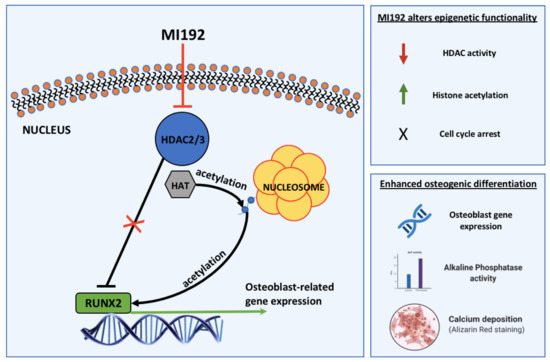
The Selective Histone Deacetylase Inhibitor MI192 Enhances the Osteogenic Differentiation Efficacy of Human Dental Pulp Stromal Cells
Abstract
:1. Introduction
2. Results
2.1. MI192 Affects hDPSCs Morphology and Viability
2.2. MI192 Altered hDPSCs HDAC Activity and Histone Acetylation
2.3. MI192 Halts hDPSCs Cell Cycle Progression
2.3.1. G0/G1 Phase
2.3.2. G2/M Phase
2.3.3. S Phase
2.4. MI192 Exhibited a Dose-Dependent Effect on hDPSCs Alkaline Phosphtase (ALP) Activity
2.5. MI192 Promoted the Osteogenic Gene and Protein Expression in hDPSCs
2.6. MI192 Enhanced hDPSCs Extracellular Matrix Mineralisation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Assay and Morphological Assessment
4.3. DNA Quantification
4.4. HDAC Activity
4.5. Histone Acetylation
4.6. Cell Cycle Analysis
4.7. MI192 Pre-Treatment and Osteogenic Induction
4.8. Alkaline Phosphatase Specific Activity (ALPSA) Assay
4.9. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-qPCR)
4.10. In-Cell Western (ICW) Assay
4.11. Alizarin Red Staining and Quantification
4.12. Von Kossa Staining
4.13. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKee, M.D. Management of segmental bony defects: The role of osteoconductive orthobiologics. J. Am. Acad. Orthop. Surg. 2006, 14, S163–S167. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, J. Repair of bone defect by using vascular bundle implantation combined with Runx II gene-transfected adipose-derived stem cells and a biodegradable matrix. Cell Tissue Res. 2013, 352, 561–571. [Google Scholar] [CrossRef]
- Chance-Larsen, K.; Backhouse, M.R.; Collier, R.; Wright, C.; Gosling, S.; Harden, B.; Marsh, S.; Kay, P.; Wyles, H.; Erwin, J.; et al. Developing a national musculoskeletal core capabilities framework for first point of contact practitioners. Rheumatol. Adv. Pract. 2019, 3, rkz036. [Google Scholar] [CrossRef] [Green Version]
- Mathoulin, C.; Gras, M.; Roukos, S. Vascularized bone grafting from the volar distal radius for carpal bones reconstruction. Chir. Main 2010, 29, S65–S76. [Google Scholar] [CrossRef]
- Solheim, E.; Pinholt, E.M.; Talsnes, O.; Larsen, T.B.; Kirkeby, O.J. Revascularisation of fresh compared with demineralised bone grafts in rats. Scand. J. Plast. Reconstr. Surg. Hand. Surg. 2001, 35, 113–116. [Google Scholar] [CrossRef]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The use of bone-graft substitutes in large bone defects: Any specific needs? Injury 2011, 42, S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Djouad, F.; Guerit, D.; Marie, M.; Toupet, K.; Jorgensen, C.; Noel, D. Mesenchymal stem cells: New insights into bone regenerative applications. J. Biomater. Tissue Eng. 2012, 2, 14–28. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Ji, K.; Kirkham, J.; Yan, Y.; Boccaccini, A.R.; Kellett, M.; Jin, Y.; Yang, X.B. Bone tissue engineering by using a combination of polymer/Bioglass composites with human adipose-derived stem cells. Cell Tissue Res. 2014, 356, 97–107. [Google Scholar] [CrossRef]
- Asatrian, G.; Pham, D.; Hardy, W.R.; James, A.W.; Peault, B. Stem cell technology for bone regeneration: Current status and potential applications. Stem Cells Cloning Adv. Appl. 2015, 8, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.T.; Chen, C.T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J. Stem cells 2014, 6, 288–295. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, R.; Yang, X.B.; Newby, P.J.; Boccaccini, A.R.; Kirkham, J. Osteogenic differentiation of human dental pulp stromal cells on 45s5 bioglass (R) based scaffolds in vitro and in vivo. Tissue Eng. Part A 2013, 19, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laino, G.; d’Aquino, R.; Graziano, A.; Lanza, V.; Carinci, F.; Naro, F.; Pirozzi, G.; Papaccio, G. A new population of human adult dental pulp stem cells: A useful source of living autologous fibrous bone tissue (LAB). J. Bone Miner. Res. 2005, 20, 1394–1402. [Google Scholar] [CrossRef]
- Ko, J.Y.; Park, S.; Im, G.I. Osteogenesis from human induced pluripotent stem cells: An in vitro and in vivo comparison with mesenchymal stem cells. Stem Cells Dev. 2014, 23, 1788–1797. [Google Scholar] [CrossRef]
- Moschidou, D.; Mukherjee, S.; Blundell, M.P.; Drews, K.; Jones, G.N.; Abdulrazzak, H.; Nowakowska, B.; Phoolchund, A.; Lay, K.; Ramasamy, T.S.; et al. Valproic acid confers functional pluripotency to human amniotic fluid stem cells in a transgene-free approach. Mol. Ther. 2012, 20, 1953–1967. [Google Scholar] [CrossRef] [Green Version]
- Tollervey, J.R.; Lunyak, V.V. Epigenetics Judge, jury and executioner of stem cell fate. Epigenetics-Us 2012, 7, 823–840. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, L.; Yang, X.B. Harnessing the HDAC-histone deacetylase enzymes, inhibitors and how these can be utilised in tissue engineering. Int. J. Oral Sci. 2019, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Bruyer, A.; Maes, K.; Herviou, L.; Kassambara, A.; Seckinger, A.; Cartron, G.; Reme, T.; Robert, N.; Requirand, G.; Boireau, S.; et al. DNMTi/HDACi combined epigenetic targeted treatment induces reprogramming of myeloma cells in the direction of normal plasma cells. Br. J. Cancer 2018, 118, 1062–1073. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.H.; Park, H.T.; Kim, Y.J.; Bae, Y.C.; Suh, K.T.; Jung, J.S. Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. J. Cell. Biochem. 2005, 96, 533–542. [Google Scholar] [CrossRef]
- Xu, Y.; Hammerick, K.E.; James, A.W.; Carre, A.L.; Leucht, P.; Giaccia, A.J.; Longaker, M.T. Inhibition of histone deacetylase activity in reduced oxygen environment enhances the osteogenesis of mouse adipose-derived stromal cells. Tissue Eng. Part A 2009, 15, 3697–3707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; De Veirman, K.; Evans, H.; Santini, G.C.; Vande Broek, I.; Leleu, X.; De Becker, A.; Van Camp, B.; Croucher, P.; Vanderkerken, K.; et al. Effect of the HDAC inhibitor vorinostat on the osteogenic differentiation of mesenchymal stem cells in vitro and bone formation in vivo. Acta Pharmacol. Sin. 2013, 34, 699–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakouros, D.; Gronthos, S. Epigenetic regulation of bone marrow stem cell aging: Revealing epigenetic signatures associated with hematopoietic and mesenchymal stem cell aging. Aging Dis. 2019, 10, 174–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.S.; Zhang, R.; Wang, G.; Zhang, Y.F. The development prospection of HDAC inhibitors as a potential therapeutic direction in Alzheimer’s disease. Transl. Neurodegener. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.M.; Kahler, R.A.; Li, X.D.; Westendorf, J.J. Histone deacetylase 3 interacts with RUNX2 to repress the osteocalcin promoter and regulate osteoblast differentiation. J. Biol. Chem. 2004, 279, 41998–42007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissinot, M.; Inman, M.; Hempshall, A.; James, S.R.; Gill, J.H.; Selby, P.; Bowen, D.T.; Grigg, R.; Cockerill, P.N. Induction of differentiation and apoptosis in leukaemic cell lines by the novel benzamide family histone deacetylase 2 and 3 inhibitor MI-192. Leuk. Res. 2012, 36, 1304–1310. [Google Scholar] [CrossRef]
- Gillespie, J.; Savic, S.; Wong, C.; Hempshall, A.; Inman, M.; Emery, P.; Grigg, R.; McDermott, M.F. Histone deacetylases are dysregulated in rheumatoid arthritis and a novel histone deacetylase 3-selective inhibitor reduces interleukin-6 production by peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Rheum-Us 2012, 64, 418–422. [Google Scholar] [CrossRef]
- Lawlor, L. The Effect of HDAC Inhibitor MI192 on Stem Cell Behaviour-The Potential of Utilising MI192 for Bone Tissue Engineering. Ph.D. Thesis, University of Leeds, Leeds, UK, 2016. [Google Scholar]
- de Boer, J.; Licht, R.; Bongers, M.; van der Klundert, T.; Arends, R.; van Blitterswijk, C. Inhibition of histone acetylation as a tool in bone tissue engineering. Tissue Eng. 2006, 12, 2927–2937. [Google Scholar] [CrossRef]
- Paino, F.; La Noce, M.; Tirino, V.; Naddeo, P.; Desiderio, V.; Pirozzi, G.; De Rosa, A.; Laino, L.; Altucci, L.; Papaccio, G. Histone deacetylase inhibition with valproic acid downregulates osteocalcin gene expression in human dental pulp stem cells and osteoblasts: Evidence for hdac2 involvement. Stem Cells 2014, 32, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Jonason, J.H.; Xiao, G.; Zhang, M.; Xing, L.; Chen, D. Post-translational regulation of runx2 in bone and cartilage. J. Dent. Res. 2009, 88, 693–703. [Google Scholar] [CrossRef]
- Lee, E.C.; Kim, Y.M.; Lim, H.M.; Ki, G.E.; Seo, Y.K. The Histone Deacetylase Inhibitor (MS-275) Promotes Differentiation of Human Dental Pulp Stem Cells into Odontoblast-Like Cells Independent of the MAPK Signaling System. Int. J. Mol. Sci. 2020, 21, 5771. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kim, T.H.; Ahn, M.Y.; Lee, J.; Jung, J.H.; Choi, W.S.; Lee, B.M.; Yoon, K.S.; Yoon, S.; Kim, H.S. Sirtinol, a class III HDAC inhibitor, induces apoptotic and autophagic cell death in MCF-7 human breast cancer cells. Int. J. Oncol. 2012, 41, 1101–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Bernardo, G.; Squillaro, T.; Dell’Aversana, C.; Miceli, M.; Cipollaro, M.; Cascino, A.; Altucci, L.; Galderisi, U. Histone deacetylase inhibitors promote apoptosis and senescence in human mesenchymal stem cells. Stem Cells Dev. 2009, 18, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Matsuyama, A.; Komatsu, Y.; Nishino, N. From discovery to the coming generation of histone deacetylase inhibitors. Curr. Med. Chem. 2003, 10, 2351–2358. [Google Scholar] [CrossRef]
- Zhu, J.X.; Gu, J.H.; Ma, J.; Xu, Z.X.; Tao, H.R. Histone deacetylase inhibitors repress chondrosarcoma cell proliferation. J. BUON 2015, 20, 269–274. [Google Scholar]
- Yamaguchi, T.; Cubizolles, F.; Zhang, Y.; Reichert, N.; Kohler, H.; Seiser, C.; Matthias, P. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Gene Dev. 2010, 24, 455–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.D.; Hsieh, J. HDAC3 controls gap 2/mitosis progression in adult neural stem/progenitor cells by regulating CDK1 levels. Proc. Natl. Acad. Sci. USA 2014, 111, 13541–13546. [Google Scholar] [CrossRef] [Green Version]
- Shafa, M.; Krawetz, R.; Rancourt, D.E. Returning to the stem state: Epigenetics of recapitulating pre-differentiation chromatin structure. Bioessays 2010, 32, 791–799. [Google Scholar] [CrossRef]
- Bacon, T.; Seiler, C.; Wolny, M.; Hughes, R.; Watson, P.; Schwabe, J.; Grigg, R.; Peckham, M. Histone deacetylase 3 indirectly modulates tubulin acetylation. Biochem. J. 2015, 472, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Huynh, N.C.; Everts, V.; Ampornaramveth, R.S. Histone deacetylases and their roles in mineralized tissue regeneration. Bone Rep. 2017, 7, 33–40. [Google Scholar] [CrossRef]
- Bae, H.S.; Yoon, W.J.; Cho, Y.D.; Islam, R.; Shin, H.R.; Kim, B.S.; Lim, J.M.; Seo, M.S.; Cho, S.A.; Choi, K.Y.; et al. An HDAC inhibitor, entinostat/MS-275, partially prevents delayed cranial suture closure in heterozygous RUNX2 null mice. J. Bone Miner. Res. 2017, 32, 951–961. [Google Scholar] [CrossRef] [Green Version]
- Jeon, E.J.; Lee, K.Y.; Choi, N.S.; Lee, M.H.; Kim, H.N.; Jin, Y.H.; Ryoo, H.M.; Choi, J.Y.; Yoshida, M.; Nishino, N.; et al. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J. Biol. Chem. 2006, 281, 16502–16511. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, T.M.; Nair, A.K.; Staggs, R.; Lamblin, A.F.; Westendorf, J.J. Gene profile analysis of osteoblast genes differentially regulated by histone deacetylase inhibitors. BMC Genom. 2007, 8, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phimphilai, M.; Zhoa, Z.R.; Boules, H.; Roca, H.; Franceschi, R.T. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Miner. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.Q.; Zhang, X.; Dai, L.H.; Zhu, J.X.; Jia, Z.Q.; Wang, W.P.; Zhou, C.Y.; Ao, Y.F. Histone deacetylase inhibitor trichostatin a promotes the osteogenic differentiation of rat adipose-derived stem cells by altering the epigenetic modifications on RUNX2 promoter in a BMP signaling-dependent manner. Stem Cells Dev. 2013, 22, 248–255. [Google Scholar] [CrossRef]
- Jin, H.; Park, J.Y.; Choi, H.; Choung, P.H. HDAC inhibitor trichostatin A promotes proliferation and odontoblast differentiation of human dental pulp stem cells. Tissue Eng. Part A 2013, 19, 613–624. [Google Scholar] [CrossRef]
- Schroeder, T.M.; Westendorf, J.J. Histone deacetylase inhibitors promote osteoblast maturation. J. Bone Miner. Res. 2005, 20, 2254–2263. [Google Scholar] [CrossRef]
- Najafipour, H.; Bagheri-Hosseinabadi, Z.; Eslaminejad, T.; Mollaei, H.R. The effect of sodium valproate on differentiation of human adipose-derived stem cells into cardiomyocyte-like cells in two-dimensional culture and fibrin scaffold conditions. Cell Tissue Res. 2019, 378, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, Y.; Jin, C.; Zhang, M.; Lv, L.; Zhang, X.; Liu, H.; Zhou, Y. Histone H3K9 Acetyltransferase PCAF Is Essential for Osteogenic Differentiation Through Bone Morphogenetic Protein Signaling and May Be Involved in Osteoporosis. Stem Cells 2016, 34, 2332–2341. [Google Scholar] [CrossRef]
- Jing, H.; Su, X.; Gao, B.; Shuai, Y.; Chen, J.; Deng, Z.; Liao, L.; Jin, Y. Epigenetic inhibition of Wnt pathway suppresses osteogenic differentiation of BMSCs during osteoporosis. Cell Death Dis. 2018, 9, 176. [Google Scholar] [CrossRef]
- Huynh, N.C.; Everts, V.; Pavasant, P.; Ampornaramveth, R.S. Inhibition of Histone Deacetylases Enhances the Osteogenic Differentiation of Human Periodontal Ligament Cells. J. Cell. Biochem. 2016, 117, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Hnilicova, J.; Hozeifi, S.; Duskova, E.; Icha, J.; Tomankova, T.; Stanek, D. Histone deacetylase activity modulates alternative splicing. PLoS ONE 2011, 6, e16727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, S.G.; Iglesias, A.H.; Teh, B.T.; Dangond, F. Modulation of splicing events in histone deacetylase 3 by various extracellular and signal transduction pathways. Gene Expr. 2003, 11, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, F.; Naujoks, C.; Laser, A.; Kelz, M.; Kersten-Thiele, P.; Berr, K.; Depprich, R.; Kubler, N.; Kogler, G.; Handschel, J. Improvement of the cell-loading efficiency of biomaterials by inoculation with stem cell-based microspheres, in osteogenesis. J. Biomater. Appl. 2012, 26, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, T.; Han, Q.; Chen, M.; You, J.; Fang, F.; Peng, L.; Wu, B. HDAC inhibitor LMK235 promotes the odontoblast differentiation of dental pulp cells. Mol. Med. Rep. 2018, 17, 1445–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, M.J.; Dennis, C.; Yang, X.B.B.; Kirkham, J. Tissue non-specific alkaline phosphatase production by human dental pulp stromal cells is enhanced by high density cell culture. Cell Tissue Res. 2015, 361, 529–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boveia, V.; Schutz-Geschwender, A. Quantitative Analysis of Signal Transduction with In-Cell Western Immunofluorescence Assays. Methods Mol. Biol. 2015, 1314, 115–130. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Wang, D.; Man, K.; Yang, X. CircRNA expression profiles in human dental pulp stromal cells undergoing oxidative stress. J. Transl. Med. 2019, 17, 327. [Google Scholar] [CrossRef]






| Gene Symbol | Description | TaqMan Assay Identification |
|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Hs99999905_m1 |
| BMP2 | Bone morphogenetic protein 2 | Hs00154192_m1 |
| ALPL | Alkaline phosphatase | Hs01029144_m1 |
| COL1A1 | Collagen, type I | Hs00164004_m1 |
| OCN/BGLAP | Osteocalcin/PMF-bone gamma-carboxyglutamate (gla) protein | Hs00609452_g1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, K.; Lawlor, L.; Jiang, L.-H.; Yang, X.B. The Selective Histone Deacetylase Inhibitor MI192 Enhances the Osteogenic Differentiation Efficacy of Human Dental Pulp Stromal Cells. Int. J. Mol. Sci. 2021, 22, 5224. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22105224
Man K, Lawlor L, Jiang L-H, Yang XB. The Selective Histone Deacetylase Inhibitor MI192 Enhances the Osteogenic Differentiation Efficacy of Human Dental Pulp Stromal Cells. International Journal of Molecular Sciences. 2021; 22(10):5224. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22105224
Chicago/Turabian StyleMan, Kenny, Liam Lawlor, Lin-Hua Jiang, and Xuebin B. Yang. 2021. "The Selective Histone Deacetylase Inhibitor MI192 Enhances the Osteogenic Differentiation Efficacy of Human Dental Pulp Stromal Cells" International Journal of Molecular Sciences 22, no. 10: 5224. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22105224