. Acad. Sci. USA 2010, 107, 17763–17767. [Google Scholar] [CrossRef] [Green Version]
- Vlassenko, A.; Gordon, B.A.; Goyal, M.S.; Su, Y.; Blazey, T.; Durbin, T.J.; Couture, L.E.; Christensen, J.J.; Jafri, H.; Morris, J.C.; et al. Aerobic glycolysis and tau deposition in preclinical Alzheimer’s disease. Neurobiol. Aging 2018, 67, 95–98. [Google Scholar] [CrossRef]
- Marcus, C.; Mena, E.; Subramaniam, R.M. Brain PET in the diagnosis of Alzheimer’s disease. Clin. Nucl. Med. 2014, 39, e413–e426. [Google Scholar] [CrossRef] [Green Version]
- Bigl, M.; Bleyl, A.-D.; Zedlick, D.; Arendt, T.; Bigl, V.; Eschrich, K. Changes of activity and isozyme pattern of phosphofructokinase in the brains of patients with Alzheimer’s disease. J. Neurochem. 1996, 67, 1164–1171. [Google Scholar] [CrossRef]
- Bigl, M.; Arendt, T.; Bigl, V.; Eschrich, K.; Brückner, M.K. Activities of key glycolytic enzymes in the brains of patients with Alzheimer’s disease. J. Neural Transm. 1999, 106, 499–511. [Google Scholar] [CrossRef]
- Hashimoto, M.; Bogdanovic, N.; Nakagawa, H.; Volkmann, I.; Aoki, M.; Winblad, B.; Sakai, J.; Tjernberg, L. Analysis of microdissected neurons by 18O mass spectrometry reveals altered protein expression in Alzheimer’s disease. J. Cell. Mol. Med. 2012, 16, 1686–1700. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.A.; Chundu, K.R.; Davies-Hill, T.; Honer, W.G.; Davies, P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann. Neurol. 1994, 35, 546–551. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Varma, V.R.; Varma, S.; Casanova, R.; Dammer, E.; Pletnikova, O.; Chia, C.W.; Egan, J.M.; Ferrucci, L.; Troncoso, J.; et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement. 2018, 14, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Bell, S.M.; Barnes, K.; Clemmens, H.; Al-Rafiah, A.R.; Al-Ofi, E.A.; Leech, V.; Bandmann, O.; Shaw, P.J.; Blackburn, D.J.; Ferraiuolo, L.; et al. Ursodeoxycholic Acid Improves Mitochondrial Function and Redistributes Drp1 in Fibroblasts from Patients with Either Sporadic or Familial Alzheimer’s Disease. J. Mol. Biol. 2018, 430, 3942–3953. [Google Scholar] [CrossRef]
- Sorbi, S.; Piacentini, S.; Latorraca, S.; Piersanti, P.; Amaducci, L. Alterations in metabolic properties in fibroblasts in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1995, 9, 73–77. [Google Scholar] [CrossRef]
- Sims, N.R.; Finegan, J.M.; Blass, J.P. Altered glucose metabolism in fibroblasts from patients with Alzheimer’s disease. N. Engl. J. Med. 1985, 313, 638–639. [Google Scholar]
- Sorbi, S.; Mortilla, M.; Piacentini, S.; Tonini, S.; Amaducci, L. Altered hexokinase activity in skin cultured fibroblasts and leukocytes from Alzheimer’s disease patients. Neurosci. Lett. 1990, 117, 165–168. [Google Scholar] [CrossRef]
- Sims, N.R.; Blass, J.P. Phosphofructokinase activity in fibroblasts from patients with Alzheimer’s disease and age- and sex-matched controls. Metab. Brain Dis. 1986, 1, 83–90. [Google Scholar] [CrossRef]
- Kaminsky, Y.G.; Reddy, V.P.; Ashraf, G.M.; Ahmad, A.; Benberin, V.V.; Kosenko, E.A.; Aliev, G. Age-related defects in erythrocyte 2,3-diphosphoglycerate metabolism in dementia. Aging Dis. 2013, 4, 244–255. [Google Scholar] [CrossRef]
- Carelli-Alinovi, C.; Dinarelli, S.; Sampaolese, B.; Misiti, F.; Girasole, M. Morphological changes induced in erythrocyte by amyloid beta peptide and glucose depletion: A combined atomic force microscopy and biochemical study. Biochim. Biophys. Acta Biomembr. 2019, 1861, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, L.A.; Kaminsky, Y.G.; Reddy, V.P.; Li, Y.; Solomadin, I.N.; Kosenko, E.A.; Aliev, G. Impact of Amyloid β25-35 on Membrane Stability, Energy Metabolism, and Antioxidant Enzymes in Erythrocytes. Am. J. Alzheimer’s Dis. Other Dement. 2014, 29, 685–695. [Google Scholar] [CrossRef]
- Yoon, S.-S.; Jo, S.A. Mechanisms of Amyloid-β Peptide Clearance: Potential Therapeutic Targets for Alzheimer’s Disease. Biomol. Ther. 2012, 20, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosenko, E.A.; Tikhonova, L.A.; Montoliu, C.; Barreto, G.E.; Aliev, G.; Kaminsky, Y.G.; Kosenko, E.A.; Tikhonova, L.A.; Montoliu, C.; Barreto, G.E.; et al. Metabolic Abnormalities of Erythrocytes as a Risk Factor for Alzheimer’s Disease. Front. Neurosci. 2018, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Casoli, T.; Di Stefano, G.; Giorgetti, B.; Grossi, Y.; Balietti, M.; Fattoretti, P.; Bertoni-Freddari, C. Release of beta-amyloid from high-density platelets: Implications for Alzheimer’s disease pathology. Ann. N. Y. Acad. Sci. 2007, 1096, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C. Stimulated release of the beta-amyloid protein of Alzheimer’s disease by normal human platelets. Neurosci. Lett. 1997, 235, 157–159. [Google Scholar] [CrossRef]
- Citron, M.; Vigo-Pelfrey, C.; Teplow, D.B.; Miller, C.; Schenk, D.; Johnston, J.; Winblad, B.; Venizelos, N.; Lannfelt, L.; Selkoe, D.J. Excessive production of amyloid beta-protein by peripheral cells of symptomatic and presymptomatic patients carrying the Swedish familial Alzheimer disease mutation. Proc. Natl. Acad. Sci. USA 1994, 91, 11993–11997. [Google Scholar] [CrossRef] [Green Version]
- Simonian, N.A.; Hyman, B.T. Functional Alterations in Alzheimer’s Disease: Selective Loss of Mitochondrial-encoded Cytochrome Oxidase mRNA in the Hippocampal Formation. J. Neuropathol. Exp. Neurol. 1994, 53, 508–512. [Google Scholar] [CrossRef]
- Song, F.; Poljak, A.; Valenzuela, M.; Mayeux, R.; Smythe, G.A.; Sachdev, P.S. Meta-analysis of plasma amyloid-β levels in Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2011, 26, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, R.A. Classic beta-amyloid deposits cluster around large diameter blood vessels rather than capillaries in sporadic Alzheimer’s disease. Curr. Neurovasc. Res. 2006, 3, 289–294. [Google Scholar] [CrossRef]
- Kosenko, E.A.; Tikhonova, L.; Li, Y.; Poghosyan, A.C.; Benberin, V.V.; Kaminsky, Y.G.; Aliev, G. Antioxidant Status and Energy State of Erythrocytes in Alzheimer Dementia—Potential Probing for Markers. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 2289–2304. [Google Scholar]
- Al-Abdi, S.Y. Decreased Glutathione S-transferase Level and Neonatal Hyperbilirubinemia Associated with Glucose-6-phosphate Dehydrogenase Deficiency: A Perspective Review. Am. J. Perinatol. 2017, 34, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Whitten, H.; Chou, Y.; Fudenberg, H.H. Immunological dysfunction in Alzheimer’s disease. J. Neuroimmunol. 1984, 7, 279–285. [Google Scholar] [CrossRef]
- Nho, K.; Kueider-Paisley, A.; Ahmad, S.; Mahmoudiandehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; et al. Association of Altered Liver Enzymes with Alzheimer Disease Diagnosis, Cognition, Neuroimaging Measures, and Cerebrospinal Fluid Biomarkers. JAMA Netw. Open 2019, 2, e197978. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; García-Barrera, T.; Vitorica, J.; Gómez-Ariza, J.L. Metabolomic investigation of systemic manifestations associated with Alzheimer’s disease in the APP/PS1 transgenic mouse model. Mol. Biosyst. 2015, 11, 2429–2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Velpen, V.; Teav, T.; Gallart-Ayala, H.; Mehl, F.; Konz, I.; Clark, C.; Oikonomidi, A.; Peyratout, G.; Henry, H.; Delorenzi, M.; et al. Systemic and central nervous system metabolic alterations in Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orešič, M.; Hyotylainen, T.; Herukka, S.-K.; Sysiaho, M.; Mattila, I.; Seppänan-Laakso, T.; Julkunen, V.; Gopalacharyulu, P.V.; Hallikainen, M.; Koikkalainen, J.; et al. Metabolome in progression to Alzheimer’s disease. Transl. Psychiatry 2011, 1, e57. [Google Scholar] [CrossRef]
- Clarke, C.E. Parkinson’s disease. BMJ 2007, 335, 441–445. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic-A Call to Action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef]
- Yang, G.; Schmiel, L.; Zhou, M.; Cintina, I.; Spencer, D.; Hogan, P. Economic Burden and Future Impact of Parkinson’s Disease; The Lewin Group, Inc.: Falls Church, VA, USA, 2019. [Google Scholar]
- Borghammer, P.; Chakravarty, M.; Jonsdottir, K.Y.; Sato, N.; Matsuda, H.; Ito, K.; Arahata, Y.; Kato, T.; Gjedde, A. Cortical hypometabolism and hypoperfusion in Parkinson’s disease is extensive: Probably even at early disease stages. Brain Struct. Funct. 2010, 214, 303–317. [Google Scholar] [CrossRef]
- Edison, P.; Ahmed, I.; Fan, Z.; Hinz, R.; Gelosa, G.; Chaudhuri, K.R.; Walker, Z.; Turkheimer, F.E.; Brooks, D.J. Microglia, amyloid, and glucose metabolism in Parkinson’s disease with and without dementia. Neuropsychopharmacology 2013, 38, 938–949. [Google Scholar] [CrossRef]
- Yang, S.-Q.; Tian, Q.; Li, D.; He, S.-Q.; Hu, M.; Liu, S.-Y.; Zou, W.; Chen, Y.-J.; Zhang, P.; Tang, X.-Q. Leptin mediates protection of hydrogen sulfide against 6-hydroxydopamine-induced Parkinson’s disease: Involving enhancement in Warburg effect. Neurochem. Int. 2020, 135, 104692. [Google Scholar] [CrossRef] [PubMed]
- Flinn, L.J.; Keatinge, M.; Bretaud, S.; Mortiboys, H.; Matsui, H.; De Felice, E.; Woodroof, H.I.; Brown, L.; McTighe, A.; Soellner, R.; et al. TigarB causes mitochondrial dysfunction and neuronal loss in PINK1 deficiency. Ann. Neurol. 2013, 74, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, K.L.R.; Simpson, J.; Watson, L.C.; Mortiboys, H.; Hautbergue, G.M.; Bandmann, O.; Highley, J.R. TIGAR inclusion pathology is specific for Lewy body diseases. Brain Res. 2019, 1706, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Milanese, C.; Payán-Gómez, C.; Galvani, M.; González, N.M.; Tresini, M.; Abdellah, S.N.; Van Roon-Mom, W.M.C.; Figini, S.; Marinus, J.; Van Hilten, J.J.; et al. Peripheral mitochondrial function correlates with clinical severity in idiopathic Parkinson’s disease. Mov. Disord. 2019, 34, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Deus, C.M.; Pereira, S.P.; Cunha-Oliveira, T.; Pereira, F.B.; Raimundo, N.; Oliveira, P.J. Mitochondrial remodeling in human skin fibroblasts from sporadic male Parkinson’s disease patients uncovers metabolic and mitochondrial bioenergetic defects. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165615. [Google Scholar] [CrossRef]
- Mortiboys, H.; Thomas, K.J.; Koopman, W.J.; Klaffke, S.; Abou-Sleiman, P.; Cookson, M.R.; Bandmann, O. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann. Neurol. 2008, 64, 555–565. [Google Scholar] [CrossRef]
- Zanellati, M.C.; Monti, V.; Barzaghi, C.; Reale, C.; Nardocci, N.; Albanese, A.; Valente, E.M.; Ghezzi, D.; Garavaglia, B. Mitochondrial dysfunction in Parkinson disease: Evidence in mutant PARK2 fibroblasts. Front. Genet. 2015, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- Requejo-Aguilar, R.; Lopez-Fabuel, I.; Fernandez, E.; Martins, L.M.; Almeida, A.; Bolaños, J.P. PINK1 deficiency sustains cell proliferation by reprogramming glucose metabolism through HIF1. Nat. Commun. 2014, 5, 4514. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef] [Green Version]
- Ambrosi, G.; Ghezzi, C.; Sepe, S.; Milanese, C.; Payan-Gomez, C.; Bombardieri, C.R.; Armentero, M.-T.; Zangaglia, R.; Pacchetti, C.; Mastroberardino, P.G.; et al. Bioenergetic and proteolytic defects in fibroblasts from patients with sporadic Parkinson’s disease. Biochim. Biophys. Acta 2014, 1842, 1385–1394. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Gandhi, S.; Burchell, V.S.; Plun-Favreau, H.; Wood, N.W.; Abramov, A.Y. Cell metabolism affects selective vulnerability in PINK1-associated Parkinson’s disease. J. Cell Sci. 2011, 124 Pt 24, 4194–4202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, G.L.; Dinsdale, D.; Macfarlane, M.; Cain, K. Switching from aerobic glycolysis to oxidative phosphorylation modulates the sensitivity of mantle cell lymphoma cells to TRAIL. Oncogene 2012, 31, 4996–5006. [Google Scholar] [CrossRef] [PubMed]
- Marion, M.-H.; Qurashi, M.; Marshall, G.; Foster, O. Is REM sleep behaviour disorder (RBD) a risk factor of dementia in idiopathic Parkinson’s disease? J. Neurol. 2008, 255, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Depp, C.; Ryan, B.J.; Johnston, G.I.; Alegre-Abarrategui, J.; Evetts, S.; Rolinski, M.; Baig, F.; Ruffmann, C.; Simon, A.K.; et al. Mitochondrial dysfunction and increased glycolysis in prodromal and early Parkinson’s blood cells. Mov. Disord. 2018, 33, 1580–1590. [Google Scholar] [CrossRef]
- Supandi, F.; van Beek, J. Computational prediction of changes in brain metabolic fluxes during Parkinson’s disease from mRNA expression. PLoS ONE 2018, 13, e0203687. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, A.D.; Kabaria, S.; Choi, D.C.; Mouradian, M.M.; Junn, E. MicroRNA-7 Promotes Glycolysis to Protect against 1-Methyl-4-phenylpyridinium-induced Cell Death. J. Biol. Chem. 2015, 290, 12425–12434. [Google Scholar] [CrossRef] [Green Version]
- Giménez-Cassina, A.; Lim, F.; Cerrato, T.; Palomo, G.M.; Díaz-Nido, J. Mitochondrial hexokinase II promotes neuronal survival and acts downstream of glycogen synthase kinase-3. J. Biol. Chem. 2009, 284, 3001–3011. [Google Scholar] [CrossRef] [Green Version]
- Corona, J.C.; Gimenez-Cassina, A.; Lim, F.; Díaz-Nido, J. Hexokinase II gene transfer protects against neurodegeneration in the rotenone and MPTP mouse models of Parkinson’s disease. J. Neurosci. Res. 2010, 88, 1943–1950. [Google Scholar] [CrossRef]
- Hong, C.T.; Chau, K.Y.; Schapira, A.H. Meclizine-induced enhanced glycolysis is neuroprotective in Parkinson disease cell models. Sci. Rep. 2016, 6, 25344. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, Y.; Simmering, J.E.; Schultz, J.L.; Li, Y.; Fernandez-Carasa, I.; Consiglio, A.; Raya, A.; Polgreen, P.M.; Narayanan, N.S.; et al. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J. Clin. Investig. 2019, 129, 4539–4549. [Google Scholar] [CrossRef] [Green Version]
- Foltynie, T. Glycolysis as a therapeutic target for Parkinson’s disease. Lancet Neurol. 2019, 18, 1072–1074. [Google Scholar] [CrossRef]
- McDermott, C.J.; Shaw, P.J. Diagnosis and management of motor neurone disease. BMJ 2008, 336, 658–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiò, A.; Calvo, A.; Mazzini, L.; Cantello, R.; Mora, G.; Moglia, C.; Corrado, L.; D’Alfonso, S.; Majounie, E.; Renton, A.; et al. Extensive genetics of ALS: A population-based study in Italy. Neurology 2012, 79, 1983–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensimon, G.; Lacomblez, L.; Meininger, V.; ALS/Riluzole Study Group. A controlled trial of riluzole in amyotrophic lateral sclerosis. N. Engl. J. Med. 1994, 330, 585–591. [Google Scholar] [CrossRef]
- Tefera, T.W.; Borges, K. Metabolic Dysfunctions in Amyotrophic Lateral Sclerosis Pathogenesis and Potential Metabolic Treatments. Front. Neurosci. 2016, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- Vandoorne, T.; De Bock, K.; Van Den Bosch, L. Energy metabolism in ALS: An underappreciated opportunity? Acta Neuropathol. 2018, 135, 489–509. [Google Scholar] [CrossRef] [Green Version]
- Floare, M.L.; Allen, S.P. Why TDP-43? Why Not? Mechanisms of Metabolic Dysfunction in Amyotrophic Lateral Sclerosis. Neurosci. Insights 2020, 15, 2633105520957302. [Google Scholar] [CrossRef]
- Bartolome, F.; Wu, H.-C.; Burchell, V.S.; Preza, E.; Wray, S.; Mahoney, C.J.; Fox, N.C.; Calvo, A.; Canosa, A.; Moglia, C.; et al. Pathogenic VCP mutations induce mitochondrial uncoupling and reduced ATP levels. Neuron 2013, 78, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.P.; Duffy, L.M.; Shaw, C.E.; Grierson, A.J. Altered age-related changes in bioenergetic properties and mitochondrial morphology in fibroblasts from sporadic amyotrophic lateral sclerosis patients. Neurobiol. Aging 2015, 36, 2893–2903. [Google Scholar] [CrossRef]
- Allen, S.P.; Rajan, S.; Duffy, L.; Mortiboys, H.; Higginbottom, A.; Grierson, A.J.; Shaw, P.J. Superoxide dismutase 1 mutation in a cellular model of amyotrophic lateral sclerosis shifts energy generation from oxidative phosphorylation to glycolysis. Neurobiol. Aging 2014, 35, 1499–1509. [Google Scholar] [CrossRef]
- Raman, R.; Allen, S.P.; Goodall, E.F.; Kramer, S.; Ponger, L.-L.; Heath, P.R.; Milo, M.; Hollinger, H.C.; Walsh, T.; Highley, J.R.; et al. Gene expression signatures in motor neurone disease fibroblasts reveal dysregulation of metabolism, hypoxia-response and RNA processing functions. Neuropathol. Appl. Neurobiol. 2015, 41, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Valbuena, G.N.; Rizzardini, M.; Cimini, S.; Siskos, A.P.; Bendotti, C.; Cantoni, L.; Keun, H.C. Metabolomic Analysis Reveals Increased Aerobic Glycolysis and Amino Acid Deficit in a Cellular Model of Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2016, 53, 2222–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzo, E.; Lorenzini, I.; Barrameda, D.; O’Conner, A.G.; Barrows, J.M.; Starr, A.; Kovalik, T.; Rabichow, B.E.; Lehmkuhl, E.M.; Shreiner, D.D.; et al. Glycolysis upregulation is neuroprotective as a compensatory mechanism in ALS. Elife 2019, 8, e45114. [Google Scholar] [CrossRef] [PubMed]
- Steyn, F.J.; Kirk, S.E.; Tefera, T.W.; Xie, T.Y.; Tracey, T.J.; Kelk, D.; Wimberger, E.; Garton, F.C.; Roberts, L.; Chapman, S.E.; et al. Altered skeletal muscle glucose-fatty acid flux in amyotrophic lateral sclerosis (ALS). bioRxiv 2020. [Google Scholar] [CrossRef]
- Kirk, S.E.; Tracey, T.J.; Steyn, F.J.; Ngo, S.T. Biomarkers of Metabolism in Amyotrophic Lateral Sclerosis. Front. Neurol. 2019, 10, 191. [Google Scholar] [CrossRef]
- Chew, S.; Atassi, N. Positron Emission Tomography Molecular Imaging Biomarkers for Amyotrophic Lateral Sclerosis. Front. Neurol. 2019, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Bauckneht, M.; Lai, R.; Miceli, A.; Schenone, D.; Cossu, V.; Donegani, M.I.; Raffa, S.; Borra, A.; Marra, S.; Campi, C.; et al. Spinal cord hypermetabolism extends to skeletal muscle in amyotrophic lateral sclerosis: A computational approach to [18F]-fluorodeoxyglucose PET/CT images. EJNMMI Res. 2020, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.P.; Hall, B.; Woof, R.; Francis, L.; Gatto, N.; Shaw, A.C.; Myszczynska, M.; Hemingway, J.; Coldicott, I.; Willcock, A.; et al. C9orf72 expansion within astrocytes reduces metabolic flexibility in amyotrophic lateral sclerosis. Brain 2019, 142, 3771–3790. [Google Scholar] [CrossRef]
- Hergesheimer, R.C.; Chami, A.A.; De Assis, D.R.; Vourc’H, P.; Andres, C.R.; Corcia, P.; Lanznaster, D.; Blasco, H. The debated toxic role of aggregated TDP-43 in amyotrophic lateral sclerosis: A resolution in sight? Brain 2019, 142, 1176–1194. [Google Scholar] [CrossRef]
- Palamiuc, L.; Schlagowski, A.; Ngo, S.T.; Vernay, A.; Dirrig-Grosch, S.; Henriques, A.; Boutillier, A.; Zoll, J.; Echaniz-Laguna, A.; Loeffler, J.-P.; et al. A metabolic switch toward lipid use in glycolytic muscle is an early pathologic event in a mouse model of amyotrophic lateral sclerosis. EMBO Mol. Med. 2015, 7, 526–546. [Google Scholar] [CrossRef]
- Dodge, J.C.; Treleaven, C.M.; Fidler, J.A.; Tamsett, T.J.; Bao, C.; Searles, M.; Taksir, T.V.; Misra, K.; Sidman, R.L.; Cheng, S.H.; et al. Metabolic signatures of amyotrophic lateral sclerosis reveal insights into disease pathogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 10812–10817. [Google Scholar] [CrossRef] [Green Version]
- Marini, C.; Cossu, V.; Bonifacino, T.; Bauckneht, M.; Torazza, C.; Bruno, S.; Castellani, P.; Ravera, S.; Milanese, M.; Venturi, C.; et al. Mechanisms underlying the predictive power of high skeletal muscle uptake of FDG in amyotrophic lateral sclerosis. EJNMMI Res. 2020, 10, 76. [Google Scholar] [CrossRef]
- Golko-Perez, S.; Amit, T.; Youdim, M.B.H.; Weinreb, O. Beneficial Effects of Multitarget Iron Chelator on Central Nervous System and Gastrocnemius Muscle in SOD1(G93A) Transgenic ALS Mice. J. Mol. Neurosci. 2016, 59, 504–510. [Google Scholar] [CrossRef]
- McCombe, P.A.; Lee, J.D.; Woodruff, T.M.; Henderson, R.D. The Peripheral Immune System and Amyotrophic Lateral Sclerosis. Front. Neurol. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostalski, H.; Leskelä, S.; Huber, N.; Katisko, K.; Cajanus, A.; Solje, E.; Marttinen, M.; Natunen, T.; Remes, A.M.; Hiltunen, M.; et al. Astrocytes and Microglia as Potential Contributors to the Pathogenesis of C9orf72 Repeat Expansion-Associated FTLD and ALS. Front. Neurosci. 2019, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Shepheard, S.; Jin, J.; Hu, F.; Zhao, X.; Xue, L.; Xiang, L.; Qi, H.; Qu, Q.; Guo, F.; et al. Urinary Extracellular Domain of Neurotrophin Receptor p75 as a Biomarker for Amyotrophic Lateral Sclerosis in a Chinese cohort. Sci. Rep. 2017, 7, 5127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matusica, D.; Alfonsi, F.; Turner, B.J.; Butler, T.J.; Shepheard, S.R.; Rogers, M.L.; Skeldal, S.; Underwood, C.K.; Mangelsdorf, M.; Coulson, E.J. Inhibition of motor neuron death in vitro and in vivo by a p75 neurotrophin receptor intracellular domain fragment. J. Cell Sci. 2016, 129, 517–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepheard, S.R.; Chataway, T.; Schultz, D.W.; Rush, R.A.; Rogers, M.-L. The extracellular domain of neurotrophin receptor p75 as a candidate biomarker for amyotrophic lateral sclerosis. PLoS ONE 2014, 9, e87398. [Google Scholar] [CrossRef] [Green Version]
- Shepheard, S.R.; Wuu, J.; Cardoso, M.; Wiklendt, L.; Dinning, P.G.; Chataway, T.; Schultz, D.; Benatar, M.; Rogers, M.L. Urinary p75(ECD): A prognostic, disease progression, and pharmacodynamic biomarker in ALS. Neurology 2017, 88, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- DiStefano, P.S.; Johnson, E.M., Jr. Identification of a truncated form of the nerve growth factor receptor. Proc. Natl. Acad. Sci. USA 1988, 85, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Pansarasa, O.; Bordoni, M.; Drufuca, L.; Diamanti, L.; Sproviero, D.; Trotti, R.; Bernuzzi, S.; La Salvia, S.; Gagliardi, S.; Ceroni, M.; et al. Lymphoblastoid cell lines as a model to understand amyotrophic lateral sclerosis disease mechanisms. Dis. Model. Mech. 2018, 11, dmm031625. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | Glycolysis Rate | Glycolytic Enzymatic Change | Disease Type | Cell Type | Number of Participants |
|---|---|---|---|---|---|
| Bell et al., 2020 [6] | (−) (↓ Glycolytic Capacity) | Not Assessed | sAD | Fibroblast | 10 sAD 10 Controls |
| Sonntag et al., 2017 [8] | ↑ | ↑ LDH ↑ PFKFB3 (gene expression) | sAD | Fibroblast | 10 AD 13 Young Controls 7 Old Controls |
| Sorbi et al., 1995 [29] | ↓ | Not assessed | sAD fAD | Fibroblast | 7 fAD 19 sAD 20 Controls |
| Allen et al., 2014 [85] | ↑ | Not assessed | fALS (SOD1) | Fibroblast | 3 Controls 3 SOD1 |
| Allen et al., 2015 [84] | ↑ | Not assessed | sALS | Fibroblast | 6 sALS 10 Controls |
| Raman et al., 2015 [86] | ↓ after complex I inhibition | (-) PGK1 | sALS | Fibroblast | 11 sALS 15 Controls |
| Konrad et al., 2017 [7] | ↑ | Not assessed | sALS | Fibroblast | 91 controls 171 sALS |
| Sorbi et al., 1990 [31] | Not assessed | ↓ HK (in fAD), (-) PFK, (-) LDH | sAD & fAD | Fibroblast Leukocyte | 5 Controls, 5 sAD, and 5 fAD (Leukocytes) 8 Controls, 6 fAD (Fibroblasts) |
| Sims et al., 1985 [30] | ↑ | Not assessed | sAD fAD | Fibroblast | 6 AD 6 Controls |
| Sims et al., 1986 [32] | Not assessed | (-) PFK | sAD | Fibroblast | 8 Controls 8 sAD |
| Deus et al., 2020 [60] | ↓ | Not assessed | sPD | Fibroblast | 5 Control 5 sPD All male |
| Milanese et al., 2019 [59] | Not assessed, theorises ↑ | Not assessed | sPD | Fibroblast | 21 Controls 47 sPD |
| Zanellati et al., 2015 [54] | No change | Not assessed | PARK2 mutant PD | Fibroblast | 4 Controls 4 fPD |
| Requejo-Aguilar et al., 2014 [63] | ↑ glycolysis and glucose uptake | ↑ GLUT1, HK-2, PDK1 and glyceraldehyde-3-phosphatase protein and mRNA, ↑ GLUT3 mRNA | PINK1 -/- Mice | Fibroblast (mouse embryonic fibroblasts) | |
| Ambrosi et al., 2014 [57] | No change | sPD | Fibroblast | 7 Controls 11 sPD | |
| Kaminsky et al., 2013 [33] | ↑ | ↑ HK, ↑ PFK, ↑ bisphosphoglycerate mutase ↑ bisphosphoglycerate phosphatase | sAD Non-AD dementia | RBC | 12 AD 13 non-AD 14 Aged matched controls 14 Young Controls |
| Carelli-Alinovi et al., 2019 [34] | ↑ | Not assessed | Controls exposed to Aβ | RBC | Not mentioned |
| Khansari et al., 1984 [46] | Not directly assessed, ↑ glucose uptake | Not assessed | sAD | B-Lymphocytes T-Lymphocytes | 12 sAD 16 Controls |
| Pansarasa et al., 2018 [106] | ↓ | Not assessed | sALS fALS (SOD1, TDP-43, FUS) | Lymphoblastoid cell lines | 4 Controls 4 sALS 3 SOD1 2 TDP-43 2-FUS |
| Smith et al., 2018 [69] | ↑ | ↓ PDK1 and LDHB | PD | Lymphocytes and Monocytes | 14 Controls 15 PD 13 RBD |
| Yao et al., 2011 [66] | No change | PINK1 -/- Mice | Muscle | ||
| Steyn et al., 2020 [89] | ↓ | Not assessed | Muscle | ||
| Dodge et al., 2013 [96] | ↑ | Not assessed | Mice with SOD1 | Muscle and Liver | 12 Controls 11 SOD1 |
| Palamiuc et al., 2015 [95] | Not assessed | ↑ PDK4, ↑ phospho-GS (gene expression) | Mice with SOD1 | Muscle | 10 Controls 13 SOD1 |
| Marini et al., 2020 [97] | ↓ PFK | Mice with SOD1 | Muscle | 15 Controls 15 SOD1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, S.M.; Burgess, T.; Lee, J.; Blackburn, D.J.; Allen, S.P.; Mortiboys, H. Peripheral Glycolysis in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8924. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21238924
Bell SM, Burgess T, Lee J, Blackburn DJ, Allen SP, Mortiboys H. Peripheral Glycolysis in Neurodegenerative Diseases. International Journal of Molecular Sciences. 2020; 21(23):8924. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21238924
Chicago/Turabian StyleBell, Simon M., Toby Burgess, James Lee, Daniel J. Blackburn, Scott P. Allen, and Heather Mortiboys. 2020. "Peripheral Glycolysis in Neurodegenerative Diseases" International Journal of Molecular Sciences 21, no. 23: 8924. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21238924