Bioaccessibility and In Vitro Intestinal Permeability of a Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) Using the Everted Intestine Assay
Abstract
:1. Introduction
2. Results
2.1. Assessment of Intestinal Tissue Viability
2.2. Bioaccessibility and Intestinal Permeability of rTBL-1
2.3. Electrophoretic Profile and Western Blot Analysis of rTBL-1 through the In Vitro and Ex Vivo Digestion
2.4. UHPLC-ESI-QTOF/MS Analysis rTBL-1 and Digested Samples
2.5. Immunohistochemical Analysis
2.6. In Silico Approach of rTBL-1 Interaction with Small Intestine Carbohydrates or Derivatives
3. Discussion
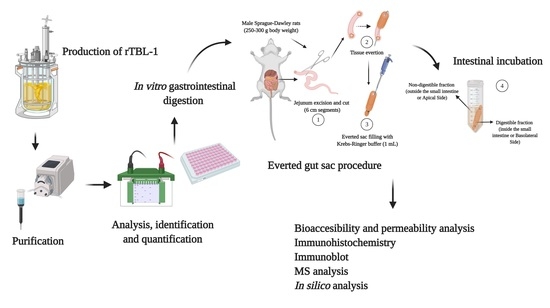
4. Materials and Methods
4.1. Production of rTBL-1
4.2. rTBL-1 Antibody Design
4.3. In Vitro and Ex Vivo Gastrointestinal Digestion
4.4. Assessment of Intestinal Tissue Viability
4.4.1. Histopathology Analysis
4.4.2. Water Flux and Glucose Transport
4.5. Effect of Gastric and Intestinal Digestion on rTBL-1
4.5.1. Bioaccessibility Measurement
4.5.2. Apparent Permeability Coefficients (Papp) and Efflux Ratio (ER)
4.6. SDS-PAGE and Western Blot
4.7. UHPLC-ESI-QTOF/MS Analysis
4.8. Immunohistochemistry Analysis of rTBL-1
4.9. In Silico Assessment of rTBL-1 Interaction with Small Intestine Ligands
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| AP | Apical side |
| BL | Basolateral side |
| CRD | Carbohydrate recognition domain |
| DF | Digestible fraction |
| ELISA | Enzyme-linked immunosorbent assay |
| ER | Efflux ratio |
| H&E | Hematoxylin and eosin |
| m/z | Mass-charge |
| NDF | Non-digestible fraction |
| IC | Intestinal content |
| NIH | National Institute of Health |
| OD | Optical density |
| Papp | Apparent permeability coefficient |
| PBST | Phosphate buffered saline with TweenTM 20 |
| PCR | Polymerase chain reaction |
| GC | Gastric content |
| RP-HPLC-MS/MS | Reverse-phase high-performance liquid chromatography coupled to mass spectrometry |
| rTBL-1 | Recombinant lectin |
| TBLF | Tepary bean lectin fraction |
| TIC | Total ion current |
| UHPLC-ESI-QTOF/MS | Ultra-high-performance liquid chromatography with electrospray ionization and Quantitative time-of-flight coupled to mass spectrometry |
| WF | Water flux |
| YPG | Yeast-peptone-glycerol extract |
References
- Liu, B.; Bian, H.; Bao, J. Plant lectins: Potential antineoplastic drugs from bench to clinic. Cancer Lett. 2010, 287, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Saha, C.; Galun, D.; Upreti, D.K.; Bayry, J.; Kaveri, S.V. European Viscum album: A potent phytotherapeutic agent with multifarious phytochemicals, pharmacological properties and clinical evidence. RSC Adv. 2016, 6, 23837–23857. [Google Scholar] [CrossRef] [Green Version]
- Ferriz-Martínez, R.; García-García, K.; Torres-Arteaga, I.; Rodriguez-Mendez, A.J.; de Guerrero-Carrillo, M.J.; Moreno-Celis, U.; Ángeles-Zaragoza, M.V.; Blanco-Labra, A.; Gallegos-Corona, M.A.; Robles-Álvarez, J.P.; et al. Tolerability assessment of a lectin fraction from Tepary bean seeds (Phaseolus acutifolius) orally administered to rats. Toxicol. Rep. 2015, 2, 63–69. [Google Scholar] [CrossRef] [Green Version]
- García-Gasca, T.; García-Cruz, M.; Hernandez-Rivera, E.; López-Matínez, J.; Castañeda-Cuevas, A.L.; Yllescas-Gasca, L.; Rodríguez-Méndez, A.J.; Mendiola-Olaya, E.; Castro-Guillén, J.L.; Blanco-Labra, A. Effects of Tepary bean (Phaseolus acutifolius) protease inhibitor and semipure lectin fractions on cancer cells. Nutr. Cancer 2012, 64, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Celis, U.; López-Martínez, F.J.; Cervantes-Jiménez, R.; Ferríz-Martínez, R.A.; Blanco-Labra, A.; García-Gasca, T. Tepary Bean (Phaseolus acutifolius) lectins induce apoptosis and cell arrest in G0/G1 by P53(Ser46) phosphorylation in colon cancer cells. Molecules 2020, 25, 1021. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Celis, U.; López-Martínez, J.; Blanco-Labra, A.; Cervantes-Jiménez, R.; Estrada-Martínez, L.E.; García-Pascalin, A.E.; Guerrero-Carrillo, M.D.J.; Rodríguez-Méndez, A.J.; Mejía, C.; Ferríz-Martínez, R.A.; et al. Phaseolus acutifolius lectin fractions exhibit apoptotic effects on colon cancer: Preclinical studies using dimethilhydrazine or azoxi-methane as cancer induction agents. Molecules 2017, 22, 1670. [Google Scholar] [CrossRef] [Green Version]
- Alatorre-Cruz, J.M.; Pita-López, W.; López-Reyes, R.G.; Ferriz-Martínez, R.A.; Cervantes-Jiménez, R.; de Jesús Guerrero Carrillo, M.; Vargas, P.J.A.; López-Herrera, G.; Rodríguez-Méndez, A.J.; Zamora-Arroyo, A.; et al. Effects of intragastrically-administered Tepary bean lectins on digestive and immune organs: Preclinical evaluation. Toxicol. Rep. 2018, 5, 56–64. [Google Scholar] [CrossRef]
- Pita-López, W.; Gomez-Garay, M.; Blanco-Labra, A.; Aguilera-Barreyro, A.; Reis-de Souza, T.C.; Olvera-Ramírez, A.; Ferriz-Martinez, R.; García-Gasca, T. Tepary bean (Phaseolus acutifolius) lectin fraction provokes reversible adverse effects on rats’ digestive tract. Toxicol. Res. 2020. [Google Scholar] [CrossRef]
- Martínez-Alarcón, D.; Varrot, A.; Fitches, E.; Gatehouse, J.A.; Cao, M.; Pyati, P.; Blanco-Labra, A.; Garcia-Gasca, T. Recombinant lectin from Tepary bean (Phaseolus acutifolius) with specific recognition for cancer-associated glycans: Production, structural characterization, and target identification. Biomolecules 2020, 10, 654. [Google Scholar] [CrossRef] [Green Version]
- Torres Arteaga, I.; Castro Guillen, J.L.; Mendiola-Olaya, E.; García-Gasca, T.; Ángeles-Zaragoza, M.V.; García-Santoyo, V.; Torres-Castillo, J.A.; Aguirre, C.; Phinney, B.; Blanco-Labra, A. Characterization of two non-fetuin-binding lectins from Tepary bean (Phaseolus acutifolius) seeds with differential cytotoxicity on colon cancer cells. J. Glycobiol. 2016, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mirkov, T.E.; Wahlstrom, J.M.; Hagiwara, K.; Finardi-Filho, F.; Kjemtrup, S.; Chrispeels, M.J. Evolutionary relationships among proteins in the phytohemagglutinin-arcelin-alpha-amylase inhibitor family of the common bean and its relatives. Plant Mol. Biol. 1994, 26, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alarcón, D.; Mora-Avilés, A.; Espinoza-Núñez, A.; Serrano Jamaica, L.M.; Cruz-Hernández, A.; Rodríguez-Torres, A.; Castro-Guillen, J.L.; Blanco-Labra, A.; García-Gasca, T. Rhizosecretion of a cisgenic lectin by genetic manipulation of Tepary bean plants (Phaseolus acutifolius). J. Biotechnol. X 2019, 3, 100013. [Google Scholar] [CrossRef]
- Nunes, R.; Silva, C.; Chaves, L. Tissue-based in vitro and ex vivo models for intestinal permeability studies. In Concepts and Models for Drug Permeability Studies; Elsevier: Amsterdam, The Netherlands, 2016; pp. 203–236. ISBN 9780081001141. [Google Scholar]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Liu, Y.; Zhao, B.; Tang, M.; Dong, H.; Zhang, L.; Lv, B.; Wei, L. Ex vivo and in situ approaches used to study intestinal absorption. J. Pharmacol. Toxicol. Methods 2013, 68, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Costa, J.; Sarmento, B.; Araújo, F. Cell-based in vitro models for intestinal permeability studies. Concepts Models Drug Permeability Stud. 2016, 57–81. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; Araújo, F.; González-Álvarez, I.; Merino-Sanjuán, M.; González-Álvarez, M.; Bermejo, M.; Sarmento, B. Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B coculture models to predict intestinal and colonic permeability compared to Caco-2 Monoculture. Mol. Pharm. 2017, 14, 1264–1270. [Google Scholar] [CrossRef]
- Antunes, F.; Andrade, F.; Ferreira, D.; Mørck Nielsen, H.; Sarmento, B. Models to predict intestinal absorption of therapeutic peptides and proteins. Curr. Drug Metab. 2012, 14, 4–20. [Google Scholar] [CrossRef]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-mohizea, A.M. Everted gut sac model as a tool in pharmaceutical research: Limitations and applications. J. Pharm. Pharmacol. 2012, 64, 326–336. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Vázquez-Sánchez, K.; López-Barrera, D.; Loarca-Piña, G.; Mendoza-Díaz, S.; Oomah, B.D. Simulated gastrointestinal digestion and in vitro colonic fermentation of spent coffee (Coffea arabica L.): Bioaccessibility and intestinal permeability. Food Res. Int. 2015, 77, 156–161. [Google Scholar] [CrossRef]
- Aguillón-Osma, J.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.; Maldonado-Celis, M.E.; Loango-Chamorro, N.; Campos-Vega, R. Impact of in vitro gastrointestinal digestion on the bioaccessibility and antioxidant capacity of bioactive compounds from Passion fruit (Passiflora edulis) leaves and juice extracts. J. Food Biochem. 2019, 43, e12879. [Google Scholar] [CrossRef] [PubMed]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Cabrera-Ramírez, Á.H.; Rodríguez-Castillo, N.; Campos-Vega, R.; Loarca-Piña, G.; Gaytán-Martínez, M. Impact of cooking and nixtamalization on the bioaccessibility and antioxidant capacity of phenolic compounds from two sorghum varieties. Food Chem. 2020, 309, 125684. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Martini, S.; Bellesia, A.; Conte, A. Identification of ACE-inhibitory peptides from Phaseolus vulgaris after in vitro gastrointestinal digestion. Int. J. Food Sci. Nutr. 2015, 66, 774–782. [Google Scholar] [CrossRef] [Green Version]
- Luna Vital, D.A.; Loarca-Piña, G.; Dia, V.P.; de Mejía, E.G. Peptides extracted from common bean (Phaseolus vulgaris L.) non-digestible fraction caused differential gene expression of HCT116 and RKO human colorectal cancer cells. Food Res. Int. 2014, 62, 193–204. [Google Scholar] [CrossRef]
- Picariello, G.; Miralles, B.; Mamone, G.; Sánchez-Rivera, L.; Recio, I.; Addeo, F.; Ferranti, P. Role of intestinal brush border peptidases in the simulated digestion of milk proteins. Mol. Nutr. Food Res. 2015, 59, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Chen, L.; Tuo, B.; Dong, H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients 2016, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Tambe, A.; Mokashi, P.; Pandita, N. Ex-vivo intestinal absorption study of boswellic acid, cyclodextrin complexes and poloxamer solid dispersions using everted gut sac technique. J. Pharm. Biomed. Anal. 2019, 167, 66–73. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Jiang, Z. Expression, distribution and role of aquaporin water channels in human and animal stomach and intestines. Int. J. Mol. Sci. 2016, 17, 1399. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Simpson, B.K.; Sun, H.; Ngadi, M.O.; Ma, Y.; Huang, T. Phaseolus vulgaris lectins: A systematic review of characteristics and health implications. Crit. Rev. Food Sci. Nutr. 2018, 58, 70–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vugmeyster, Y. Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges. World J. Biol. Chem. 2012, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; Sindelar, R.D.; Meibohm, B. Pharmaceutical Biotechnology; Crommelin, D.J.A., Sindelar, R.D., Meibohm, B., Eds.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-6485-3. [Google Scholar]
- Crotti, S.; Posocco, B.; Marangon, E.; Nitti, D.; Toffoli, G.; Agostini, M. Mass spectrometry in the pharmacokinetic studies of anticancer natural products. Mass Spectrom. Rev. 2017, 36, 213–251. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Eisenbraun, J.; Miletzki, B.; Adler, M.; Scheer, R.; Klein, R.; Gleiter, C.H. Pharmacokinetics of natural mistletoe lectins after subcutaneous injection. Eur. J. Clin. Pharmacol. 2010, 66, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Barton, C.; Kouokam, J.; Hurst, H.; Palmer, K. Pharmacokinetics of the Antiviral Lectin Griffithsin Administered by Different Routes Indicates Multiple Potential Uses. Viruses 2016, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Anupama, S.; Preeti, L.; Mamta, S.; Kamal, P.; Sanjay, B.; Arvind, D.; Vikram, G.; Rajiv, D.K.; Lu-Gang, Y.; Jonathan, M.R.; et al. Pharmacokinetics, biodistribution and antitumour effects of Sclerotium rolfsii lectin in mice. Oncol. Rep. 2017, 37, 2803–2810. [Google Scholar] [CrossRef] [Green Version]
- Lajolo, F.M.; Genovese, M.I. Nutritional Significance of Lectins and Enzyme Inhibitors from Legumes. J. Agric. Food Chem. 2002, 50, 6592–6598. [Google Scholar] [CrossRef]
- Pusztai, A.; Bardocz, S. Biological Effects of Plant Lectins on the Gastrointestinal Tract: Metabolic Consequences and Applications. Trends Glycosci. Glycotechnol. 1996, 8, 149–165. [Google Scholar] [CrossRef]
- Hara, T.; Mukunoki, Y.; Tsukamoto, I.; Miyoshi, M.; Hasegawa, K. Susceptibility of Kintoki bean lectin to digestive enzymes in vitro and its behavior in the digestive organs of mouse in vivo. J. Nutr. Sci. Vitaminol. 1984, 30, 381–394. [Google Scholar] [CrossRef]
- Nakata, S.; Kimura, T. Effect of ingested toxic bean lectins on the gastrointestinal tract in the rat. J. Nutr. 1985, 115, 1621–1629. [Google Scholar] [CrossRef]
- Cabrera-Ramírez, A.H.; Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Morales-Sánchez, E.; Campos-Vega, R.; Gaytán-Martínez, M. Effect of the nixtamalization process on the protein bioaccessibility of white and red sorghum flours during in vitro gastrointestinal digestion. Food Res. Int. 2020, 134, 109234. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar] [CrossRef]
- Ramadass, B.; Dokladny, K.; Moseley, P.L.; Patel, Y.R.; Lin, H.C. Sucrose Co-administration Reduces the Toxic Effect of Lectin on Gut Permeability and Intestinal Bacterial Colonization. Dig. Dis. Sci. 2010, 55, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Mishra, G.; Jaggi, M.; Khar, R.K.; Talegaonkar, S. Effect of P-glycoprotein inhibitor, verapamil, on oral bioavailability and pharmacokinetics of irinotecan in rats. Eur. J. Pharm. Sci. 2009, 36, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Awortwe, C.; Fasinu, P.S.; Rosenkranz, B. Application of Caco-2 Cell line in Herb-Drug interaction studies: Current approaches and challenges. J. Pharm. Pharm. Sci. 2014, 17, 1. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein digestion of different protein sources using the INFOGEST static digestion model. Food Res. Int. 2020, 130, 108996. [Google Scholar] [CrossRef]
- Dimitrijevic, R.; Jadranin, M.; Burazer, L.; Ostojic, S.; Gavrovic-Jankulovic, M. Evaluation of the thermal stability and digestibility of heterologously produced banana lectin. Food Chem. 2010, 120, 1113–1118. [Google Scholar] [CrossRef]
- Dodd, R.B.; Drickamer, K. Lectin-like proteins in model organisms: Implications for evolution of carbohydrate-binding activity. Glycobiology 2001, 11, 71R–79R. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Alarcón, D.; Blanco-Labra, A.; García-Gasca, T. Expression of lectins in heterologous systems. Int. J. Mol. Sci. 2018, 19, 616. [Google Scholar] [CrossRef] [Green Version]
- Gabor, F.; Bogner, E.; Weissenboeck, A.; Wirth, M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; Iacomino, G.; Mamone, G.; Ferranti, P.; Fierro, O.; Gianfrani, C.; Di Luccia, A.; Addeo, F. Transport across Caco-2 monolayers of peptides arising from in vitro digestion of bovine milk proteins. Food Chem. 2013, 139, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Krippendorff, B.-F.; Kuester, K.; Kloft, C.; Huisinga, W. Nonlinear pharmacokinetics of therapeutic proteins resulting from receptor mediated endocytosis. J. Pharmacokinet. Pharmacodyn. 2009, 36, 239–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khemiss, F.; Saidane, D.; Moshtaghie, A.A. Effect of aqueous extract of Prasium majus‘s L. leaves on water and electrolytes transport in rat intestine. Pakistan J. Biol. Sci. 2005, 8, 1424–1429. [Google Scholar] [CrossRef] [Green Version]
- Caicedo-Lopez, L.H.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.L.; Campos-Vega, R.; Mendoza, S.; Loarca-Piña, G. Effect of the in vitro gastrointestinal digestion on free-phenolic compounds and mono/oligosaccharides from Moringa oleifera leaves: Bioaccessibility, intestinal permeability and antioxidant capacity. Food Res. Int. 2019, 120, 631–642. [Google Scholar] [CrossRef]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Weiss, M.; Gonzalez de Mejia, E. Anthocyanins from Purple Corn Ameliorated Tumor Necrosis Factor-α-Induced Inflammation and Insulin Resistance in 3T3-L1 Adipocytes via Activation of Insulin Signaling and Enhanced GLUT4 Translocation. Mol. Nutr. Food Res. 2017, 61, 1–13. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.A.J.A. Autodock vina: Improving the speed and accuracy of docking. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]










| Ligands | Binding Energy (kcal/mol) |
|---|---|
| β-D-Mannose | −5.70 |
| −5.40 | |
| −5.40 | |
| N-acetyl β-D glucosamine | −6.10 |
| −5.80 | |
| −5.70 | |
| Acid sialic | −6.70 |
| −6.30 | |
| −6.30 | |
| N-acetyl galactosamine | −6.10 |
| −5.80 | |
| −5.60 | |
| β-D-Galactose | −5.60 |
| −5.40 | |
| −5.50 | |
| α-D-Glucose | −5.40 |
| −5.40 | |
| −5.30 | |
| −5.4 | |
| α-L-Fucose | −5.4 |
| −5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Rojas, L.J.; Luzardo-Ocampo, I.; Mosqueda, J.; Palmerín-Carreño, D.M.; Escobedo-Reyes, A.; Blanco-Labra, A.; Escobar-García, K.; García-Gasca, T. Bioaccessibility and In Vitro Intestinal Permeability of a Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) Using the Everted Intestine Assay. Int. J. Mol. Sci. 2021, 22, 1049. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031049
Vega-Rojas LJ, Luzardo-Ocampo I, Mosqueda J, Palmerín-Carreño DM, Escobedo-Reyes A, Blanco-Labra A, Escobar-García K, García-Gasca T. Bioaccessibility and In Vitro Intestinal Permeability of a Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) Using the Everted Intestine Assay. International Journal of Molecular Sciences. 2021; 22(3):1049. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031049
Chicago/Turabian StyleVega-Rojas, Lineth Juliana, Ivan Luzardo-Ocampo, Juan Mosqueda, Dulce María Palmerín-Carreño, Antonio Escobedo-Reyes, Alejandro Blanco-Labra, Konisgmar Escobar-García, and Teresa García-Gasca. 2021. "Bioaccessibility and In Vitro Intestinal Permeability of a Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) Using the Everted Intestine Assay" International Journal of Molecular Sciences 22, no. 3: 1049. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031049