Flavonoids in Skin Senescence Prevention and Treatment
Abstract
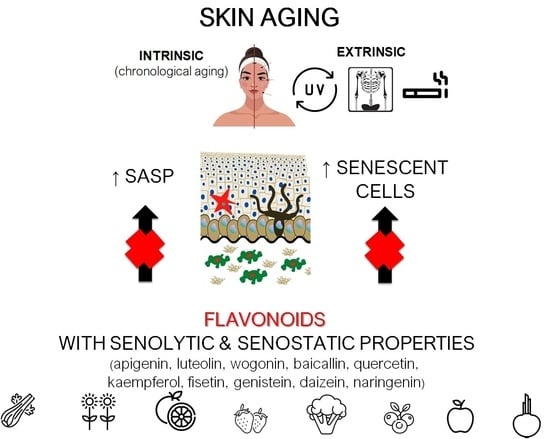
:1. Introduction
2. Skin Aging and Senescence
2.1. Keratinocytes
2.2. Fibroblasts
2.3. Melanocytes
2.4. Langerhans Cells
3. The Influence of Senescent Cells and SASP on Skin Function
3.1. Cellular Senescence and Wound Healing
3.2. Skin Senescence and Cancerogenesis
4. Therapeutic Strategies Targeting Skin Senescence
5. Flavonoids as a Senostatic and Senolytic Strategy
5.1. Flavones
5.1.1. Apigenin
5.1.2. Baicalin
5.1.3. Luteolin
5.1.4. Wogonin
5.2. Flavonols
5.2.1. Quercetin
5.2.2. Kaempferol
5.2.3. Fisetin
5.3. Isoflavones
Daidzein and Genistein
5.4. Flavanones
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Jesus, B.B.; De Blasco, M.A. Europe PMC Funders Group Assessing Cell and Organ Senescence Biomarkers. Circ. Res. 2016, 111, 97–109. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Kaur, J.; Farr, J.N. Cellular senescence in age-related disorders. Transl. Res. 2020, 226, 96–104. [Google Scholar] [CrossRef]
- Tacutu, R.; Budovsky, A.; Yanai, H.; Fraifeld, V.E. Molecular links between cellular senescence, longevity and age-related diseases—A systems biology perspective. Aging 2011, 3, 1178–1191. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.E.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Laberge, R.-M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef] [Green Version]
- De Kreutzenberg, S.V.; Ceolotto, G.; Cattelan, A.; Pagnin, E.; Mazzucato, M.; Garagnani, P.; Borelli, V.; Bacalini, M.G.; Franceschi, C.; Fadini, G.P.; et al. Metformin improves putative longevity effectors in peripheral mononuclear cells from subjects with prediabetes. A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 686–693. [Google Scholar] [CrossRef]
- Kraig, E.; Linehan, L.A.; Liang, H.; Romo, T.Q.; Liu, Q.; Wu, Y.; Benavides, A.D.; Curiel, T.J.; Javors, M.A.; Musi, N.; et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp. Gerontol. 2018, 105, 53–69. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Campbell, K.L.; Lichtensteiger, C.A. Structure and Function of The Skin; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 9781437711462. [Google Scholar]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; Van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [Green Version]
- Ritschka, B.; Storer, M.; Mas, A.; Heinzmann, F.; Ortells, M.C.; Morton, J.; Sansom, O.J.; Zender, L.; Keyes, W.M. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017, 31, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical cAMP Manipulation. Molecules 2014, 19, 6202. [Google Scholar] [CrossRef]
- Begović, L.; Antunovic, M.; Matic, I.; Furcic, I.; Baricevic, A.; Parcina, V.V.; Štefanić, P.P.; Nagy, B.; Marijanovic, I. Effect of UVC radiation on mouse fibroblasts deficient for FAS-associated protein with death domain. Int. J. Radiat. Biol. 2016, 92, 475–482. [Google Scholar] [CrossRef]
- Wang, S.-C.; Chen, S.-F.; Lee, Y.-M.; Chuang, C.-L.; Bau, D.-T.; Lin, S.-S. Baicalin scavenges reactive oxygen species and protects human keratinocytes against UVC-induced cytotoxicity. In Vivo 2013, 27, 707–714. [Google Scholar]
- Cho, S.; Shin, M.H.; Kim, Y.K.; Seo, J.-E.; Lee, Y.M.; Park, C.-H.; Chung, J.H. Effects of Infrared Radiation and Heat on Human Skin Aging In vivo. J. Investig. Dermatol. Symp. Proc. 2009, 14, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, H.-O.; Lee, Y.-K.; Kim, J.-M.; Yoon, G. From cell senescence to age-related diseases: Differential mechanisms of action of senescence-associated secretory phenotypes. BMB Rep. 2015, 48, 549–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, K.; Capell, B.C. The Senescence-Associated Secretory Phenotype: Critical Effector in Skin Cancer and Aging. J. Investig. Dermatol. 2016, 136, 2133–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Choi, S.; Roh, W.; Lee, J.; Kim, T.-G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Norsgaard, H.; Clark, B.F.; Rattan, S.I. Distinction between differentiation and senescence and the absence of increased apoptosis in human keratinocytes undergoing cellular aging in vitro. Exp. Gerontol. 1996, 31, 563–570. [Google Scholar] [CrossRef]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- Nassour, J.; Abbadie, C. A novel role for DNA single-strand breaks in senescence and neoplastic escape of epithelial cells. Mol. Cell. Oncol. 2016, 3, e1190885. [Google Scholar] [CrossRef] [Green Version]
- Kemp, M.G.; Spandau, D.F.; Travers, J.B. Impact of Age and Insulin-Like Growth Factor-1 on DNA Damage Responses in UV-Irradiated Human Skin. Molecules 2017, 22, 356. [Google Scholar] [CrossRef]
- Matsumura, H.; Mohri, Y.; Morinaga, H.; Fukuda, M.; Kurata, S.; Nishimura, E.K. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. J. Dermatol. Sci. 2017, 86, e53. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Campisi, J.; Andersen, J.K.; Kapahi, P.; Melov, S. Cellular senescence: A link between cancer and age-related degenerative disease? Semin. Cancer Biol. 2011, 21, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Terlecki-Zaniewicz, L.; Pils, V.; Bobbili, M.R.; Lämmermann, I.; Perrotta, I.; Grillenberger, T.; Schwestka, J.; Weiß, K.; Pum, D.; Arcalis, E.; et al. Extracellular Vesicles in Human Skin: Cross-Talk from Senescent Fibroblasts to Keratinocytes by miRNAs. J. Investig. Dermatol. 2019, 139, 2425–2436.e5. [Google Scholar] [CrossRef] [Green Version]
- Dong, K.; Goyarts, E.; Rella, A.; Pelle, E.; Wong, Y.H.; Pernodet, N. Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage. Int. J. Mol. Sci. 2020, 21, 326. [Google Scholar] [CrossRef] [Green Version]
- Kalfalah, F.; Seggewiß, S.; Walter, R.; Tigges, J.; Moreno-Villanueva, M.; Bürkle, A.; Ohse, S.; Busch, H.; Boerries, M.; Hildebrandt, B.; et al. Structural chromosome abnormalities, increased DNA strand breaks and DNA strand break repair deficiency in dermal fibroblasts from old female human donors. Aging 2015, 7, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Hearing, V.J. Melanocytes and Their Diseases. Cold Spring Harb. Perspect. Med. 2014, 4, a017046. [Google Scholar] [CrossRef] [Green Version]
- Weinmüllner, R.; Zbiral, B.; Becirovic, A.; Stelzer, E.M.; Nagelreiter, F.; Schosserer, M.; Lämmermann, I.; Liendl, L.; Lang, M.; Terlecki-Zaniewicz, L.; et al. Organotypic human skin culture models constructed with senescent fibroblasts show hallmarks of skin aging. NPJ Aging Mech. Dis. 2020, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Victorelli, S.; Lagnado, A.; Halim, J.; Moore, W.; Talbot, D.; Barrett, K.; Chapman, J.; Birch, J.; Ogrodnik, M.; Meves, A.; et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 2019, 38, e101982. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.; Dearman, R.J.; Kimber, I. Influence of ageing on Langerhans cell migration in mice: Identification of a putative deficiency of epidermal interleukin-1beta. Immunology 2002, 105, 466–477. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Dearman, R.J.; Kimber, I.; Griffiths, C.E.M. Langerhans cells express human β-defensin 3: Relevance for immunity during skin ageing. Br. J. Dermatol. 2018, 179, 1170–1171. [Google Scholar] [CrossRef] [Green Version]
- De Maria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.-M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.; et al. An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, J.-I.; Lau, L.F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 2010, 12, 676–685. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Huang, Y.; Zhou, Y.; Sheng, X.; Jiang, Q.; Wang, Y.; Luo, P.; Luo, M.; Shi, C. Senolytics (DQ) Mitigates Radiation Ulcers by Removing Senescent Cells. Front. Oncol. 2020, 9, 1576. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10. [Google Scholar] [CrossRef]
- Salama, R.; Sadaie, M.; Hoare, M.; Narita, M. Cellular senescence and its effector programs. Genes Dev. 2014, 28, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.-Y.; Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef] [Green Version]
- Takasugi, M.; Okada, R.; Takahashi, A.; Chen, D.V.; Watanabe, S.; Hara, E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 2017, 8, 15729. [Google Scholar] [CrossRef]
- Terlecki-Zaniewicz, L.; Lämmermann, I.; Latreille, J.; Bobbili, M.R.; Pils, V.; Schosserer, M.; Weinmüllner, R.; Dellago, H.; Skalicky, S.; Pum, D.; et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging 2018, 10, 1103–1132. [Google Scholar] [CrossRef]
- Elkhattouti, A.; Hassan, M.; Gomez, C.R. Stromal Fibroblast in Age-Related Cancer: Role in Tumorigenesis and Potential as Novel Therapeutic Target. Front. Oncol. 2015, 5, 158. [Google Scholar] [CrossRef] [Green Version]
- Velarde, M.; De Maria, M. Targeting Senescent Cells: Possible Implications for Delaying Skin Aging: A Mini-Review. Gerontology 2016, 62, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Regulski, M.J. Cellular Senescence: What, Why and How. Wounds 2017, 29, 168–174. [Google Scholar]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell. Signal. 2012, 24, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Abraham, A.; Roga, G. Topical steroid-damaged skin. Indian J. Dermatol. 2014, 59, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuryłowicz, A. The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment—A Narrative Review. Int. J. Mol. Sci. 2020, 22, 218. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H.P. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem. Pharmacol. 2015, 96, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- Laura, V.; Mattia, F.; Roberta, G.; Federico, I.; Emi, D.; Chiara, T.; Luca, B.; Elena, C. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability. Adv Nutr. 2017, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.A.; Parama, D.; Daimari, E.; Girisa, S.; Banik, K.; Harsha, C.; Dutta, U.; Kunnumakkara, A.B. Rationalizing the therapeutic potential of apigenin against cancer. Life Sci. 2021, 267, 118814. [Google Scholar] [CrossRef]
- Bridgeman, B.B.; Wang, P.; Ye, B.; Pelling, J.C.; Volpert, O.V.; Tong, X. Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes: A new implication of skin cancer prevention. Cell. Signal. 2016, 28, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Mirzoeva, S.; Veliceasa, D.; Bridgeman, B.B.; Fitchev, P.; Cornwell, M.L.; Crawford, S.E.; Pelling, J.C.; Volpert, O.V. Chemopreventive apigenin controls UVB-induced cutaneous proliferation and angiogenesis through HuR and thrombospondin-1. Oncotarget 2014, 5, 11413–11427. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Youn, J.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; An, I.-S.; Kwon, S.; Youn, H.J.; et al. Apigenin inhibits UVA-induced cytotoxicity in vitro and prevents signs of skin aging in vivo. Int. J. Mol. Med. 2016, 38, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Britto, S.M.; Shanthakumari, D.; Agilan, B.; Radhiga, T.; Kanimozhi, G.; Prasad, N.R. Apigenin prevents ultraviolet-B radiation induced cyclobutane pyrimidine dimers formation in human dermal fibroblasts. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017, 821, 28–35. [Google Scholar] [CrossRef]
- Mirzoeva, S.; Tong, X.; Bridgeman, B.B.; Plebanek, M.P.; Volpert, O.V. Apigenin Inhibits UVB-Induced Skin Carcinogenesis: The Role of Thrombospondin-1 as an Anti-Inflammatory Factor. Neoplasia 2018, 20, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Perrott, K.M.; Wiley, C.D.; Desprez, P.-Y.; Campisi, J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. GeroScience 2017, 39, 161–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Das, J.; Paul, A.; Samadder, A.; Khuda-Bukhsh, A.R. Apigenin, a Bioactive Flavonoid from Lycopodium clavatum, Stimulates Nucleotide Excision Repair Genes to Protect Skin Keratinocytes from Ultraviolet B-Induced Reactive Oxygen Species and DNA Damage. J. Acupunct. Meridian Stud. 2013, 6, 252–262. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, J.; Cheng, X.; Yi, B.; Zhang, X.; Li, Q. Apigenin induces dermal collagen synthesis via smad2/3 signaling pathway. Eur. J. Histochem. 2015, 59, 2467. [Google Scholar] [CrossRef]
- Ve, A.S.G. Apigenin as an Anti-Aging Skin Treatment. J. Clin. Cosmet. Dermatol. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Bing-Rong, Z.; Song-Liang, J.; Xiao, E.C.; Xiang-Fei, L.; Bao-Xiang, C.; Jie, G.; Dan, L. Protective effect of the Baicalin against DNA damage induced by ultraviolet B irradiation to mouse epidermis. Photodermatol. Photoimmunol. Photomed. 2008, 24, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, H.K.; Park, S.; Kim, J.Y.; Zou, Y.; Cho, K.H.; Kim, Y.S.; Kim, D.H.; Yu, B.P.; Choi, J.S.; et al. Short-term feeding of baicalin inhibits age-associated NF-κB activation. Mech. Ageing Dev. 2006, 127, 719–725. [Google Scholar] [CrossRef]
- Zhang, J.-A.; Yin, Z.; Ma, L.-W.; Yin, Z.-Q.; Hu, Y.-Y.; Xu, Y.; Wu, D.; Permatasari, F.; Luo, D.; Zhou, B.-R. The Protective Effect of Baicalin against UVB Irradiation Induced Photoaging: An In Vitro and In Vivo Study. PLoS ONE 2014, 9, e99703. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. BioFactors 2020, 47, 170–180. [Google Scholar] [CrossRef]
- Wölfle, U.; Haarhaus, B.; Schempp, C.M. The Photoprotective and Antioxidative Properties of Luteolin are Synergistically Augmented by Tocopherol and Ubiquinone. Planta Med. 2013, 79, 963–965. [Google Scholar] [CrossRef]
- Wölfle, U.; Heinemann, A.; Esser, P.R.; Haarhaus, B.; Martin, S.F.; Schempp, C.M. Luteolin Prevents Solar Radiation-Induced Matrix Metalloproteinase-1 Activation in Human Fibroblasts: A Role for p38 Mitogen-Activated Protein Kinase and Interleukin-20 Released from Keratinocytes. Rejuvenat. Res. 2012, 15, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Averbeck, M.; Gebhardt, C.A.; Voigt, S.; Beilharz, S.; Anderegg, U.; Termeer, C.C.; Sleeman, J.P.; Simon, J.C. Differential Regulation of Hyaluronan Metabolism in the Epidermal and Dermal Compartments of Human Skin by UVB Irradiation. J. Investig. Dermatol. 2007, 127, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.P.; Oh, K.N.; Yun, H.J.; Jeong, H.G. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J. Dermatol. Sci. 2011, 61, 23–31. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Kim, H.G.; Choi, J.M.; Hwang, S.K.; Chung, Y.C.; Jeong, H.G. Cultivated ginseng suppresses ultraviolet B–induced collagenase activation via mitogen-activated protein kinases and nuclear factor κB/activator protein-1–dependent signaling in human dermal fibroblasts. Nutr. Res. 2012, 32, 428–438. [Google Scholar] [CrossRef]
- Yang, D.; Guo, Q.; Liang, Y.; Zhao, Y.; Tian, X.; Ye, Y.; Tian, J.; Wu, T.; Lu, N. Wogonin induces cellular senescence in breast cancer via suppressing TXNRD2 expression. Arch. Toxicol. 2020, 94, 3433–3447. [Google Scholar] [CrossRef]
- Chi, Y.; Kim, H. Suppression of cyclooxygenase-2 expression of skin fibroblasts by wogonin, a plant flavone from Scutellaria radix. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 59–66. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Cho, J.-G.; Hwang, E.-S.; Yang, J.-E.; Gao, W.; Fang, M.-Z.; Zheng, S.-D.; Yi, T.-H. Enhancement of Protective Effects of Radix Scutellariae on UVB-induced Photo Damage in Human HaCaT Keratinocytes. Appl. Biochem. Biotechnol. 2018, 184, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Heo, M.Y.; Park, H.; Kim, H.P. Inhibition of TPA-induced cyclooxygenase-2 expression and skin inflammation in mice by wogonin, a plant flavone from Scutellaria radix. Eur. J. Pharmacol. 2001, 425, 153–157. [Google Scholar] [CrossRef]
- Chi, Y.S.; Lim, H.; Park, H.; Kim, H.P. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol. 2003, 66, 1271–1278. [Google Scholar] [CrossRef]
- Company, A.; Lloret-Fillol, J.; Costas, M. Small Molecule Models for Nonporphyrinic Iron and Manganese Oxygenases; Elsevier BV: Amsterdam, The Nethrelands, 2013; Volume 3, pp. 487–564. ISBN 9780-08096-5291. [Google Scholar]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.-G.; Byun, S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef] [Green Version]
- Wermuth, P.; Addya, S.; Jimenez, S.A. Effect of Protein Kinase C delta (PKC-δ) Inhibition on the Transcriptome of Normal and Systemic Sclerosis Human Dermal Fibroblasts In Vitro. PLoS ONE 2011, 6, e27110. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Bloniarz, D.; Olszowka, J.; Kulpa-Greszta, M.; Litwinienko, G.; Tomaszewska, A.; Wnuk, M.; Pazik, R. AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe3O4 nanoparticles during oxidant-induced senescence in human fibroblasts. Redox Biol. 2020, 28, 101337. [Google Scholar] [CrossRef]
- Vicentini, F.; He, T.; Shao, Y.; Fonseca, M.J.; Verri, W.; Fisher, G.J.; Xu, Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-κB pathway. J. Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef]
- García-Mediavilla, M.V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.-H.; Jeong, G.-S. Fisetin inhibits TNF-α-induced inflammatory action and hydrogen peroxide-induced oxidative damage in human keratinocyte HaCaT cells through PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int. Immunopharmacol. 2015, 29, 246–253. [Google Scholar] [CrossRef]
- Chiang, H.-M.; Chan, S.-Y.; Chu, Y.; Wen, K.-C. Fisetin Ameliorated Photodamage by Suppressing the Mitogen-Activated Protein Kinase/Matrix Metalloproteinase Pathway and Nuclear Factor-κB Pathways. J. Agric. Food Chem. 2015, 63, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-Y.; Lyu, J.-L.; Liu, Y.-J.; Chien, T.-Y.; Hsu, H.-C.; Wen, K.-C.; Chiang, H.-M. Fisetin Regulates Nrf2 Expression and the Inflammation-Related Signaling Pathway to Prevent UVB-Induced Skin Damage in Hairless Mice. Int. J. Mol. Sci. 2017, 18, 2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alleviation by Fisetin of Frailty, Inflammation, and Related Measures in Older Adults—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03675724 (accessed on 24 May 2021).
- Barnes, S. The Biochemistry, Chemistry and Physiology of the Isoflavones in Soybeans and their Food Products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-Y.; Kim, S.-J.; Lee, J.-Y.; Kim, W.-G.; Park, W.-S.; Sim, Y.-C.; Lee, S.-J. Protective effects of dietary soy isoflavones against UV-induced skin-aging in hairless mouse model. J. Am. Coll. Nutr. 2004, 23, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shi, Y.; Dang, Y.; Zhai, Y.; Ye, X. Daidzein stimulates collagen synthesis by activating the TGF-β/smad signal pathway. Australas. J. Dermatol. 2014, 56, e7–e14. [Google Scholar] [CrossRef]
- Oh, H.-J.; Kang, Y.-G.; Na, T.-Y.; Kim, H.-J.; Park, J.S.; Cho, W.-J.; Lee, M.-O. Identification of daidzein as a ligand of retinoic acid receptor that suppresses expression of matrix metalloproteinase-9 in HaCaT cells. Mol. Cell. Endocrinol. 2013, 376, 107–113. [Google Scholar] [CrossRef]
- Huang, P.-H.; Tseng, C.-H.; Lin, C.-Y.; Lee, C.-W.; Yen, F.-L. Preparation, characterizations and anti-pollutant activity of 7,3′,4′-trihydroxyisoflavone nanoparticles in particulate matter-induced HaCaT keratinocytes. Int. J. Nanomed. 2018, 13, 3279–3293. [Google Scholar] [CrossRef] [Green Version]
- Widyarini, S.; Spinks, N.; Husband, A.J.; Reeve, V.E. Isoflavonoid Compounds from Red Clover (Trifolium pratense) Protect from Inflammation and Immune Suppression Induced by UV Radiation. Photochem. Photobiol. 2001, 74, 465. [Google Scholar] [CrossRef]
- Reeve, V.E.; Widyarini, S.; Domanski, D.; Chew, E.; Barnes, K. Protection Against Photoaging in the Hairless Mouse by the Isoflavone Equol. Photochem. Photobiol. 2005, 81, 1548–1553. [Google Scholar] [CrossRef]
- Polito, F.; Marini, H.R.; Bitto, A.; Irrera, N.; Vaccaro, M.; Adamo, E.B.; Micali, A.; Squadrito, F.; Minutoli, L.; Altavilla, D. Genistein aglycone, a soy-derived isoflavone, improves skin changes induced by ovariectomy in rats. Br. J. Pharmacol. 2011, 165, 994–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na Wang, Y.; Wu, W.; Chen, H.C.; Fang, H. Genistein protects against UVB-induced senescence-like characteristics in human dermal fibroblast by p66Shc down-regulation. J. Dermatol. Sci. 2010, 58, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Iovine, B.; Iannella, M.L.; Gasparri, F.; Monfrecola, G.; Bevilacqua, M.A. Synergic Effect of Genistein and Daidzein on UVB-Induced DNA Damage: An Effective Photoprotective Combination. J. Biomed. Biotechnol. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, K.; Hanamizu, T.; Iizuka, R.; Chiba, K. Genistein and Daidzein Stimulate Hyaluronic Acid Production in Transformed Human Keratinocyte Culture and Hairless Mouse Skin. Ski. Pharmacol. Physiol. 2002, 15, 175–183. [Google Scholar] [CrossRef]
- Izumi, T.; Saito, M.; Obata, A.; Arii, M.; Yamaguchi, H.; Matsuyama, A. Oral intake of soy isoflavone aglycone improves the aged skin of adult women. J. Nutr. Sci. Vitaminol. 2007, 53, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraes, A.B.; Haidar, M.A.; Soares, J.M.; Simões, M.J.; Baracat, E.C.; Patriarca, M.T. The effects of topical isoflavones on postmenopausal skin: Double-blind and randomized clinical trial of efficacy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 188–192. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy, M.A.; Zhu, Q.; Wang, Q.-E.; Wani, G.; Patnaik, S.; Zhao, Q.; Arafa, E.-S.; Barakat, B.; Mir, S.N.; Wani, A.A. Naringenin Protects HaCaT Human Keratinocytes Against UVB-induced Apoptosis and Enhances the Removal of Cyclobutane Pyrimidine Dimers from the Genome. Photochem. Photobiol. 2007, 84, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Silva, T.C.C.; Caviglione, C.V.; Bottura, C.; Fonseca, M.J.V.; Vicentini, F.T.M.C.; Vignoli, J.A.; Baracat, M.M.; et al. Topical Formulation Containing Naringenin: Efficacy against Ultraviolet B Irradiation-Induced Skin Inflammation and Oxidative Stress in Mice. PLoS ONE 2016, 11, e0146296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.H.; Kim, G.R. Inhibitory effect of naringenin on LPS-induced skin senescence by SIRT1 regulation in HDFs. Biomed. Dermatol. 2018, 2, 26. [Google Scholar] [CrossRef] [Green Version]

| Flavonoid | Route of Administration | Disease Entity | Research Model | Mechanism | Conclusion | Reference |
|---|---|---|---|---|---|---|
| Flavones | ||||||
| Apigenin | in vitro | UVA & UVB-induced skin ageing | human dermal fibroblasts | ↓ NF-κB pathway ↓ MAPK ↓ MMP-1 ↓ ROS | ↑ viability ↑ collagen synthesis ↑ DNA repair | [70] [74] [71] |
| in vitro | UVB-induced skin damage | human keratinocytes | ↑ TSP-1 | ↓ UVB-induced carcinogenesis | [69] | |
| topical (25 μmol/μL) | UVB-induced acute skin damage | mice | ↑ TSP-1 ↓ IL-6, -12 ↓ inflammatory infiltrates | restoration of skin damage caused by UVB radiation | [72] | |
| topical (1.5–3 mg/cm2) | UVA-induced skin ageing | mice | ↓ NF-κB pathway ↓ MAPK ↓ MMP-1 ↓ ROS | ↑ dermal thickness ↑ collagen deposition | [74] | |
| in vitro | bleomycin-induced cellular senescence | human dermal fibroblasts | ↓ NF-κB pathway ↓ IL-1α, -1β, -6, -8 ↓ GM-CSF, CXCL1 ↓ MCP-2, MMP-3 | ↓ SASP secretion | [62] | |
| in vitro | ionizing radiation-induced cellular senescence | human dermal fibroblasts | ↓ NF-κB pathway ↓ MAPK ↓ IP10 | ↓ SASP secretion | [73] | |
| topical (2 g of 1% cream for 4 weeks) | UVA-induced skin ageing | healthy individuals | ↓ MMP-1 | ↑ dermal density and elasticity ↓ fine wrinkle length | [70] | |
| Baicalin | in vitro | UVB-induced skin ageing | human dermal fibroblasts human skin samples | ↓ MMP-1, MMP-3 ↓ ROS ↓ p16, p21,p53 | ↑ collagen synthesis ↓ DNA damage ↓ apoptosis ↑ viability | [79] |
| in vitro | UVC-induced cytotoxicity | human keratinocytes | ↓ ROS | ↓ DNA damage | [21] | |
| topical (0.5–1 mg/cm2) | UVB-induced skin damage | Balb/C mice | ↓ p53 in epidermis | ↓ DNA damage ↓ apoptosis | [77] | |
| Luteolin | in vitro | UVB-induced skin damage | human keratinocytes | ↓ p38/MAPK ↓ ROS ↓ COX-2 | ↓ DNA damage | [82] |
| topical (0.25–4 mg/mL) | human skin explants | |||||
| in vitro | UVA-induced skin ageing | human dermal fibroblasts human keratinocytes | ↓ p38/MAPK ↓ ROS ↓ MMP-1 ↓ IL-6, -20 | ↓ SASP secretion ↓ collagen degradation ↓ hylauronic acid degradation | [81] | |
| topical (8 mg/mL) | human skin explants | |||||
| in vitro | UVB-induced skin ageing | human dermal fibroblasts | ↓ MAPK/AP-1 ↓ NF-κB pathway ↓ MMP-1 | ↓ SASP secretion ↓ collagen degradation | [85] | |
| Apigenin & Luteolin | UVA-induced skin damage | human keratinocytes | ↓ MAPK/AP-1 ↓ MMP-1 | ↓ SASP secretion ↓ collagen degradation | [84] | |
| Wogonin | in vitro | mouse dermal fibroblasts | ↓ COX-2 | ↓ SASP secretion | [87] | |
| in vitro | UVB-induced skin damage | human keratinocytes | ↓ MAPK/AP-1 ↓ NF-κB pathway ↓ IL-6 ↓ MMP-1 ↓ TGF-β ↓ Nrf2 | ↓ SASP secretion ↑ collagen synthesis ↑ antioxidants | [88] | |
| Flavonols | ||||||
| Quercetin | in vitro | UV-induced skin ageing | human dermal fibroblasts human skin explants | ↓ MAPK/AP-1 ↓ NF-κB pathway ↓ JAK2/STAT3 ↓ COX-2 ↓ MMP-1 | ↓ SASP secretion ↓ collagen degradation | [92] |
| in vitro | hydrogen peroxide-induced skin ageing | human dermal fibroblasts | ↑ AMPK ↓ IL-8, IFN-β | ↓ SASP secretion ↓ senescent cells number | [94] | |
| in vitro | UV-induced skin damage | human keratinocytes | ↓ NF-κB ↓ IL-1β, -6, -8,TNF-α =MMP-1, -3 | ↓ SASP secretion | [95] | |
| Kaempferol | in vitro | bleomycin-induced senescence | fibroblasts | ↓ NF-κB pathway | ↓ SASP secretion | [62] |
| Fisetin | in vitro | hydrogen peroxide-induced skin damage | human keratinocytes | ↓ ROS ↓ NF-κB pathway ↓ iNOS ↓ COX-2 ↓ IL-1β, -6, TNF-α | ↑ viability ↓ SASP secretion | [98] |
| in vitro | UVB-induced skin damage | human dermal fibroblasts | ↓ MAPK/AP-1/MMP ↓ ROS | ↓ SASP secretion ↓ collagen degradation | [99] | |
| topical (50–200 μmol/daily) | photoinflammation | hairless mice | ↓ iNOS ↓ MMP-1, -2 ↓ COX-2 ↑ filaggrin ↑ aquaporins | ↓ SASP secretion ↓ collagen degradation ↓ photo inflammation ↓ skin-drying | [100] | |
| Isoflavones | ||||||
| Daidzein & Genistein | in vitro | UVB-induced skin damage | human keratinocytes | ↓ MMP-1, -2 | ↓ collagen degradation | [103] |
| systematically (500 mg of soy extract/kg/day) | hairless mice | ↓ wrinkle length | [103] | |||
| in vitro | human keratinocytes | ↑ hyaluronic acid | [112] | |||
| topicaly (0.1 mL of 10 μmol equol solution) | hairless mice | ↓ fine wrinkle | [108] | |||
| in vitro | UVB-induced skin damage | human dermal fibroblasts | ↓ COX-2 ↓ Gadd45 | ↑ genomic and mitochondrial DNA repair | [110] [111] | |
| systemically (40 mg of soy isoflavone aglycone per day) | estrogen deficiency | middle-aged women | ↓ fine wrinkles ↑ skin elasticity | [113] | ||
| Daidzein | in vitro | UV-induced skin damage | human dermal fibroblasts | ↑ TGF-β/Smad2/3 | ↓ collagen degradation | [104] |
| in vitro | particulate matter-exposure | human keratinocytes | ↓ MAPK ↓ COX-2 ↓ MMP-9 | ↓ SASP | [106] | |
| Genistein | in vitro | UV-induced skin damage | human keratinocytes | ↓ COX-2 | ↓ SASP | [107] |
| topical (0.1 mL of 10 μmol equol solution) | UVB-induced skin damage | hairless mice | ↓ DNA pyrimidine dimer formation ↓ ROS | ↓ DNA damage | [108] | |
| systematically (1 mg/kg sc) | estrogen deficiency | ovariectomized rats | ↑ TGF-β/Smad2/3 ↑TIMP ↓ TGF-β, MMP-2,-9 | ↓ collagen degradation | [109] | |
| Flavanones | ||||||
| naringenin | in vitro | UVB-induced apoptosis | human keratinocytes | ↑ caspase cascade pathway | ↓ apoptosis | [115] |
| intraperitoneal (10–100 mg/kg) | UVB-induced inflammation | hairless mice | ↓ ROS ↓ MMP-9, ↓ TNF-α, IFN-γ, ↓ IL-1β, -4,-5,-6,-12, -13, -17, -22, -23 | ↓ SASP ↓ inflammatory infiltrations | [116] | |
| topical (0.5% solution) | UVB-induced skin damage | hairless mice | ↓ ROS ↓ TNF-α, IL-1β, -6, -10 | ↓ SASP | [117] | |
| in vitro | LPS-induced skin damage | human dermal fibroblasts | ↓ NF-κB pathway ↓ MMP-1,-3 | ↓ SASP ↓collagen degradation | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domaszewska-Szostek, A.; Puzianowska-Kuźnicka, M.; Kuryłowicz, A. Flavonoids in Skin Senescence Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 6814. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22136814
Domaszewska-Szostek A, Puzianowska-Kuźnicka M, Kuryłowicz A. Flavonoids in Skin Senescence Prevention and Treatment. International Journal of Molecular Sciences. 2021; 22(13):6814. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22136814
Chicago/Turabian StyleDomaszewska-Szostek, Anna, Monika Puzianowska-Kuźnicka, and Alina Kuryłowicz. 2021. "Flavonoids in Skin Senescence Prevention and Treatment" International Journal of Molecular Sciences 22, no. 13: 6814. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22136814