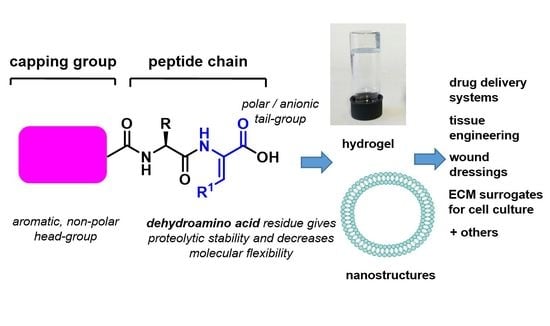
Dehydropeptide Supramolecular Hydrogels and Nanostructures as Potential Peptidomimetic Biomedical Materials
Abstract
:1. Introduction
1.1. Supramolecular Peptide Hydrogels
1.2. Structure of Dehydroamino Acids and Dehydropeptides
1.3. Occurrence in Nature and Pharmaceutics
1.4. Pharmacological Considerations
2. Dehydropeptide Hydrogels and Other Nanostructures
2.1. Dehydropeptides without Capping Groups (Free Amine and Acid Groups at N-Terminus and C-Terminus)
2.1.1. Nanostructures from Uncapped Dehydropeptides
2.1.2. Hydrogels from Uncapped Dehydropeptides
2.2. Dehydropeptides Modified at the C-Terminus
2.3. Dehydropeptides Modified at the N-Terminus
2.3.1. N-Conjugated with Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
2.3.2. N-Conjugated with Carboxybenzyl (Benzyloxycarbonyl) Groups
3. Synthesis of Dehydropeptides
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Pang, Y.; Su, Y.; Zhu, X. Supramolecular hydrogels: Synthesis, properties and their biomedical applications. Biomater. Sci. 2015, 3, 937–954. [Google Scholar] [CrossRef]
- Johnson, E.K.; Adams, D.J.; Cameron, P.J. Peptide based low molecular weight gelators. J. Mater. Chem. 2011, 21, 2024–2027. [Google Scholar] [CrossRef] [Green Version]
- Frederix, P.W.J.M.; Scott, G.G.; Abul-Haija, Y.M.; Kalafatovic, D.; Pappas, C.G.; Javid, N.; Hunt, N.T.; Ulijn, R.V.; Tuttle, T. Exploring the sequence space for (tri-)peptide self-assembly to design and discover new hydrogels. Nat. Chem. 2015, 7, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, J.K.; Nazareth, C.; VandenBerg, M.A.; Webber, M.J. Self-assembly of amphiphilic tripeptides with sequence-dependent nanostructure. Biomater. Sci. 2017, 5, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.Y.; Hauser, C.A.E. Short to ultrashort peptide hydrogels for biomedical uses. Mater. Today 2014, 17, 381–388. [Google Scholar] [CrossRef]
- Chen, J.; Zou, X. Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Dipeptide and Tripeptide Conjugates as Low-Molecular-Weight Hydrogelators. Macromol. Biosci. 2011, 11, 160–173. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Liang, G.; Xu, B. Enzymatic Hydrogelation of Small Molecules. Acc. Chem. Res. 2008, 41, 315–326. [Google Scholar] [CrossRef]
- Shi, J.; Gao, Y.; Zhang, Y.; Pan, Y.; Xu, B. Calcium Ions to Cross-Link Supramolecular Nanofibers to Tune the Elasticity of Hydrogels over Orders of Magnitude. Langmuir 2011, 27, 14425–14431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draper, E.R.; Adams, D.J. Controlling the Assembly and Properties of Low-Molecular-Weight Hydrogelators. Langmuir 2019, 35, 6506–6521. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, Q.; Zhu, S.; Liu, H.; Chen, J. Preparation and applications of peptide-based injectable hydrogels. RSC Adv. 2019, 9, 28299–28311. [Google Scholar] [CrossRef] [Green Version]
- Fichman, G.; Gazit, E. Self-assembly of short peptides to form hydrogels: Design of building blocks, physical properties and technological applications. Acta Biomater. 2014, 10, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef]
- Shah, A.; Malik, M.S.; Khan, G.S.; Nosheen, E.; Iftikhar, F.J.; Khan, F.A.; Shukla, S.S.; Akhter, M.S.; Kraatz, H.-B.; Aminabhavi, T.M. Stimuli-responsive peptide-based biomaterials as drug delivery systems. Chem. Eng. J. 2018, 353, 559–583. [Google Scholar] [CrossRef]
- Martin, A.D.; Thordarson, P. Beyond Fmoc: A review of aromatic peptide capping groups. J. Mater. Chem. B 2020, 8, 863–877. [Google Scholar] [CrossRef]
- Adams, D.J.; Butler, M.F.; Frith, W.J.; Kirkland, M.; Mullen, L.; Sanderson, P. A new method for maintaining homogeneity during liquid–hydrogel transitions using low molecular weight hydrogelators. Soft Matter 2009, 5, 1856–1862. [Google Scholar] [CrossRef]
- VandenBerg, M.A.; Sahoo, J.K.; Zou, L.; McCarthy, W.; Webber, M.J. Divergent Self-Assembly Pathways to Hierarchically Organized Networks of Isopeptide-Modified Discotics under Kinetic Control. ACS Nano 2020, 14, 5491–5505. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Chauhan, M.K.; Chauhan, V.S. Short to ultrashort peptide-based hydrogels as a platform for biomedical applications. Biomater. Sci. 2020, 8, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Carpino, L.A.; Han, G.Y. 9-Fluorenylmethoxycarbonyl amino-protecting group. J. Org. Chem. 1972, 37, 3404–3409. [Google Scholar] [CrossRef]
- Truong, W.T.; Su, Y.; Gloria, D.; Braet, F.; Thordarson, P. Dissolution and degradation of Fmoc-diphenylalanine self-assembled gels results in necrosis at high concentrations in vitro. Biomater. Sci. 2015, 3, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Swanekamp, R.J.; Welch, J.J.; Nilsson, B.L. Proteolytic stability of amphipathic peptide hydrogels composed of self-assembled pleated β-sheet or coassembled rippled β-sheet fibrils. Chem. Commun. 2014, 50, 10133–10136. [Google Scholar] [CrossRef]
- Michele, M.; Katie, E.S.; Silvia, M. The Unexpected Advantages of Using D-Amino Acids for Peptide Self- Assembly into Nanostructured Hydrogels for Medicine. Curr. Top. Med. Chem. 2016, 16, 2009–2018. [Google Scholar] [CrossRef] [Green Version]
- Luca, G.; Rossella De, M.; Lucia, C. Chemical Modifications Designed to Improve Peptide Stability: Incorporation of Non-Natural Amino Acids, Pseudo-Peptide Bonds, and Cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. [Google Scholar] [CrossRef]
- Li, X.; Du, X.; Li, J.; Gao, Y.; Pan, Y.; Shi, J.; Zhou, N.; Xu, B. Introducing d-Amino Acid or Simple Glycoside into Small Peptides to Enable Supramolecular Hydrogelators to Resist Proteolysis. Langmuir 2012, 28, 13512–13517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Liang, G.; Xu, B. Supramolecular hydrogels based on β-amino acid derivatives. Chem. Commun. 2006, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandary, A.; Nilsson, B.L. Investigating the effects of peptoid substitutions in self-assembly of Fmoc-diphenylalanine derivatives. Pept. Sci. 2017, 108, e22994. [Google Scholar] [CrossRef]
- Bogart, J.W.; Bowers, A.A. Dehydroamino acids: Chemical multi-tools for late-stage diversification. Org. Biomol. Chem. 2019, 17, 3653–3669. [Google Scholar] [CrossRef]
- Buczek, A.; Siodłak, D.; Bujak, M.; Makowski, M.; Kupka, T.; Broda, M.A. Impact of the ΔPhe configuration on the Boc-Gly-ΔPhe-NHMe conformation: Experiment and theory. Struct. Chem. 2019, 30, 1685–1697. [Google Scholar] [CrossRef] [Green Version]
- Joaquin, D.; Lee, M.A.; Kastner, D.W.; Singh, J.; Morrill, S.T.; Damstedt, G.; Castle, S.L. Impact of Dehydroamino Acids on the Structure and Stability of Incipient 310-Helical Peptides. J. Org. Chem. 2020, 85, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.M.; Rajashankar, K.R.; Ramakumar, S.; Chauhan, V.S. First Observation of Left-Handed Helical Conformation in a Dehydro Peptide Containing Two l-Val Residues. Crystal and Solution Structure of Boc-l-Val-ΔPhe-ΔPhe-ΔPhe-l-Val-OMe. J. Am. Chem. Soc. 1997, 119, 3205–3211. [Google Scholar] [CrossRef]
- Mathur, P.; Ramakumar, S.; Chauhan, V.S. Peptide design using α,β-dehydro amino acids: From β-turns to helical hairpins. Pept. Sci. 2004, 76, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Chauhan, V.S. De novo design of α,β-didehydrophenylalanine containing peptides: From models to applications. Biopolymers 2011, 95, 161–173. [Google Scholar] [CrossRef]
- Gupta, M.; Acharya, R.; Mishra, A.; Ramakumar, S.; Ahmed, F.; Chauhan, V.S. Dehydrophenylalanine (ΔPhe) as a β Breaker: Extended Structure Terminated by a ΔPhe-Induced Turn in the Pentapeptide Boc-Phe1-Ala2-Ile3-ΔPhe4-Ala5-OMe. ChemBioChem 2008, 9, 1375–1378. [Google Scholar] [CrossRef]
- Paul, M.; Donk, W.A.v.d. Chemical and Enzymatic Synthesis of Lanthionines. Mini-Rev. Org. Chem. 2005, 2, 23–37. [Google Scholar] [CrossRef]
- Siodłak, D. α,β-Dehydroamino acids in naturally occurring peptides. Amino Acids 2015, 47, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Zhang, Q. Linaridin natural products. Nat. Prod. Rep. 2020, 37, 1152–1163. [Google Scholar] [CrossRef]
- Gross, E.; Morell, J.L. Structure of nisin. J. Am. Chem. Soc. 1971, 93, 4634–4635. [Google Scholar] [CrossRef] [PubMed]
- Sandu, C.; Chandramouli, N.; Glickman, J.F.; Molina, H.; Kuo, C.-L.; Kukushkin, N.; Goldberg, A.L.; Steller, H. Thiostrepton interacts covalently with Rpt subunits of the 19S proteasome and proteasome substrates. J. Cell. Mol. Med. 2015, 19, 2181–2192. [Google Scholar] [CrossRef] [Green Version]
- Demain, A.L.; Elander, R.P. The β-lactam antibiotics: Past, present, and future. Antonie Leeuwenhoek 1999, 75, 5–19. [Google Scholar] [CrossRef]
- Pastel, D.A. Imipenem-Cilastatin Sodium, A Broad-Spectrum Carbapenem Antibiotic Combination. Am. J. Hosp. Pharm. 1986, 43, 2630–2644. [Google Scholar] [CrossRef]
- Balfour, J.A.; Bryson, H.M.; Brogden, R.N. Imipenem/Cilastatin. Drugs 1996, 51, 99–136. [Google Scholar] [CrossRef]
- Blayney, D.W.; Bazhenova, L.; Lloyd, G.K.; Huang, L.; Mohanlal, R. Plinabulin, a Novel Small Molecule That Ameliorates Chemotherapy-Induced Neutropenia, Is Administered on the Same Day of Chemotherapy and Has Anticancer Efficacy. Blood 2016, 128, 2508. [Google Scholar] [CrossRef]
- Schorlemmer, H.-U.; Opitz, W.; Etschenberg, E.; Bitter-Suermann, D.; Hadding, U. Killing of Tumor Cells in vitro by Macrophages from Mice Treated with Synthetic Dehydrodipeptides. Cancer Res. 1979, 39, 1847–1853. [Google Scholar] [PubMed]
- Latajka, R.; Makowski, M.; Jewgiński, M.; Pawełczak, M.; Koroniak, H.; Kafarski, P. Peptide p-nitrophenylanilides containing (E)-dehydrophenylalanine—synthesis, structural studies and evaluation of their activity towards cathepsin C. New J. Chem. 2006, 30, 1009–1018. [Google Scholar] [CrossRef]
- Latajka, R.; Jewginski, M.; Makowski, M.; Pawełczak, M.; Huber, T.; Sewald, N.; Kafarski, P. Pentapeptides containing two dehydrophenylalanine residues-synthesis, structural studies and evaluation of their activity towards cathepsin C. J. Pept. Sci. 2008, 14, 1084–1095. [Google Scholar] [CrossRef]
- Makowski, M.; Lenartowicz, P.; Oszywa, B.; Jewgiński, M.; Pawełczak, M.; Kafarski, P. Synthesis of dehydrodipeptide esters and their evaluation as inhibitors of cathepsin C. Med. Chem. Res. 2015, 24, 3157–3165. [Google Scholar] [CrossRef] [Green Version]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Gupta, M.; Bagaria, A.; Mishra, A.; Mathur, P.; Basu, A.; Ramakumar, S.; Chauhan, V.S. Self-Assembly of a Dipeptide- Containing Conformationally Restricted Dehydrophenylalanine Residue to Form Ordered Nanotubes. Adv. Mater. 2007, 19, 858–861. [Google Scholar] [CrossRef]
- Mishra, A.; Panda, J.J.; Basu, A.; Chauhan, V.S. Nanovesicles Based on Self-Assembly of Conformationally Constrained Aromatic Residue Containing Amphiphilic Dipeptides. Langmuir 2008, 24, 4571–4576. [Google Scholar] [CrossRef]
- Alam, S.; Panda, J.J.; Chauhan, V.S. Novel dipeptide nanoparticles for effective curcumin delivery. Int. J. Nanomed. 2012, 7, 4207–4222. [Google Scholar] [CrossRef] [Green Version]
- Panda, J.J.; Varshney, A.; Chauhan, V.S. Self-assembled nanoparticles based on modified cationic dipeptides and DNA: Novel systems for gene delivery. J. Nanobiotechnol. 2013, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Khatri, A.; Mishra, A.; Chauhan, V.S. Characterization of DNA Condensation by Conformationally Restricted Dipeptides and Gene Delivery. J. Biomed. Nanotechnol. 2017, 13, 35–53. [Google Scholar] [CrossRef]
- Baskar, G.; Ravi, M.; Panda, J.J.; Khatri, A.; Dev, B.; Santosham, R.; Sathiya, S.; Babu, C.S.; Chauhan, V.S.; Rayala, S.K.; et al. Efficacy of Dipeptide-Coated Magnetic Nanoparticles in Lung Cancer Models Under Pulsed Electromagnetic Field. Cancer Investig. 2017, 35, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Varshney, A.; Panda, J.J.; Singh, A.K.; Yadav, N.; Bihari, C.; Biswas, S.; Sarin, S.K.; Chauhan, V.S. Targeted delivery of microRNA-199a-3p using self-assembled dipeptide nanoparticles efficiently reduces hepatocellular carcinoma in mice. Hepatology 2018, 67, 1392–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, J.J.; Kaul, A.; Kumar, S.; Alam, S.; Mishra, A.K.; Kundu, G.C.; Chauhan, V.S. Modified dipeptide-based nanoparticles: Vehicles for targeted tumor drug delivery. Nanomedicine 2013, 8, 1927–1942. [Google Scholar] [CrossRef] [PubMed]
- Parween, S.; Misra, A.; Ramakumar, S.; Chauhan, V.S. Self-assembled dipeptide nanotubes constituted by flexible β-phenylalanine and conformationally constrained α,β-dehydrophenylalanine residues as drug delivery system. J. Mater. Chem. B 2014, 2, 3096–3106. [Google Scholar] [CrossRef] [PubMed]
- Panda, J.J.; Mishra, A.; Basu, A.; Chauhan, V.S. Stimuli Responsive Self-Assembled Hydrogel of a Low Molecular Weight Free Dipeptide with Potential for Tunable Drug Delivery. Biomacromolecules 2008, 9, 2244–2250. [Google Scholar] [CrossRef]
- Panda, J.J.; Dua, R.; Mishra, A.; Mittra, B.; Chauhan, V.S. 3D Cell Growth and Proliferation on a RGD Functionalized Nanofibrillar Hydrogel Based on a Conformationally Restricted Residue Containing Dipeptide. ACS Appl. Mater. Interfaces 2010, 2, 2839–2848. [Google Scholar] [CrossRef]
- Thota, C.K.; Yadav, N.; Chauhan, V.S. A novel highly stable and injectable hydrogel based on a conformationally restricted ultrashort peptide. Sci. Rep. 2016, 6, 31167. [Google Scholar] [CrossRef] [Green Version]
- Thota, C.K.; Berger, A.A.; Elomaa, L.; Nie, C.; Böttcher, C.; Koksch, B. Coassembly Generates Peptide Hydrogel with Wound Dressing Material Properties. ACS Omega 2020, 5, 8557–8563. [Google Scholar] [CrossRef] [Green Version]
- Mahato, M.; Arora, V.; Pathak, R.; Gautam, H.K.; Sharma, A.K. Fabrication of nanostructures through molecular self-assembly of small amphiphilic glyco-dehydropeptides. Mol. Biosyst. 2012, 8, 1742–1749. [Google Scholar] [CrossRef]
- Shivhare, K.; Garg, C.; Priyam, A.; Gupta, A.; Sharma, A.K.; Kumar, P. Enzyme sensitive smart inulin-dehydropeptide conjugate self-assembles into nanostructures useful for targeted delivery of ornidazole. Int. J. Biol. Macromol. 2018, 106, 775–783. [Google Scholar] [CrossRef]
- Deka, S.R.; Yadav, S.; Kumar, D.; Garg, S.; Mahato, M.; Sharma, A.K. Self-assembled dehydropeptide nano carriers for delivery of ornidazole and curcumin. Colloids Surf. B Biointerfaces 2017, 155, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.; Patrignani, P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J. Pain. Res. 2015, 8, 105–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.C. COX-2 inhibitors in cancer treatment and prevention, a recent development. Anti Cancer Drugs 2002, 13, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Jervis, P.J.; Amorim, C.; Pereira, T.; Martins, J.A.; Ferreira, P.M.T. Exploring the properties and potential biomedical applications of NSAID-capped peptide hydrogels. Soft Matter 2020, 16, 10001–10012. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, Y.; Gao, Y.; Du, X.; Shi, J.; Xu, B. d-Amino Acids Boost the Selectivity and Confer Supramolecular Hydrogels of a Nonsteroidal Anti-Inflammatory Drug (NSAID). J. Am. Chem. Soc. 2013, 135, 542–545. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Xing, L.; Fan, Q.; Cheetham, A.G.; Lin, R.; Holt, B.; Chen, L.; Xiao, Y.; Cui, H. Drug-Bearing Supramolecular Filament Hydrogels as Anti-Inflammatory Agents. Theranostics 2017, 7, 2003–2014. [Google Scholar] [CrossRef] [Green Version]
- Majumder, J.; Das, M.R.; Deb, J.; Jana, S.S.; Dastidar, P. β-Amino Acid and Amino-Alcohol Conjugation of a Nonsteroidal Anti-Inflammatory Drug (NSAID) Imparts Hydrogelation Displaying Remarkable Biostability, Biocompatibility, and Anti-Inflammatory Properties. Langmuir 2013, 29, 10254–10263. [Google Scholar] [CrossRef]
- Huang, Y.; Meng, L.; Nie, Q.; Zhou, Y.; Chen, L.; Yang, S.; Fung, Y.M.E.; Li, X.; Huang, C.; Cao, Y.; et al. Selection of DNA-encoded chemical libraries against endogenous membrane proteins on live cells. Nat. Chem. 2021, 13, 77–88. [Google Scholar] [CrossRef]
- Vilaça, H.; Pereira, G.; Castro, T.G.; Hermenegildo, B.F.; Shi, J.; Faria, T.Q.; Micaêlo, N.; Brito, R.M.M.; Xu, B.; Castanheira, E.M.S.; et al. New self-assembled supramolecular hydrogels based on dehydropeptides. J. Mater. Chem. B 2015, 3, 6355–6367. [Google Scholar] [CrossRef]
- Vilaça, H.; Hortelão, A.C.L.; Castanheira, E.M.S.; Queiroz, M.-J.R.P.; Hilliou, L.; Hamley, I.W.; Martins, J.A.; Ferreira, P.M.T. Dehydrodipeptide Hydrogelators Containing Naproxen N-Capped Tryptophan: Self-Assembly, Hydrogel Characterization, and Evaluation as Potential Drug Nanocarriers. Biomacromolecules 2015, 16, 3562–3573. [Google Scholar] [CrossRef]
- Vilaça, H.; Castro, T.; Costa, F.M.G.; Melle-Franco, M.; Hilliou, L.; Hamley, I.W.; Castanheira, E.M.S.; Martins, J.A.; Ferreira, P.M.T. Self-assembled RGD dehydropeptide hydrogels for drug delivery applications. J. Mater. Chem. B 2017, 5, 8607–8617. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.; Gallo, J.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Hilliou, L.; Ferreira, P.M.T.; Bañobre-López, M.; Martins, J.A. Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications. Nanomaterials 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veloso, S.R.S.; Martins, J.A.; Hilliou, L.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Jervis, P.J.; Moreira, R.; Pereira, D.M.; Coutinho, P.J.G.; et al. Dehydropeptide-based plasmonic magnetogels: A supramolecular composite nanosystem for multimodal cancer therapy. J. Mater. Chem. B 2020, 8, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Jervis, P.J.; Carvalho, A.; Ferreira, P.M.T.; Martins, J.A.; Valentão, P.; Andrade, P.B.; Pereira, D.M. Biological Evaluation of Naproxen–Dehydrodipeptide Conjugates with Self-Hydrogelation Capacity as Dual LOX/COX Inhibitors. Pharmaceutics 2020, 12, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veloso, S.R.S.; Jervis, P.J.; Silva, J.F.G.; Hilliou, L.; Moura, C.; Pereira, D.M.; Coutinho, P.J.G.; Martins, J.A.; Castanheira, E.M.S.; Ferreira, P.M.T. Supramolecular ultra-short carboxybenzyl-protected dehydropeptide-based hydrogels for drug delivery. Mater. Sci. Eng. C 2021, 122, 111869. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.M.; Chamberlin, A.R. Chemical Synthesis of Natural Product Peptides: Coupling Methods for the Incorporation of Noncoded Amino Acids into Peptides. Chem. Rev. 1997, 97, 2243–2266. [Google Scholar] [CrossRef]
- Bonauer, C.; Walenzyk, T.; König, B. α,β-Dehydroamino Acids. Synthesis 2006, 2006, 1–20. [Google Scholar] [CrossRef]
- Itoh, H.; Miura, K.; Kamiya, K.; Yamashita, T.; Inoue, M. Solid-Phase Total Synthesis of Yaku’amide B Enabled by Traceless Staudinger Ligation. Angew. Chem. Int. Ed. 2020, 59, 4564–4571. [Google Scholar] [CrossRef] [PubMed]
- Kuranaga, T.; Mutoh, H.; Sesoko, Y.; Goto, T.; Matsunaga, S.; Inoue, M. Elucidation and Total Synthesis of the Correct Structures of Tridecapeptides Yaku’amides A and B. Synthesis-Driven Stereochemical Reassignment of Four Amino Acid Residues. J. Am. Chem. Soc. 2015, 137, 9443–9451. [Google Scholar] [CrossRef] [PubMed]
- Wołczański, G.; Lisowski, M. A general method for preparation of N-Boc-protected or N-Fmoc-protected α,β-didehydropeptide building blocks and their use in the solid-phase peptide synthesis. J. Pept. Sci. 2018, 24, e3091. [Google Scholar] [CrossRef]
- Makowski, M.; Rzeszotarska, B.; Kubica, Z.; Pietrzyńnski, G. Synthesis of Peptides with α,β-Dehydroamino Acids, II. Synthesis of tert-Butyloxycarbonyldipeptides of Dehydroalanine and Dehydrophenylalanine. Liebigs Ann. Chem. 1985, 1985, 893–900. [Google Scholar] [CrossRef]
- Buck, R.T.; Clarke, P.A.; Coe, D.M.; Drysdale, M.J.; Ferris, L.; Haigh, D.; Moody, C.J.; Pearson, N.D.; Swann, E. The Carbenoid Approach to Peptide Synthesis. Chem. A Eur. J. 2000, 6, 2160–2167. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Kasiński, A.; Zielińska-Pisklak, M. Smart Hydrogels-Synthetic Stimuli-Responsive Antitumor Drug Release Systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Lu, H.; Yuan, L.; Yu, X.; Wu, C.; He, D.; Deng, J. Recent advances of on-demand dissolution of hydrogel dressings. Burns Trauma 2018, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jervis, P.J.; Amorim, C.; Pereira, T.; Martins, J.A.; Ferreira, P.M.T. Dehydropeptide Supramolecular Hydrogels and Nanostructures as Potential Peptidomimetic Biomedical Materials. Int. J. Mol. Sci. 2021, 22, 2528. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052528
Jervis PJ, Amorim C, Pereira T, Martins JA, Ferreira PMT. Dehydropeptide Supramolecular Hydrogels and Nanostructures as Potential Peptidomimetic Biomedical Materials. International Journal of Molecular Sciences. 2021; 22(5):2528. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052528
Chicago/Turabian StyleJervis, Peter J., Carolina Amorim, Teresa Pereira, José A. Martins, and Paula M. T. Ferreira. 2021. "Dehydropeptide Supramolecular Hydrogels and Nanostructures as Potential Peptidomimetic Biomedical Materials" International Journal of Molecular Sciences 22, no. 5: 2528. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052528