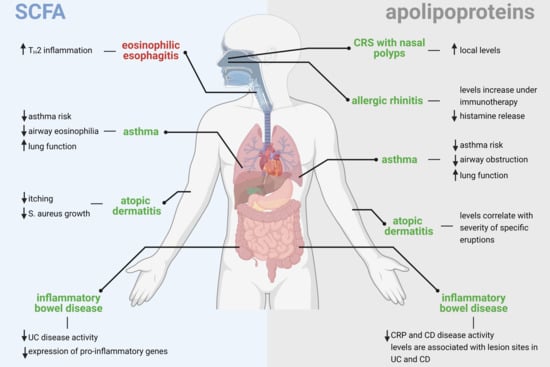
Role of Short Chain Fatty Acids and Apolipoproteins in the Regulation of Eosinophilia-Associated Diseases
Abstract
:1. Introduction
2. Cellular Mechanism Underlying Eosinophilic Inflammation
3. Apolipoproteins
3.1. Apolipoprotein A-I
3.2. Apolipoprotein A-IV
3.3. Effects of Apolipoproteins on Eosinophil Cellular Function
3.4. The Role of Apolipoproteins in Mouse Models of Eosinophilia-Associated Diseases
3.4.1. Mouse Models of Allergic Lung Inflammation
3.4.2. Mouse Models of Inflammatory Bowel Disease
3.5. The Role of Apolipoprotein A-I in Eosinophilia-Associated Diseases
3.5.1. Allergic Rhinitis
3.5.2. Asthma
3.5.3. Atopic Dermatitis
3.5.4. Inflammatory Bowel Disease
4. Short Chain Fatty Acids
4.1. Effects of SCFA on Eosinophil Cellular Function
4.2. The Role of SCFAs in Mouse Models of Eosinophilia-Associated Diseases
4.2.1. Mouse models of Airway Inflammation
4.2.2. Mouse Models of Atopic Dermatitis
4.2.3. Mouse Models of Inflammatory Bowel Disease
4.2.4. Mouse Models of Eosinophilic Esophagitis
4.3. The Role of SCFAs in Eosinophilia-Associated Diseases
4.3.1. Asthma
4.3.2. Atopic Dermatitis
4.3.3. Inflammatory Bowel Diseases
4.3.4. Eosinophilic Esophagitis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Marichal, T.; Mesnil, C.; Bureau, F. Homeostatic Eosinophils: Characteristics and Functions. Front. Med. Lausanne 2017, 4, 101. [Google Scholar] [CrossRef] [Green Version]
- Kienzl, M.; Hasenoehrl, C.; Valadez-Cosmes, P.; Maitz, K.; Sarsembayeva, A.; Sturm, E.; Heinemann, A.; Kargl, J.; Schicho, R. IL-33 reduces tumor growth in models of colorectal cancer with the help of eosinophils. Oncoimmunology 2020, 9, 1776059. [Google Scholar] [CrossRef]
- Reichman, H.; Itan, M.; Rozenberg, P.; Yarmolovski, T.; Brazowski, E.; Varol, C.; Gluck, N.; Shapira, S.; Arber, N.; Qimron, U.; et al. Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer. Cancer Immunol. Res. 2019, 7, 388–400. [Google Scholar] [CrossRef] [Green Version]
- Munitz, A.; Hogan, S.P. Alarming eosinophils to combat tumors. Nat. Immunol. 2019, 20, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Wang, M.; Wang, S.; Jeong, J.M.; Xu, L.; Wen, Y.; Emontzpohl, C.; Atkins, C.L.; Duong, K.; et al. Eosinophils attenuate hepatic ischemia-reperfusion injury in mice through ST2-dependent IL-13 production. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, C.; Liu, T.; Deng, Z.; Fang, W.; Zhang, X.; Li, J.; Huang, Q.; Liu, C.; Wang, Y.; et al. Eosinophils improve cardiac function after myocardial infarction. Nat. Commun. 2020, 11, 6396. [Google Scholar] [CrossRef]
- Falchi, L.; Verstovsek, S. Eosinophilia in Hematologic Disorders. Immunol. Allergy Clin. N. Am. 2015, 35, 439–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, E.M.; Nutman, T.B. Eosinophilia in Infectious Diseases. Immunol. Allergy. Clin. N. Am. 2015, 35, 493–522. [Google Scholar] [CrossRef] [Green Version]
- Fulkerson, P.C.; Rothenberg, M.E. Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Haddad, S.; Riddell, R.H. The role of eosinophils in inflammatory bowel disease. Gut 2005, 54, 1674–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujieda, S.; Imoto, Y.; Kato, Y.; Ninomiya, T.; Tokunaga, T.; Tsutsumiuchi, T.; Yoshida, K.; Kidoguchi, M.; Takabayashi, T. Eosinophilic chronic rhinosinusitis. Allergol. Int. 2019, 68, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.R.; Ackerman, S.J. Eosinophil granule proteins: Form and function. J. Biol. Chem. 2014, 289, 17406–17415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoine, F.; Lacy, P. Eosinophil cytokines, chemokines, and growth factors: Emerging roles in immunity. Front. Immunol. 2014, 5, 570. [Google Scholar] [CrossRef] [Green Version]
- Miyata, J.; Fukunaga, K.; Kawashima, Y.; Ohara, O.; Arita, M. Cysteinyl leukotriene metabolism of human eosinophils in allergic disease. Allergol. Int. 2020, 69, 28–34. [Google Scholar] [CrossRef]
- Tani, Y.; Isobe, Y.; Imoto, Y.; Segi-Nishida, E.; Sugimoto, Y.; Arai, H.; Arita, M. Eosinophils control the resolution of inflammation and draining lymph node hypertrophy through the proresolving mediators and CXCL13 pathway in mice. FASEB J. 2014, 28, 4036–4043. [Google Scholar] [CrossRef]
- Isobe, Y.; Arita, M.; Matsueda, S.; Iwamoto, R.; Fujihara, T.; Nakanishi, H.; Taguchi, R.; Masuda, K.; Sasaki, K.; Urabe, D.; et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012, 287, 10525–10534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, J.; Fukunaga, K.; Iwamoto, R.; Isobe, Y.; Niimi, K.; Takamiya, R.; Takihara, T.; Tomomatsu, K.; Suzuki, Y.; Oguma, T.; et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J. Allergy Clin. Immunol. 2013, 131, 353–360. [Google Scholar] [CrossRef]
- Lacy, P.; Abdel-Latif, D.; Steward, M.; Musat-Marcu, S.; Man, S.F.; Moqbel, R. Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects. J. Immunol. 2003, 170, 2670–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, T.; Rothenberg, M.E. The Regulatory Function of Eosinophils. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkstrom, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, J.B.; Hirano, I. Biological therapies for eosinophilic gastrointestinal diseases. J. Allergy Clin. Immunol. 2018, 142, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armandi, A.; Bonetto, S.; Pellicano, R.; Caviglia, G.P.; Astegiano, M.; Saracco, G.M.; Ribaldone, D.G. Dupilumab to target interleukin 4 for inflammatory bowel disease? Hypothesis based on a translational message. Minerva Biotecnol. 2019, 31, 93–99. [Google Scholar] [CrossRef]
- Dellon, E.S.; Peterson, K.A.; Murray, J.A.; Falk, G.W.; Gonsalves, N.; Chehade, M.; Genta, R.M.; Leung, J.; Khoury, P.; Klion, A.D.; et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N. Engl. J. Med. 2020, 383, 1624–1634. [Google Scholar] [CrossRef]
- Kumari, M.; Kozyrskyj, A.L. Gut microbial metabolism defines host metabolism: An emerging perspective in obesity and allergic inflammation. Obes. Rev. 2017, 18, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Roula, D.; Theiler, A.; Luschnig, P.; Sturm, G.J.; Tomazic, P.V.; Marsche, G.; Heinemann, A.; Sturm, E.M. Apolipoprotein A-IV acts as an endogenous anti-inflammatory protein and is reduced in treatment-naive allergic patients and allergen-challenged mice. Allergy 2020, 75, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Trakaki, A.; Sturm, G.J.; Pregartner, G.; Scharnagl, H.; Eichmann, T.O.; Trieb, M.; Knuplez, E.; Holzer, M.; Stadler, J.T.; Heinemann, A.; et al. Allergic rhinitis is associated with complex alterations in high-density lipoprotein composition and function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1280–1292. [Google Scholar] [CrossRef]
- Yao, X.; Gordon, E.M.; Figueroa, D.M.; Barochia, A.V.; Levine, S.J. Emerging Roles of Apolipoprotein E and Apolipoprotein A-I in the Pathogenesis and Treatment of Lung Disease. Am. J. Respir. Cell Mol. Biol. 2016, 55, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Gordon, E.M.; Figueroa, D.M.; Barochia, A.V.; Yao, X.; Levine, S.J. High-density Lipoproteins and Apolipoprotein A-I: Potential New Players in the Prevention and Treatment of Lung Disease. Front. Pharmacol. 2016, 7, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulkerson, P.C.; Rothenberg, M.E. Eosinophil Development, Disease Involvement, and Therapeutic Suppression. Adv. Immunol. 2018, 138, 1–34. [Google Scholar] [PubMed]
- Johnston, L.K.; Hsu, C.L.; Krier-Burris, R.A.; Chhiba, K.D.; Chien, K.B.; McKenzie, A.; Berdnikovs, S.; Bryce, P.J. IL-33 Precedes IL-5 in Regulating Eosinophil Commitment and Is Required for Eosinophil Homeostasis. J. Immunol. 2016, 197, 3445–3453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna-Gomes, T.; Bozza, P.T.; Bandeira-Melo, C. Eosinophil recruitment and activation: The role of lipid mediators. Front. Pharmacol. 2013, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, W.S.; Rokach, J. The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor. Prog. Lipid Res. 2013, 52, 651–665. [Google Scholar] [CrossRef] [Green Version]
- Kitaura, M.; Nakajima, T.; Imai, T.; Harada, S.; Combadiere, C.; Tiffany, H.L.; Murphy, P.M.; Yoshie, O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J. Biol. Chem. 1996, 271, 7725–7730. [Google Scholar] [CrossRef] [Green Version]
- Heath, H.; Qin, S.; Rao, P.; Wu, L.; LaRosa, G.; Kassam, N.; Ponath, P.D.; Mackay, C.R. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J. Clin. Investig. 1997, 99, 178–184. [Google Scholar] [CrossRef]
- Garcia-Zepeda, E.A.; Combadiere, C.; Rothenberg, M.E.; Sarafi, M.N.; Lavigne, F.; Hamid, Q.; Murphy, P.M.; Luster, A.D. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J. Immunol. 1996, 157, 5613–5626. [Google Scholar]
- DiScipio, R.G.; Daffern, P.J.; Jagels, M.A.; Broide, D.H.; Sriramarao, P. A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transendothelial migration in vitro and in vivo. J. Immunol. 1999, 162, 1127–1136. [Google Scholar]
- Conus, S.; Straumann, A.; Bettler, E.; Simon, H.U. Mepolizumab does not alter levels of eosinophils, T cells, and mast cells in the duodenal mucosa in eosinophilic esophagitis. J. Allergy Clin. Immunol. 2010, 126, 175–177. [Google Scholar] [CrossRef]
- Youngblood, B.A.; Brock, E.C.; Leung, J.; Falahati, R.; Bochner, B.S.; Rasmussen, H.S.; Peterson, K.; Bebbington, C.; Tomasevic, N. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Peinhaupt, M.; Roula, D.; Theiler, A.; Sedej, M.; Schicho, R.; Marsche, G.; Sturm, E.M.; Sabroe, I.; Rothenberg, M.E.; Heinemann, A. DP1 receptor signaling prevents the onset of intrinsic apoptosis in eosinophils and functions as a transcriptional modulator. J. Leukoc. Biol. 2018, 104, 159–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.; Kim, Y.M.; Lee, H.R.; Mun, J.; Sim, S.; Lee, D.H.; Pham, D.L.; Kim, S.H.; Shin, Y.S.; Lee, S.W.; et al. Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma. Allergy 2020, 75, 95–103. [Google Scholar] [CrossRef]
- Hwang, C.S.; Park, S.C.; Cho, H.J.; Park, D.J.; Yoon, J.H.; Kim, C.H. Eosinophil extracellular trap formation is closely associated with disease severity in chronic rhinosinusitis regardless of nasal polyp status. Sci. Rep. 2019, 9, 8061. [Google Scholar] [CrossRef] [Green Version]
- Simon, D.; Simon, H.U.; Yousefi, S. Extracellular DNA traps in allergic, infectious, and autoimmune diseases. Allergy 2013, 68, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueki, S.; Konno, Y.; Takeda, M.; Moritoki, Y.; Hirokawa, M.; Matsuwaki, Y.; Honda, K.; Ohta, N.; Yamamoto, S.; Takagi, Y.; et al. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J. Allergy Clin. Immunol. 2016, 137, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, M.; Deng, J.; Wang, K.; Shi, J.; Sun, Y. Elevated Levels of Activated and Pathogenic Eosinophils Characterize Moderate-Severe House Dust Mite Allergic Rhinitis. J. Immunol. Res. 2020, 2020, 8085615. [Google Scholar] [CrossRef] [PubMed]
- Bakakos, A.; Loukides, S.; Bakakos, P. Severe Eosinophilic Asthma. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.; Braathen, L.R.; Simon, H.U. Eosinophils and atopic dermatitis. Allergy 2004, 59, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Gomez Torrijos, E.; Gonzalez-Mendiola, R.; Alvarado, M.; Avila, R.; Prieto-Garcia, A.; Valbuena, T.; Borja, J.; Infante, S.; Lopez, M.P.; Marchan, E.; et al. Eosinophilic Esophagitis: Review and Update. Front. Med. Lausanne 2018, 5, 247. [Google Scholar] [CrossRef]
- Alhmoud, T.; Gremida, A.; Colom Steele, D.; Fallahi, I.; Tuqan, W.; Nandy, N.; Ismail, M.; Aburajab Altamimi, B.; Xiong, M.J.; Kerwin, A.; et al. Outcomes of inflammatory bowel disease in patients with eosinophil-predominant colonic inflammation. BMJ Open Gastroenterol. 2020, 7, e000373. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.; van der Steeg, W.A.; Holme, I.; Gaffney, M.; Cater, N.B.; Barter, P.; Deedwania, P.; Olsson, A.G.; Boekholdt, S.M.; Demicco, D.A.; et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008, 117, 3002–3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [PubMed] [Green Version]
- Chan, D.C.; Watts, G.F. Apolipoproteins as markers and managers of coronary risk. QJM 2006, 99, 277–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, L.; Yi, J.; Li, W.; Zheng, X.; Liu, J.; Wang, J.; Du, G. Apolipoproteins and cancer. Cancer Med. 2019, 8, 7032–7043. [Google Scholar] [CrossRef] [Green Version]
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Noor, R.; Shuaib, U.; Wang, C.X.; Todd, K.; Ghani, U.; Schwindt, B.; Shuaib, A. High-density lipoprotein cholesterol regulates endothelial progenitor cells by increasing eNOS and preventing apoptosis. Atherosclerosis 2007, 192, 92–99. [Google Scholar] [CrossRef]
- Barter, P.J.; Nicholls, S.; Rye, K.A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 2000, 275, 33053–33058. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.M.; Davidson, W.S. Apolipoprotein A-I mimetics and high-density lipoprotein function. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 109–114. [Google Scholar] [CrossRef]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011, 8, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Georgila, K.; Vyrla, D.; Drakos, E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Ghiselli, G.; Krishnan, S.; Beigel, Y.; Gotto, A.M., Jr. Plasma metabolism of apolipoprotein A-IV in humans. J. Lipid Res. 1986, 27, 813–827. [Google Scholar] [CrossRef]
- Lu, S.; Yao, Y.; Cheng, X.; Mitchell, S.; Leng, S.; Meng, S.; Gallagher, J.W.; Shelness, G.S.; Morris, G.S.; Mahan, J.; et al. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J. Biol. Chem. 2006, 281, 3473–3483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, T.; Cook, V.R.; Rao, A.; Weinberg, R.B. Impact of murine intestinal apolipoprotein A-IV expression on regional lipid absorption, gene expression, and growth. J. Lipid Res. 2011, 52, 1984–1994. [Google Scholar] [CrossRef] [Green Version]
- Stein, O.; Stein, Y.; Lefevre, M.; Roheim, P.S. The role of apolipoprotein A-IV in reverse cholesterol transport studied with cultured cells and liposomes derived from an ether analog of phosphatidylcholine. Biochim. Biophys. Acta 1986, 878, 7–13. [Google Scholar] [CrossRef]
- Kohan, A.B.; Wang, F.; Lo, C.M.; Liu, M.; Tso, P. ApoA-IV: Current and emerging roles in intestinal lipid metabolism, glucose homeostasis, and satiety. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G472–G481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duverger, N.; Tremp, G.; Caillaud, J.M.; Emmanuel, F.; Castro, G.; Fruchart, J.C.; Steinmetz, A.; Denefle, P. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science 1996, 273, 966–968. [Google Scholar] [CrossRef]
- Geronimo, F.R.B.; Barter, P.J.; Rye, K.A.; Heather, A.K.; Shearston, K.D.; Rodgers, K.J. Plaque stabilizing effects of apolipoprotein A-IV. Atherosclerosis 2016, 251, 39–46. [Google Scholar] [CrossRef]
- Qin, X.; Swertfeger, D.K.; Zheng, S.; Hui, D.Y.; Tso, P. Apolipoprotein AIV: A potent endogenous inhibitor of lipid oxidation. Am. J. Physiol. 1998, 274, H1836–H1840. [Google Scholar] [CrossRef] [PubMed]
- Vowinkel, T.; Mori, M.; Krieglstein, C.F.; Russell, J.; Saijo, F.; Bharwani, S.; Turnage, R.H.; Davidson, W.S.; Tso, P.; Granger, D.N.; et al. Apolipoprotein A-IV inhibits experimental colitis. J. Clin. Investig. 2004, 114, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.L.; Lou, B.; Ma, J.; Wu, M.P. Neutrophils activation can be diminished by apolipoprotein A-I. Life Sci. 2005, 77, 325–335. [Google Scholar] [CrossRef]
- Blackburn, W.D., Jr.; Dohlman, J.G.; Venkatachalapathi, Y.V.; Pillion, D.J.; Koopman, W.J.; Segrest, J.P.; Anantharamaiah, G.M. Apolipoprotein A-I decreases neutrophil degranulation and superoxide production. J. Lipid Res. 1991, 32, 1911–1918. [Google Scholar] [CrossRef]
- Murphy, A.J.; Woollard, K.J.; Suhartoyo, A.; Stirzaker, R.A.; Shaw, J.; Sviridov, D.; Chin-Dusting, J.P. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlaneto, C.J.; Ribeiro, F.P.; Hatanaka, E.; Souza, G.M.; Cassatella, M.A.; Campa, A. Apolipoproteins A-I and A-II downregulate neutrophil functions. Lipids 2002, 37, 925–928. [Google Scholar] [CrossRef]
- Sharifov, O.F.; Xu, X.; Gaggar, A.; Tabengwa, E.M.; White, C.R.; Palgunachari, M.N.; Anantharamaiah, G.M.; Gupta, H. L-4F inhibits lipopolysaccharide-mediated activation of primary human neutrophils. Inflammation 2014, 37, 1401–1412. [Google Scholar]
- Kopprasch, S.; Pietzsch, J.; Graessler, J. The protective effects of HDL and its constituents against neutrophil respiratory burst activation by hypochlorite-oxidized LDL. Mol. Cell Biochem. 2004, 258, 121–127. [Google Scholar] [CrossRef]
- Madenspacher, J.H.; Azzam, K.M.; Gong, W.; Gowdy, K.M.; Vitek, M.P.; Laskowitz, D.T.; Remaley, A.T.; Wang, J.M.; Fessler, M.B. Apolipoproteins and apolipoprotein mimetic peptides modulate phagocyte trafficking through chemotactic activity. J. Biol. Chem. 2012, 287, 43730–43740. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.D.; Lim, H.Y.; Lee, H.G.; Yoon, D.Y.; Choe, Y.K.; Choi, I.; Paik, S.G.; Kim, Y.S.; Yang, Y.; Lim, J.S. Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem. Biophys. Res. Commun. 2005, 338, 1126–1136. [Google Scholar] [CrossRef]
- Diederich, W.; Orso, E.; Drobnik, W.; Schmitz, G. Apolipoprotein AI and HDL(3) inhibit spreading of primary human monocytes through a mechanism that involves cholesterol depletion and regulation of CDC42. Atherosclerosis 2001, 159, 313–324. [Google Scholar] [CrossRef]
- Murphy, A.J.; Hoang, A.; Aprico, A.; Sviridov, D.; Chin-Dusting, J. Anti-inflammatory functions of apolipoprotein A-I and high-density lipoprotein are preserved in trimeric apolipoprotein A-I. J. Pharmacol. Exp. Ther. 2013, 344, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyka, N.; Dayer, J.M.; Modoux, C.; Kohno, T.; Edwards, C.K., 3rd; Roux-Lombard, P.; Burger, D. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 2001, 97, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, W.Z.; Wu, M.P. Apolipoprotein A-I inhibits chemotaxis, adhesion, activation of THP-1 cells and improves the plasma HDL inflammatory index. Cytokine 2010, 49, 194–200. [Google Scholar] [CrossRef]
- Tabet, F.; Lambert, G.; Cuesta Torres, L.F.; Hou, L.; Sotirchos, I.; Touyz, R.M.; Jenkins, A.J.; Barter, P.J.; Rye, K.A. Lipid-free apolipoprotein A-I and discoidal reconstituted high-density lipoproteins differentially inhibit glucose-induced oxidative stress in human macrophages. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1192–1200. [Google Scholar] [CrossRef] [Green Version]
- Hamid, T.; Ismahil, M.A.; Bansal, S.S.; Patel, B.; Goel, M.; White, C.R.; Anantharamaiah, G.M.; Prabhu, S.D. The Apolipoprotein A-I Mimetic L-4F Attenuates Monocyte Activation and Adverse Cardiac Remodeling after Myocardial Infarction. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.J.; Barrett, T.J.; Taylor, L.; McNeill, E.; Manmadhan, A.; Recio, C.; Carmineri, A.; Brodermann, M.H.; White, G.E.; Cooper, D.; et al. Acute exposure to apolipoprotein A1 inhibits macrophage chemotaxis in vitro and monocyte recruitment in vivo. eLife 2016, 5. [Google Scholar] [CrossRef]
- Song, X.; Shi, Y.; You, J.; Wang, Z.; Xie, L.; Zhang, C.; Xiong, J. D-4F, an apolipoprotein A-I mimetic, suppresses IL-4 induced macrophage alternative activation and pro-fibrotic TGF-beta1 expression. Pharm. Biol. 2019, 57, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Wu, Y.; Lu, Q.; Wang, S.; Xing, D. Endogenous ApoA-I expression in macrophages: A potential target for protection against atherosclerosis. Clin. Chim. Acta 2020, 505, 55–59. [Google Scholar] [CrossRef]
- Smythies, L.E.; White, C.R.; Maheshwari, A.; Palgunachari, M.N.; Anantharamaiah, G.M.; Chaddha, M.; Kurundkar, A.R.; Datta, G. Apolipoprotein A-I mimetic 4F alters the function of human monocyte-derived macrophages. Am. J. Physiol. Cell Physiol. 2010, 298, C1538–C1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada Calvo, F.; Fillet, M.; Renaut, J.; Crahay, C.; Gueders, M.; Hacha, J.; Paulissen, G.; Foidart, J.M.; Noel, A.; Rocks, N.; et al. Potential therapeutic target discovery by 2D-DIGE proteomic analysis in mouse models of asthma. J. Proteome Res. 2011, 10, 4291–4301. [Google Scholar] [CrossRef]
- Dai, C.; Yao, X.; Keeran, K.J.; Zywicke, G.J.; Qu, X.; Yu, Z.X.; Dagur, P.K.; McCoy, J.P.; Remaley, A.T.; Levine, S.J. Apolipoprotein A-I attenuates ovalbumin-induced neutrophilic airway inflammation via a granulocyte colony-stimulating factor-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 2012, 47, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Dai, C.; Fredriksson, K.; Dagur, P.K.; McCoy, J.P.; Qu, X.; Yu, Z.X.; Keeran, K.J.; Zywicke, G.J.; Amar, M.J.; et al. 5A, an apolipoprotein A-I mimetic peptide, attenuates the induction of house dust mite-induced asthma. J. Immunol. 2011, 186, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Nandedkar, S.D.; Weihrauch, D.; Xu, H.; Shi, Y.; Feroah, T.; Hutchins, W.; Rickaby, D.A.; Duzgunes, N.; Hillery, C.A.; Konduri, K.S.; et al. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J. Lipid Res. 2011, 52, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.W.; Lee, E.H.; Lee, E.J.; Kim, H.J.; Bae, D.J.; Han, S.; Kim, D.; Jang, A.S.; Uh, S.T.; Kim, Y.H.; et al. Apolipoprotein A1 potentiates lipoxin A4 synthesis and recovery of allergen-induced disrupted tight junctions in the airway epithelium. Clin. Exp. Allergy 2013, 43, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Sappati Biyyani, R.S.; Putka, B.S.; Mullen, K.D. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J. Clin. Lipidol. 2010, 4, 478–482. [Google Scholar] [CrossRef]
- Ripolles Piquer, B.; Nazih, H.; Bourreille, A.; Segain, J.P.; Huvelin, J.M.; Galmiche, J.P.; Bard, J.M. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: Consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism 2006, 55, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Meriwether, D.; Sulaiman, D.; Wagner, A.; Grijalva, V.; Kaji, I.; Williams, K.J.; Yu, L.; Fogelman, S.; Volpe, C.; Bensinger, S.J.; et al. Transintestinal transport of the anti-inflammatory drug 4F and the modulation of transintestinal cholesterol efflux. J. Lipid Res. 2016, 57, 1175–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerster, R.; Eloranta, J.J.; Hausmann, M.; Ruiz, P.A.; Cosin-Roger, J.; Terhalle, A.; Ziegler, U.; Kullak-Ublick, G.A.; von Eckardstein, A.; Rogler, G. Anti-inflammatory Function of High-Density Lipoproteins via Autophagy of IkappaB Kinase. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 171–187. [Google Scholar] [CrossRef] [Green Version]
- Gkouskou, K.K.; Ioannou, M.; Pavlopoulos, G.A.; Georgila, K.; Siganou, A.; Nikolaidis, G.; Kanellis, D.C.; Moore, S.; Papadakis, K.A.; Kardassis, D.; et al. Apolipoprotein A-I inhibits experimental colitis and colitis-propelled carcinogenesis. Oncogene 2016, 35, 2496–2505. [Google Scholar] [CrossRef]
- Meriwether, D.; Sulaiman, D.; Volpe, C.; Dorfman, A.; Grijalva, V.; Dorreh, N.; Solorzano-Vargas, R.S.; Wang, J.; O’Connor, E.; Papesh, J.; et al. Apolipoprotein A-I mimetics mitigate intestinal inflammation in COX2-dependent inflammatory bowel disease model. J. Clin. Investig. 2019, 129, 3670–3685. [Google Scholar] [CrossRef]
- Nowacki, T.M.; Remaley, A.T.; Bettenworth, D.; Eisenblatter, M.; Vowinkel, T.; Becker, F.; Vogl, T.; Roth, J.; Tietge, U.J.; Lugering, A.; et al. The 5A apolipoprotein A-I (apoA-I) mimetic peptide ameliorates experimental colitis by regulating monocyte infiltration. Br. J. Pharmacol. 2016, 173, 2780–2792. [Google Scholar] [CrossRef] [Green Version]
- Tucker, B.; Sawant, S.; McDonald, H.; Rye, K.A.; Patel, S.; Ong, K.L.; Cochran, B.J. The association of serum lipid and lipoprotein levels with total and differential leukocyte counts: Results of a cross-sectional and longitudinal analysis of the UK Biobank. Atherosclerosis 2020, 319, 1–9. [Google Scholar] [CrossRef]
- Karedal, M.H.; Mortstedt, H.; Jeppsson, M.C.; Kronholm Diab, K.; Nielsen, J.; Jonsson, B.A.; Lindh, C.H. Time-dependent proteomic iTRAQ analysis of nasal lavage of hairdressers challenged by persulfate. J. Proteome Res. 2010, 9, 5620–5628. [Google Scholar] [CrossRef] [PubMed]
- Tomazic, P.V.; Birner-Gruenberger, R.; Leitner, A.; Darnhofer, B.; Spoerk, S.; Lang-Loidolt, D. Apolipoproteins have a potential role in nasal mucus of allergic rhinitis patients: A proteomic study. Laryngoscope 2015, 125, E91–E96. [Google Scholar] [CrossRef]
- Tomazic, P.V.; Birner-Gruenberger, R.; Leitner, A.; Obrist, B.; Spoerk, S.; Lang-Loidolt, D. Nasal mucus proteomic changes reflect altered immune responses and epithelial permeability in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2014, 133, 741–750. [Google Scholar] [CrossRef]
- Makino, Y.; Noguchi, E.; Takahashi, N.; Matsumoto, Y.; Kubo, S.; Yamada, T.; Imoto, Y.; Ito, Y.; Osawa, Y.; Shibasaki, M.; et al. Apolipoprotein A-IV is a candidate target molecule for the treatment of seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2010, 126, 1163–1169. [Google Scholar] [CrossRef]
- Tomazic, P.V.; Birner-Grünberger, R.; Britta, O.; Spörk, S.; Leitner, A.; Lang-Loidolt, D. The (potential) role of apolipoproteins in nasal mucus of allergic rhinitis patients. Clin. Transl. Allergy 2014, 4, P19. [Google Scholar] [CrossRef] [Green Version]
- Ekmekci, O.B.; Donma, O.; Ekmekci, H.; Yildirim, N.; Uysal, O.; Sardogan, E.; Demirel, H.; Demir, T. Plasma paraoxonase activities, lipoprotein oxidation, and trace element interaction in asthmatic patients. Biol. Trace Elem. Res. 2006, 111, 41–52. [Google Scholar] [CrossRef]
- Choi, G.S.; Kim, J.H.; Shin, Y.S.; Ye, Y.M.; Kim, S.H.; Park, H.S. Eosinophil activation and novel mediators in the aspirin-induced nasal response in AERD. Clin. Exp. Allergy 2013, 43, 730–740. [Google Scholar] [CrossRef]
- Mehrani, H.; Ghanei, M.; Aslani, J.; Golmanesh, L. Bronchoalveolar lavage fluid proteomic patterns of sulfur mustard-exposed patients. Proteom. Clin. Appl. 2009, 3, 1191–1200. [Google Scholar] [CrossRef]
- Nagel, G.; Weiland, S.K.; Rapp, K.; Link, B.; Zoellner, I.; Koenig, W. Association of apolipoproteins with symptoms of asthma and atopy among schoolchildren. Int. Arch Allergy Immunol. 2009, 149, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, D.J.; Agrawal, Y.; Cassano, P.A. Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2002, 155, 842–848. [Google Scholar] [CrossRef] [Green Version]
- Barochia, A.V.; Kaler, M.; Cuento, R.A.; Gordon, E.M.; Weir, N.A.; Sampson, M.; Fontana, J.R.; MacDonald, S.; Moss, J.; Manganiello, V.; et al. Serum apolipoprotein A-I and large high-density lipoprotein particles are positively correlated with FEV1 in atopic asthma. Am. J. Respir. Crit. Care Med. 2015, 191, 990–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, Y.; Moriyama, K.; Yasuda, C.; Miyata, S.; Aihara, M.; Ikezawa, Z.; Miyazaki, K. New horny layer marker proteins for evaluating skin condition in atopic dermatitis. Int. Arch. Allergy Immunol. 2009, 150, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Haberman, Y.; Tickle, T.L.; Dexheimer, P.J.; Kim, M.O.; Tang, D.; Karns, R.; Baldassano, R.N.; Noe, J.D.; Rosh, J.; Markowitz, J.; et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J. Clin. Investig. 2014, 124, 3617–3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Shen, B.; Zhuge, L.; Xie, Y. Identification of differentially expressed genes between the colon and ileum of patients with inflammatory bowel disease by gene co-expression analysis. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [Green Version]
- Broedl, U.C.; Schachinger, V.; Lingenhel, A.; Lehrke, M.; Stark, R.; Seibold, F.; Goke, B.; Kronenberg, F.; Parhofer, K.G.; Konrad-Zerna, A. Apolipoprotein A-IV is an independent predictor of disease activity in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 391–397. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.N.; Chassard, C.; Zimmermann, M.; Muller, P.; Stinca, S.; Lacroix, C. The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutr. Diabetes 2011, 1, e12. [Google Scholar] [CrossRef] [Green Version]
- Markowiak-Kopec, P.; Slizewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Mascolo, N.; Rajendran, V.M.; Binder, H.J. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology 1991, 101, 331–338. [Google Scholar] [CrossRef]
- Goncalves, P.; Martel, F. Butyrate and colorectal cancer: The role of butyrate transport. Curr. Drug Metab. 2013, 14, 994–1008. [Google Scholar] [CrossRef]
- Merezhinskaya, N.; Ogunwuyi, S.A.; Mullick, F.G.; Fishbein, W.N. Presence and localization of three lactic acid transporters (MCT1, -2, and -4) in separated human granulocytes, lymphocytes, and monocytes. J. Histochem. Cytochem. 2004, 52, 1483–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloemen, J.G.; Venema, K.; van de Poll, M.C.; Olde Damink, S.W.; Buurman, W.A.; Dejong, C.H. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 2009, 28, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomare, E.W.; Branch, W.J.; Cummings, J.H. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J. Clin. Investig. 1985, 75, 1448–1454. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]
- Kumari, R.; Ahuja, V.; Paul, J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. 2013, 19, 3404–3414. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef]
- Ulven, T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front. Endocrinol. Lausanne 2012, 3, 111. [Google Scholar] [CrossRef] [Green Version]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Tazoe, H.; Otomo, Y.; Karaki, S.; Kato, I.; Fukami, Y.; Terasaki, M.; Kuwahara, A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed. Res. 2009, 30, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [Green Version]
- Wilkerson, E.M.; Johansson, M.W.; Hebert, A.S.; Westphall, M.S.; Mathur, S.K.; Jarjour, N.N.; Schwantes, E.A.; Mosher, D.F.; Coon, J.J. The Peripheral Blood Eosinophil Proteome. J. Proteome Res. 2016, 15, 1524–1533. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Aronow, B.J.; Rochman, Y.; Rochman, M.; Kc, K.; Dexheimer, P.J.; Putnam, P.; Mukkada, V.; Foote, H.; Rehn, K.; et al. Single-cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J. Clin. Investig. 2019, 129, 2014–2028. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal. Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [Green Version]
- Bolden, J.E.; Peart, M.J.; Johnstone, R.W. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 2006, 5, 769–784. [Google Scholar] [CrossRef]
- Lin, K.T.; Wang, Y.W.; Chen, C.T.; Ho, C.M.; Su, W.H.; Jou, Y.S. HDAC inhibitors augmented cell migration and metastasis through induction of PKCs leading to identification of low toxicity modalities for combination cancer therapy. Clin. Cancer Res. 2012, 18, 4691–4701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Tao, J.; Chen, P.; Chen, L.; Sharma, S.; Wang, G.; Dong, Q. Sodium Butyrate Inhibits Colorectal Cancer Cell Migration by Downregulating Bmi-1 Through Enhanced miR-200c Expression. Mol. Nutr. Food Res. 2018, 62, e1700844. [Google Scholar] [CrossRef]
- Kankaanranta, H.; Janka-Junttila, M.; Ilmarinen-Salo, P.; Ito, K.; Jalonen, U.; Ito, M.; Adcock, I.M.; Moilanen, E.; Zhang, X. Histone deacetylase inhibitors induce apoptosis in human eosinophils and neutrophils. J. Inflamm. Lond. 2010, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, M.; Kotani, J.; Usami, M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 2010, 26, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Y.; He, L.; Wu, L.; Wang, X.; Liu, Z. Effects of the intestinal microbial metabolite butyrate on the development of colorectal cancer. J. Cancer 2018, 9, 2510–2517. [Google Scholar] [CrossRef]
- Toki, S.; Goleniewska, K.; Reiss, S.; Zhou, W.; Newcomb, D.C.; Bloodworth, M.H.; Stier, M.T.; Boyd, K.L.; Polosukhin, V.V.; Subramaniam, S.; et al. The histone deacetylase inhibitor trichostatin A suppresses murine innate allergic inflammation by blocking group 2 innate lymphoid cell (ILC2) activation. Thorax 2016, 71, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H.; Oh, S.W.; Kang, M.S.; Kwon, H.J.; Oh, G.T.; Kim, D.Y. Trichostatin A attenuates airway inflammation in mouse asthma model. Clin. Exp. Allergy 2005, 35, 89–96. [Google Scholar] [CrossRef]
- Saito, H.; Bourinbaiar, A.; Ginsburg, M.; Minato, K.; Ceresi, E.; Yamada, K.; Machover, D.; Breard, J.; Mathe, G. Establishment and characterization of a new human eosinophilic leukemia cell line. Blood 1985, 66, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Fischkoff, S.A.; Condon, M.E. Switch in differentiative response to maturation inducers of human promyelocytic leukemia cells by prior exposure to alkaline conditions. Cancer Res. 1985, 45, 2065–2069. [Google Scholar]
- Beekman, J.M.; Verhagen, L.P.; Geijsen, N.; Coffer, P.J. Regulation of myelopoiesis through syntenin-mediated modulation of IL-5 receptor output. Blood 2009, 114, 3917–3927. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K.; Takahashi, A.; Kaneko, M.; Sugeno, H.; Hirasawa, N.; Hong, J.; Zee, O.; Ohuchi, K. Differentiation of eosinophilic leukemia EoL-1 cells into eosinophils induced by histone deacetylase inhibitors. Life Sci. 2007, 80, 1213–1220. [Google Scholar] [CrossRef]
- Theiler, A.; Barnthaler, T.; Platzer, W.; Richtig, G.; Peinhaupt, M.; Rittchen, S.; Kargl, J.; Ulven, T.; Marsh, L.M.; Marsche, G.; et al. Butyrate ameliorates allergic airway inflammation by limiting eosinophil trafficking and survival. J. Allergy Clin. Immunol. 2019, 144, 764–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, T.; Sichelstiel, A.; Schar, C.; Yadava, K.; Burki, K.; Cahenzli, J.; McCoy, K.; Marsland, B.J.; Harris, N.L. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am. J. Respir. Crit. Care Med. 2011, 184, 198–205. [Google Scholar] [CrossRef]
- Hogenkamp, A.; Thijssen, S.; van Vlies, N.; Garssen, J. Supplementing pregnant mice with a specific mixture of nondigestible oligosaccharides reduces symptoms of allergic asthma in male offspring. J. Nutr. 2015, 145, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Verheijden, K.A.; Willemsen, L.E.; Braber, S.; Leusink-Muis, T.; Delsing, D.J.; Garssen, J.; Kraneveld, A.D.; Folkerts, G. Dietary galacto-oligosaccharides prevent airway eosinophilia and hyperresponsiveness in a murine house dust mite-induced asthma model. Respir. Res. 2015, 16, 17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Shi, L.; Pang, W.; Liu, W.; Li, J.; Wang, H.; Shi, G. Dietary Fiber Intake Regulates Intestinal Microflora and Inhibits Ovalbumin-Induced Allergic Airway Inflammation in a Mouse Model. PLoS ONE 2016, 11, e0147778. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Shi, L.; Pang, W.; Wang, X.; Li, J.; Wang, H.; Shi, G. Is a high-fiber diet able to influence ovalbumin-induced allergic airway inflammation in a mouse model? Allergy Rhinol. Provid. 2016, 7, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Cait, A.; Hughes, M.R.; Antignano, F.; Cait, J.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal. Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef]
- Thio, C.L.; Chi, P.Y.; Lai, A.C.; Chang, Y.J. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J. Allergy Clin. Immunol. 2018, 142, 1867–1883. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G.; Wang, B.; Shafiei Jahani, P.; Hurrell, B.P.; Banie, H.; Aleman Muench, G.R.; Maazi, H.; Helou, D.G.; Howard, E.; Galle-Treger, L.; et al. Dietary Fiber-Induced Microbial Short Chain Fatty Acids Suppress ILC2-Dependent Airway Inflammation. Front. Immunol. 2019, 10, 2051. [Google Scholar] [CrossRef] [Green Version]
- Onishi, N.; Kawamoto, S.; Nishimura, M.; Nakano, T.; Aki, T.; Shigeta, S.; Shimizu, H.; Hashimoto, K.; Ono, K. A new immunomodulatory function of low-viscous konjac glucomannan with a small particle size: Its oral intake suppresses spontaneously occurring dermatitis in NC/Nga mice. Int. Arch. Allergy Immunol. 2005, 136, 258–265. [Google Scholar] [CrossRef]
- Onishi, N.; Kawamoto, S.; Nishimura, M.; Nakano, T.; Aki, T.; Shigeta, S.; Shimizu, H.; Hashimoto, K.; Ono, K. The ability of konjac-glucomannan to suppress spontaneously occurring dermatitis in NC/Nga mice depends upon the particle size. Biofactors 2004, 21, 163–166. [Google Scholar] [CrossRef]
- Fujiwara, R.; Takemura, N.; Watanabe, J.; Sonoyama, K. Maternal consumption of fructo-oligosaccharide diminishes the severity of skin inflammation in offspring of NC/Nga mice. Br. J. Nutr. 2010, 103, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, L.J.; Oh, E.; Cho, C.; Kwon, H.; Lee, C.G.; Jeon, J.; Lee, H.; Choi, S.; Han, S.J.; Nam, J.; et al. 3’-Sialyllactose prebiotics prevents skin inflammation via regulatory T cell differentiation in atopic dermatitis mouse models. Sci. Rep. 2020, 10, 5603. [Google Scholar] [CrossRef] [PubMed]
- Hino, T.; Suzuki, K.; Ohkawara, S.; Miwa, T.; Asanuma, N. Effects of oral administration of Butyrivibrio fibrisolvens MDT-1 on the development and healing of atopic dermatitis in NC/Nga mice. Eur. J. Dermatol. 2012, 22, 211–217. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.H.; Kim, I.S.; Yu, D.Y.; Kim, S.C.; Lee, S.H.; Lee, S.S.; Yun, C.H.; Choi, I.S.; Cho, K.K. Anti-Inflammatory Effects of a Mixture of Lactic Acid Bacteria and Sodium Butyrate in Atopic Dermatitis Murine Model. J. Med. Food 2018, 21, 716–725. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Kim, W. Cream Cheese-Derived Lactococcus chungangensis CAU 28 Modulates the Gut Microbiota and Alleviates Atopic Dermatitis in BALB/c Mice. Sci. Rep. 2019, 9, 446. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Li, L.; Zhao, J.; Zhang, H.; Lee, Y.K.; Lu, W.; Chen, W. Bifidobacteria adolescentis regulated immune responses and gut microbial composition to alleviate DNFB-induced atopic dermatitis in mice. Eur. J. Nutr. 2020, 59, 3069–3081. [Google Scholar] [CrossRef]
- Kang, J.; Im, D.S. FFA2 Activation Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in Mice. Biomol. Ther. Seoul 2020, 28, 267–271. [Google Scholar] [CrossRef]
- Nagy-Szakal, D.; Hollister, E.B.; Luna, R.A.; Szigeti, R.; Tatevian, N.; Smith, C.W.; Versalovic, J.; Kellermayer, R. Cellulose supplementation early in life ameliorates colitis in adult mice. PLoS ONE 2013, 8, e56685. [Google Scholar] [CrossRef]
- Islam, J.; Koseki, T.; Watanabe, K.; Ardiansyah; Budijanto, S.; Oikawa, A.; Alauddin, M.; Goto, T.; Aso, H.; Komai, M.; et al. Dietary Supplementation of Fermented Rice Bran Effectively Alleviates Dextran Sodium Sulfate-Induced Colitis in Mice. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Su, L.; Mao, C.; Wang, X.; Li, L.; Tong, H.; Mao, J.; Ji, D.; Lu, T.; Hao, M.; Huang, Z.; et al. The Anti-colitis Effect of Schisandra chinensis Polysaccharide Is Associated with the Regulation of the Composition and Metabolism of Gut Microbiota. Front. Cell Infect. Microbiol. 2020, 10, 519479. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, W.; Deng, Q.; Huang, Q.; Wang, X.; Yang, C.; Huang, F. Flaxseed oligosaccharides alleviate DSS-induced colitis through modulation of gut microbiota and repair of the intestinal barrier in mice. Food Funct. 2020, 11, 8077–8088. [Google Scholar] [CrossRef]
- Tang, S.; Liu, W.; Zhao, Q.; Li, K.; Zhu, J.; Yao, W.; Gao, X. Combination of polysaccharides from Astragalus membranaceus and Codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J. Ethnopharmacol. 2021, 264, 113280. [Google Scholar] [CrossRef]
- Zou, Q.; Zhang, X.; Liu, X.; Li, Y.; Tan, Q.; Dan, Q.; Yuan, T.; Liu, X.; Liu, R.H.; Liu, Z. Ficus carica polysaccharide attenuates DSS-induced ulcerative colitis in C57BL/6 mice. Food Funct. 2020, 11, 6666–6679. [Google Scholar] [CrossRef]
- Zha, Z.; Lv, Y.; Tang, H.; Li, T.; Miao, Y.; Cheng, J.; Wang, G.; Tan, Y.; Zhu, Y.; Xing, X.; et al. An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis. Int. J. Biol. Macromol. 2020, 156, 1217–1233. [Google Scholar] [CrossRef]
- Shao, S.; Wang, D.; Zheng, W.; Li, X.; Zhang, H.; Zhao, D.; Wang, M. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. Int. Immunopharmacol. 2019, 71, 411–422. [Google Scholar] [CrossRef]
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 2019, 9, 12294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.K.; Han, D.H.; Jang, Y.J.; Park, S.; Jang, S.J.; Lee, G.; Han, H.S.; Ko, G. Alleviation of DSS-induced colitis via Lactobacillus acidophilus treatment in mice. Food Funct. 2020. [Google Scholar] [CrossRef]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Shastri, S.; Southam, B.; Eri, R.; Stanley, R. Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD. Nutrients 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Q.; Zhang, Q.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. The synergistic effect of Lactobacillus plantarum CCFM242 and zinc on ulcerative colitis through modulating intestinal homeostasis. Food Funct. 2019, 10, 6147–6156. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, X.; Hao, Y.; Ding, J.; Shen, J.; Xue, Z.; Qi, W.; Li, Z.; Song, Y.; Zhang, T.; et al. Protective effects of a novel probiotic strain, Lactococcus lactis ML2018, in colitis: In vivo and in vitro evidence. Food Funct. 2019, 10, 1132–1145. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Nishida, A.; Ohno, M.; Imaeda, H.; Shimada, T.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Enterococcus durans TN-3 Induces Regulatory T Cells and Suppresses the Development of Dextran Sulfate Sodium (DSS)-Induced Experimental Colitis. PLoS ONE 2016, 11, e0159705. [Google Scholar] [CrossRef]
- Fujiwara, M.; Kaneko, T.; Iwana, H.; Taketomo, N.; Tsunoo, H.; Kanno, J.; Ohkusa, T.; Okayasu, I. Inhibitory effects of Bifidobacterium longum on experimental ulcerative colitis induced in mice by synthetic dextran sulfate sodium. Digestion 2003, 67, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.S.; Lee, J.Y.; Kim, Y.T.; Kim, S.H.; Lee, J.H. Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes 2020, 12, 1785803. [Google Scholar] [CrossRef]
- Branning, C.E.; Nyman, M.E. Malt in combination with Lactobacillus rhamnosus increases concentrations of butyric acid in the distal colon and serum in rats compared with other barley products but decreases viable counts of cecal bifidobacteria. J. Nutr. 2011, 141, 101–107. [Google Scholar] [CrossRef]
- Mishiro, T.; Kusunoki, R.; Otani, A.; Ansary, M.M.; Tongu, M.; Harashima, N.; Yamada, T.; Sato, S.; Amano, Y.; Itoh, K.; et al. Butyric acid attenuates intestinal inflammation in murine DSS-induced colitis model via milk fat globule-EGF factor 8. Lab. Investig. 2013, 93, 834–843. [Google Scholar] [CrossRef]
- Simeoli, R.; Mattace Raso, G.; Pirozzi, C.; Lama, A.; Santoro, A.; Russo, R.; Montero-Melendez, T.; Berni Canani, R.; Calignano, A.; Perretti, M.; et al. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 2017, 174, 1484–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Xu, Q.; Sun, L.; Ye, Y.; Ji, G. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J. Nutr. Biochem. 2018, 57, 103–109. [Google Scholar] [CrossRef]
- Holvoet, S.; Doucet-Ladeveze, R.; Perrot, M.; Barretto, C.; Nutten, S.; Blanchard, C. Beneficial effect of Lactococcus lactis NCC 2287 in a murine model of eosinophilic esophagitis. Allergy 2016, 71, 1753–1761. [Google Scholar] [CrossRef]
- Garcia-Meseguer, M.J.; Delicado-Soria, A.; Serrano-Urrea, R. Fiber Patterns in Young Adults Living in Different Environments (USA, Spain, and Tunisia). Anthropometric and Lifestyle Characteristics. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkitt, D.P.; Walker, A.R.; Painter, N.S. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet 1972, 2, 1408–1412. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Cait, A.; Cardenas, E.; Dimitriu, P.A.; Amenyogbe, N.; Dai, D.; Cait, J.; Sbihi, H.; Stiemsma, L.; Subbarao, P.; Mandhane, P.J.; et al. Reduced genetic potential for butyrate fermentation in the gut microbiome of infants who develop allergic sensitization. J. Allergy Clin. Immunol. 2019, 144, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Ivashkin, V.; Zolnikova, O.; Potskherashvili, N.; Trukhmanov, A.; Kokina, N.; Dzhakhaya, N.; Sedova, A.; Bueverova, E. Metabolic activity of intestinal microflora in patients with bronchial asthma. Clin. Pract. 2019, 9, 1126. [Google Scholar] [CrossRef] [Green Version]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Berthon, B.S.; Macdonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology 2013, 18, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Halnes, I.; Baines, K.J.; Berthon, B.S.; MacDonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLoughlin, R.; Berthon, B.S.; Rogers, G.B.; Baines, K.J.; Leong, L.E.X.; Gibson, P.G.; Williams, E.J.; Wood, L.G. Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine 2019, 46, 473–485. [Google Scholar] [CrossRef]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penders, J.; Gerhold, K.; Stobberingh, E.E.; Thijs, C.; Zimmermann, K.; Lau, S.; Hamelmann, E. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 2013, 132, 601–607. [Google Scholar] [CrossRef]
- Petersen, E.B.M.; Skov, L.; Thyssen, J.P.; Jensen, P. Role of the Gut Microbiota in Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2019, 99, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Boehm, G.; Wienz, F.; Stahl, B.; Bertino, E. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J. Biol. Regul. Homeost. Agents 2012, 26 (Suppl. 3), 49–59. [Google Scholar]
- Gruber, C.; van Stuijvenberg, M.; Mosca, F.; Moro, G.; Chirico, G.; Braegger, C.P.; Riedler, J.; Boehm, G.; Wahn, U.; Group, M.W. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J. Allergy Clin. Immunol. 2010, 126, 791–797. [Google Scholar] [CrossRef]
- Shibata, R.; Kimura, M.; Takahashi, H.; Mikami, K.; Aiba, Y.; Takeda, H.; Koga, Y. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clin. Exp. Allergy 2009, 39, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Van Hoffen, E.; Ruiter, B.; Faber, J.; M’Rabet, L.; Knol, E.F.; Stahl, B.; Arslanoglu, S.; Moro, G.; Boehm, G.; Garssen, J. A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Allergy 2009, 64, 484–487. [Google Scholar] [CrossRef]
- Makrgeorgou, A.; Leonardi-Bee, J.; Bath-Hextall, F.J.; Murrell, D.F.; Tang, M.L.; Roberts, A.; Boyle, R.J. Probiotics for treating eczema. Cochrane Database Syst. Rev. 2018, 11, CD006135. [Google Scholar] [CrossRef] [PubMed]
- Nylund, L.; Nermes, M.; Isolauri, E.; Salminen, S.; de Vos, W.M.; Satokari, R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 2015, 70, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Aranami, A.; Ishige, A.; Watanabe, K.; Benno, Y. LKM512 yogurt consumption improves the intestinal environment and induces the T-helper type 1 cytokine in adult patients with intractable atopic dermatitis. Clin. Exp. Allergy 2007, 37, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Traisaeng, S.; Herr, D.R.; Kao, H.J.; Chuang, T.H.; Huang, C.M. A Derivative of Butyric Acid, the Fermentation Metabolite of Staphylococcus epidermidis, Inhibits the Growth of a Staphylococcus aureus Strain Isolated from Atopic Dermatitis Patients. Toxins 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Roessler, A.; Forssten, S.D.; Glei, M.; Ouwehand, A.C.; Jahreis, G. The effect of probiotics on faecal microbiota and genotoxic activity of faecal water in patients with atopic dermatitis: A randomized, placebo-controlled study. Clin. Nutr. 2012, 31, 22–29. [Google Scholar] [CrossRef]
- Burkitt, D.P. Epidemiology of large bowel disease: The role of fibre. Proc. Nutr. Soc. 1973, 32, 145–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamba, T.; Kanauchi, O.; Andoh, A.; Fujiyama, Y. A new prebiotic from germinated barley for nutraceutical treatment of ulcerative colitis. J. Gastroenterol. Hepatol. 2002, 17, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanauchi, O.; Iwanaga, T.; Mitsuyama, K. Germinated barley foodstuff feeding. A novel neutraceutical therapeutic strategy for ulcerative colitis. Digestion 2001, 63 (Suppl. 1), 60–67. [Google Scholar] [CrossRef]
- Komiyama, Y.; Mitsuyama, K.; Masuda, J.; Yamasaki, H.; Takedatsu, H.; Andoh, A.; Tsuruta, O.; Fukuda, M.; Kanauchi, O. Prebiotic treatment in experimental colitis reduces the risk of colitic cancer. J. Gastroenterol. Hepatol. 2011, 26, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernandez, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Zallot, C.; Quilliot, D.; Chevaux, J.B.; Peyrin-Biroulet, C.; Gueant-Rodriguez, R.M.; Freling, E.; Collet-Fenetrier, B.; Williet, N.; Ziegler, O.; Bigard, M.A.; et al. Dietary beliefs and behavior among inflammatory bowel disease patients. Inflamm. Bowel Dis. 2013, 19, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Owczarek, D.; Rodacki, T.; Domagala-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Peng, L.; Zhong, Y.; Wang, A.; Jiang, Z. Probiotics combined with aminosalicylic acid affiliates remission of ulcerative colitis: A meta-analysis of randomized controlled trial. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J. Cell Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef]
- Asto, E.; Mendez, I.; Audivert, S.; Farran-Codina, A.; Espadaler, J. The Efficacy of Probiotics, Prebiotic Inulin-Type Fructans, and Synbiotics in Human Ulcerative Colitis: A Systematic Review and Meta-Analysis. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henn, M.R.; O’Brien, E.J.; Diao, L.; Feagan, B.G.; Sandborn, W.J.; Huttenhower, C.; Wortman, J.R.; McGovern, B.H.; Wang-Weigand, S.; Lichter, D.I.; et al. A Phase 1b Safety Study of SER-287, a Spore-Based Microbiome Therapeutic, for Active Mild to Moderate Ulcerative Colitis. Gastroenterology 2021, 160, 115–127. [Google Scholar] [CrossRef]
- Curro, D.; Ianiro, G.; Pecere, S.; Bibbo, S.; Cammarota, G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br. J. Pharmacol. 2017, 174, 1426–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treem, W.R.; Ahsan, N.; Shoup, M.; Hyams, J.S. Fecal short-chain fatty acids in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 159–164. [Google Scholar] [CrossRef]
- Kim, Y.I. Short-chain fatty acids in ulcerative colitis. Nutr. Rev. 1998, 56, 17–24. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, G.; Mazzacca, G. Short-chain fatty acid in the human colon. Relation to inflammatory bowel diseases and colon cancer. Adv. Exp. Med. Biol. 1999, 472, 149–158. [Google Scholar] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Bjerrum, J.T.; Wang, Y.; Hao, F.; Coskun, M.; Ludwig, C.; Gunther, U.; Nielsen, O.H. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics 2015, 11, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Ashton, J.J.; Colquhoun, C.M.; Cleary, D.W.; Coelho, T.; Haggarty, R.; Mulder, I.; Batra, A.; Afzal, N.A.; Beattie, R.M.; Scott, K.P.; et al. 16S sequencing and functional analysis of the fecal microbiome during treatment of newly diagnosed pediatric inflammatory bowel disease. Med. Baltim. 2017, 96, e7347. [Google Scholar] [CrossRef]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Breuer, R.I.; Buto, S.K.; Christ, M.L.; Bean, J.; Vernia, P.; Paoluzi, P.; Di Paolo, M.C.; Caprilli, R. Rectal irrigation with short-chain fatty acids for distal ulcerative colitis. Preliminary report. Dig. Dis Sci. 1991, 36, 185–187. [Google Scholar] [CrossRef]
- Breuer, R.I.; Soergel, K.H.; Lashner, B.A.; Christ, M.L.; Hanauer, S.B.; Vanagunas, A.; Harig, J.M.; Keshavarzian, A.; Robinson, M.; Sellin, J.H.; et al. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: A randomised, placebo controlled trial. Gut 1997, 40, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patz, J.; Jacobsohn, W.Z.; Gottschalk-Sabag, S.; Zeides, S.; Braverman, D.Z. Treatment of refractory distal ulcerative colitis with short chain fatty acid enemas. Am. J. Gastroenterol. 1996, 91, 731–734. [Google Scholar]
- Scheppach, W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas. A placebo-controlled trial. German-Austrian SCFA Study Group. Dig. Dis. Sci. 1996, 41, 2254–2259. [Google Scholar] [CrossRef]
- Steinhart, A.H.; Hiruki, T.; Brzezinski, A.; Baker, J.P. Treatment of left-sided ulcerative colitis with butyrate enemas: A controlled trial. Aliment. Pharmacol. Ther. 1996, 10, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Assisi, R.F.; GISDI Study Group. Combined butyric acid/mesalazine treatment in ulcerative colitis with mild-moderate activity. Results of a multicentre pilot study. Minerva Gastroenterol. Dietol. 2008, 54, 231–238. [Google Scholar]
- Magnusson, M.K.; Isaksson, S.; Ohman, L. The Anti-inflammatory Immune Regulation Induced by Butyrate Is Impaired in Inflamed Intestinal Mucosa from Patients with Ulcerative Colitis. Inflammation 2020, 43, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Corning, B.; Copland, A.P.; Frye, J.W. The Esophageal Microbiome in Health and Disease. Curr. Gastroenterol. Rep. 2018, 20, 39. [Google Scholar] [CrossRef]
- Choksi, Y.; Vaezi, M.F. Preliminary esophageal microbiome studies prompt important scientific questions. Clin. Transl. Gastroenterol. 2018, 9, 156. [Google Scholar] [CrossRef]
- Dellon, E.S. The Esophageal Microbiome in Eosinophilic Esophagitis. Gastroenterology 2016, 151, 364–365. [Google Scholar] [CrossRef]
- Hunt, R.H.; Yaghoobi, M. The Esophageal and Gastric Microbiome in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 121–141. [Google Scholar] [CrossRef]
- Harris, J.K.; Fang, R.; Wagner, B.D.; Choe, H.N.; Kelly, C.J.; Schroeder, S.; Moore, W.; Stevens, M.J.; Yeckes, A.; Amsden, K.; et al. Esophageal microbiome in eosinophilic esophagitis. PLoS ONE 2015, 10, e0128346. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, S.K. Exploring Esophageal Microbiomes in Esophageal Diseases: A Systematic Review. J. Neurogastroenterol. Motil. 2020, 26, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.J.; Hoffmann, C.; Muir, A.B.; Dods, K.K.; Spergel, J.M.; Bushman, F.D.; Wang, M.L. Inflammation-associated microbiota in pediatric eosinophilic esophagitis. Microbiome 2015, 3, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mennini, M.; Tambucci, R.; Riccardi, C.; Rea, F.; De Angelis, P.; Fiocchi, A.; Assa’ad, A. Eosinophilic Esophagitis and Microbiota: State of the Art. Front. Immunol. 2021, 12, 595762. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Dellon, E.S.; McCoy, A.N.; Sun, S.; Jensen, E.T.; Fodor, A.A.; Keku, T.O. Lack of association of the esophageal microbiome in adults with eosinophilic esophagitis compared with non-EoE controls. J. Gastrointestin. Liver Dis. 2021, 30, 17–24. [Google Scholar] [CrossRef]
- Nobel, Y.R.; Snider, E.J.; Compres, G.; Freedberg, D.E.; Khiabanian, H.; Lightdale, C.J.; Toussaint, N.C.; Abrams, J.A. Increasing Dietary Fiber Intake Is Associated with a Distinct Esophageal Microbiome. Clin. Transl. Gastroenterol. 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.C.; Johnson, S.; Geno, D.M.; Lekatz, H.R.; Lavey, C.; Alexander, J.A.; Chen, J.; Katzka, D.A. A decreased abundance of clostridia characterizes the gut microbiota in eosinophilic esophagitis. Physiol. Rep. 2019, 7, e14261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckalbar, W.L.; Erle, D.J. Singling out Th2 cells in eosinophilic esophagitis. J. Clin. Investig. 2019, 129, 1830–1832. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturm, E.M.; Knuplez, E.; Marsche, G. Role of Short Chain Fatty Acids and Apolipoproteins in the Regulation of Eosinophilia-Associated Diseases. Int. J. Mol. Sci. 2021, 22, 4377. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094377
Sturm EM, Knuplez E, Marsche G. Role of Short Chain Fatty Acids and Apolipoproteins in the Regulation of Eosinophilia-Associated Diseases. International Journal of Molecular Sciences. 2021; 22(9):4377. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094377
Chicago/Turabian StyleSturm, Eva Maria, Eva Knuplez, and Gunther Marsche. 2021. "Role of Short Chain Fatty Acids and Apolipoproteins in the Regulation of Eosinophilia-Associated Diseases" International Journal of Molecular Sciences 22, no. 9: 4377. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094377