Functional Dental Pulp Regeneration: Basic Research and Clinical Translation
Abstract
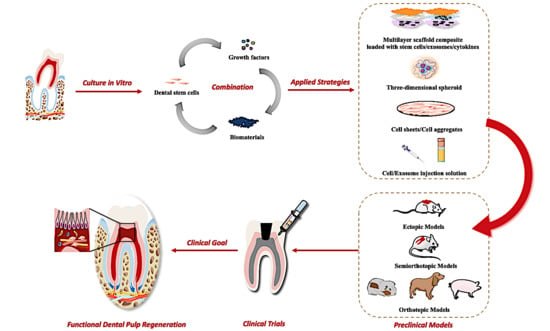
:1. Introduction
2. Literature Search and Scope of the Review
3. Treatment Status and Development Trend of Pulpal and Periapical Diseases
3.1. RCT
- Technology sensitivity: it is difficult for general dentists to properly prepare and fill the complex and changeable root canal systems [17]; thus, they are likely to cause complications, such as perforation, instrument fracture, underfilling, and overfilling.
- Reinfection: root canal reinfection caused by root canal sealant dissolution and crown microleakage accounts for approximately 60% of RCT complications [18].
- Loss of pulp function: along with large hard tissue defects, denervation and avascularity, damaged teeth after RCT lose almost all physiological pulp functions, such as sense, nutrition, and defence, and are susceptible to fractures [19].
- Age limitations: immature permanent teeth cannot continue to develop after RCT; in addition, calcification and obliteration of root canals in elderly individuals may inevitably increase operative difficulty [20].
3.2. Pulp Revascularisation
3.3. Clinical Goals of Pulp Regeneration
4. Biological Studies of Pulp Regeneration
- (1)
- Biomaterials can not only provide a three-dimensional growth space for cells but can also regulate the paracrine secretion of stem cells and the phenotypic switching of macrophages in the local niche.
- (2)
- Growth factors enhance the regenerative effect and regulatory function of stem cells and niche cells.
- (3)
- We can choose different combinations of these elements that are applicable to different situations to achieve the best therapeutic effect (Table 2).
4.1. Stem Cells Applicable for Pulp Regeneration
4.1.1. DPSCs and Other Stem Cell Sources
4.1.2. Surface Markers, Subpopulations and Side Populations of DPSCs
4.1.3. Molecular Mechanism Underlying the Multipotent Differentiation of DPSCs
- (1)
- Transcriptional regulation: KLF4 could directly upregulate the expression levels of odontoblastic-related genes in DPSCs, such as Dmp1, Dspp, and Sp7, by binding to their promoters during odontoblastic differentiation of DPSCs [71,72]. In addition, nuclear factor I-C (NFIC) could bind directly to the Klf4 promoter and stimulate Klf4 transcriptional activity, thereby regulating Dmp1/Dspp signalling during odontoblast differentiation [73]. Similarly, the transcription factor SP1 could regulate KLF4 through a binding site lying in a CpG island in the promoter region of Klf4 [74].
- (2)
- Posttranscriptional regulation: Competitive endogenous RNAs (ceRNAs), a group of transcripts, can affect Klf4 mRNA by competitively binding to miRNA response elements. Sp1 functions as a ceRNA of Klf4 during odontoblast differentiation by competing for miR-7a, miR-29b, and miR-135a [75].
- (3)
- Epigenetic modification: As mentioned above, transcription factors such as KLF4 and SP1 mainly exert their functions by binding to specific DNA motifs. Epigenetic modifications of these motifs might alter DNA accessibility and thereafter affect the expression of binding transcription factors and downstream genes [74]. For example, the demethylation of the SP1/KLF4 binding motif during odontoblastic differentiation enhanced the efficiency of SP1 binding and transcriptional regulation of KLF4. Additionally, KLF4 could recruit the histone acetylases P300 and HDAC3, which relaxed and provided a more open chromatin structure to transactivate the expression of Dmp1 and Sp7 [76]. Furthermore, a recent study indicated that TET1 can potentially promote odontoblast differentiation by inhibiting FAM20C hydroxymethylation and subsequent transcription [77].
4.2. Biomaterials
4.2.1. Natural and Naturally Derived Substances
4.2.2. Synthetic Polymer Materials
4.3. Growth Factors
- (1)
- (2)
- (3)
- Neuronal regeneration: nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) [112].
5. Preclinical Exploration and Clinical Status of Pulp Regeneration
5.1. Animal Models and Research Patterns
- (1)
- Ectopic regenerative models: The dorsum subcutaneous space of immunocompromised rodent species could simulate the deficient blood supply of the dental pulp cavity. In this model, exogenous stem cells are seeded in HA/TCP scaffolds under the dorsal subcutaneous space of mice or rats to determine whether they possess the ability to form pulp/dentin in vivo [35,36]. In consideration of ready availability, easy operation, and low expenses, ectopic transplantation is supposed to be the first and basic modality for studying ectopic pulp regeneration.
- (2)
- Semiorthotopic regenerative models: The tooth slice/fragment scaffold can be chosen as an active carrier to deliver stem cells and/or growth factors. Since they were implanted together into an ectopic location in immunodeficient animals, regeneration occurred inside a real tooth. Therefore, these tooth slice/fragment models are considered semiorthotopic for pulp regeneration [41,42,118]. Such an approach is relatively simple and has the advantage of providing an orthotopic-like regenerative environment as well as minimising experimental variables. Unfortunately, there are obvious disadvantages: (a) the blood supply and operative procedures are significantly different from clinical conditions, and (b) the regenerated tissues are mainly produced and populated by mouse cells.
- (3)
- Orthotopic regenerative models: The pulp tissues of large nonprimate animals, such as ferrets [119,120], dogs [121,122], and swine [123,124], are more accessible and similar to humans. Thus, the regenerative performance following root canals in these animals can completely mimic clinical conditions [123]. Additionally, the single-rooted cuspid of ferrets and dogs, as well as single-/multirooted teeth of swine, provides a relatively larger space for model establishment and image-taking. Therefore, this model holds the highest value in various preclinical efficacy and safety tests.
5.2. Safety Assessment of DPSC-Based Pulp Regeneration
5.2.1. Immunorejection and Systemic Inflammatory Response
5.2.2. Tumorigenicity
5.3. Optimised Strategies for Pulp Regeneration
5.3.1. Novel Culture Methods for DPSCs
5.3.2. Exosome-Mediated Pulp Regeneration
5.3.3. Cell Homing-Based Pulp Regeneration
5.4. Clinical Trials
6. Future Prospects of Pulp Regeneration
6.1. Challenge #1: The Reconstruction of Precisely Layered and Highly Ordered Dental Pulp Structures
6.2. Challenge #2: Specific Therapeutic Procedures with Different Indications
6.3. Challenge #3: Personalised Cellular Therapy for an Elderly Individual or an Individual with Severe Systemic Disease
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAE | The American Association of Endodontists |
| ADSCs | Adipose-derived stem cells |
| BDNF | Brain-derived neurotrophic factor |
| bFGF | Basic fibroblast growth factor |
| BMP7 | Bone morphogenetic protein 7 |
| BMSCs | Bone marrow-derived stem cells |
| CBCT | Cone beam computed tomography |
| CD105 | Endoglin |
| CD271/LNGFR | Low-affinity nerve growth factor receptor |
| CD73 | 5′-ectonucleotidase |
| CD90 (Thy-1) | Glycosylphosphatidylinositol-anchored glycoprotein |
| CeRNAs | Competitive endogenous RNAs |
| CH | Calcium hydroxide |
| DDM | Demineralised dentin matrix |
| DFPCs | Dental follicle precursor cells |
| DLA | Dog leukocyte antigen |
| DPSC-exos | DPSC-derived exosomes |
| DPSC-OD-exos | Exosomes derived from osteogenic DPSCs |
| DPSCs | Dental pulp stem cells |
| ECM | Extracellular matrix |
| ERK | Extracellular-signal regulated kinase |
| FBS | Foetal bovine serum |
| FDA | Food and Drug Administration |
| Flk-1/VEGF-R2 | Vascular endothelial cell growth factor receptor-2 |
| G-CSF | Granulocyte-colony stimulating factor |
| Gdf11 | Growth/differentiation factor 11 |
| GelMA | Gelatine methacryloyl |
| GMP | Good manufacturing practice |
| HA/TCP | Hydroxyapatite/tricalcium phosphate |
| HERS | Hertwig’s epithelial root sheath |
| HLA | Human leukocyte antigen |
| iPS cells | Induced pluripotent stem cells |
| KLF4 | Küppel-like factor 4 |
| LOX | Lysyl oxidase |
| L-PRF | Leukocyte platelet-rich fibrin |
| MDPSCs | Multipotent dental pulp regenerative stem cells |
| MDPSCs | Mobilised dental pulp stem cells |
| MEK | Mitogen-activated protein kinase |
| MTA | Mineral trioxide aggregate |
| NF | Nanofibrous |
| NFIC | Nuclear factor I-C |
| NF-SMS | Nanofibrous spongy microspheres |
| NGF | Nerve growth factor |
| PCL | Polycaprolactone |
| PD-1 | Programmed cell death-1 |
| PDGF | Platelet-derived growth factor |
| PDLSCs | Periodontal ligament stem cells |
| PEO | Polyethylene oxide |
| PGA | Polyglycolic acid |
| PHB | Polyhydroxybutyrate |
| PI3K | Phosphatidylinositol 3 kinase |
| PLA | Polylactic acid |
| PLG | poly-d,l-lactide/glycolide |
| PLGA | Poly(d,l-lactide-coglycolide) |
| PLLA | Poly(l-lactic) acid |
| PRP | Platelet-rich plasma |
| PTEN | Phosphatase and tensin homologue |
| RCT | Root canal therapy |
| RET | Regenerative endodontic treatment |
| rhBMP-2 | Recombinant human bone morphogenetic protein-2 |
| SCAPs | Stem cells from the apical papilla |
| SCF | Stem cell factor |
| SDF-1 | Stromal cell-derived factor-1 |
| SHEDs | Stem cells from human exfoliated deciduous teeth |
| TDMP | Treated dentin matrix paste |
| VEGF | Vascular endothelial growth factor |
| α-SMA | α-smooth muscle actin |
| β-GP | β-glycerophosphate |
References
- Jakovljevic, A.; Nikolic, N.; Jaćimović, J.; Pavlovic, O.; Milicic, B.; Beljic-Ivanovic, K.; Miletic, M.; Andric, M.; Milasin, J. Prevalence of Apical Periodontitis and Conventional Nonsurgical Root Canal Treatment in General Adult Population: An Updated Systematic Review and Meta-analysis of Cross-sectional Studies Published between 2012 and 2020. J. Endod. 2020, 46, 1371–1386.e8. [Google Scholar] [CrossRef]
- Tibúrcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2020, 54, 712–735. [Google Scholar] [CrossRef]
- Goldberg, M.; Njeh, A.; Uzunoglu, E. Is Pulp Inflammation a Prerequisite for Pulp Healing and Regeneration? Mediat. Inflamm. 2015, 2015, 347649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Wang, C.; Ye, L. Healing Rate and Post-obturation Pain of Single- versus Multiple-visit Endodontic Treatment for Infected Root Canals: A Systematic Review. J. Endod. 2011, 37, 125–132. [Google Scholar] [CrossRef]
- Duggal, M.; Tong, H.J.; Alansary, M.; Twati, W.; Day, P.F.; Nazzal, H. Interventions for the endodontic management of non-vital traumatised immature permanent anterior teeth in children and adolescents: A systematic review of the evidence and guidelines of the European Academy of Paediatric Dentistry. Eur. Arch. Paediatr. Dent. 2017, 18, 139–151. [Google Scholar] [CrossRef]
- Murray, P.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative Endodontics: A Review of Current Status and a Call for Action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- American Association of Endodontists (Aae). Clinical Considerations for a Regenerative Procedure. Revised 2016; American association of Endodontists: Chicago, IL, USA, 2016; Available online: http://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/currentregenerativeendodonticconsiderations.pdf (accessed on 10 August 2021).
- American Association of Endodontists (Aae). Clinical Considerations for a Regenerative Procedure. Revised 2018; American association of Endodontists: Chicago, IL, USA, 2018; Available online: https://www.aae.org/specialty/wp-content/uploads/sites/2/2018/06/ConsiderationsForRegEndo_AsOfApril2018.pdf (accessed on 10 August 2021).
- Iwaya, S.-I.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent. Traumatol. 2001, 17, 185–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigler, R.; Kaufman, A.Y.; Lin, S.; Steinbock, N.; Hazan-Molina, H.; Torneck, C.D. Revascularization: A Treatment for Permanent Teeth with Necrotic Pulp and Incomplete Root Development. J. Endod. 2013, 39, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Altaii, M.; Richards, L.; Rossi-Fedele, G. Histological assessment of regenerative endodontic treatment in animal studies with different scaffolds: A systematic review. Dent. Traumatol. 2017, 33, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Song, M.; Kim, E.; Shon, W.; Chugal, N.; Bogen, G.; Lin, L.; Kim, R.H.; Park, N.H.; Kang, M.K. Pulp-Dentin Regeneration: Current State and Future Prospects. J. Dent. Res. 2015, 94, 1544–1551. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K. Regeneration of Dental Pulp by Stem Cells. Adv. Dent. Res. 2011, 23, 313–319. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2018, 13, 58–75. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of Dental-Pulp-like Tissue by Chemotaxis-Induced Cell Homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Li, Z.; Liu, A.; Liu, X.; Guo, H.; Wu, M.; Yang, X.; Han, B.; Xuan, K. Microenvironment Influences Odontogenic Mesenchymal Stem Cells Mediated Dental Pulp Regeneration. Front. Physiol. 2021, 12, 656588. [Google Scholar] [CrossRef]
- Meirinhos, J.; Martins, J.N.R.; Pereira, A.B.D.C.R.J.; Baruwa, A.; Gouveia, J.; Quaresma, S.A.; Monroe, A.; Ginjeira, A. Prevalence of apical periodontitis and its association with previous root canal treatment, root canal filling length and type of coronal restoration—A cross-sectional study. Int. Endod. J. 2019, 53, 573–584. [Google Scholar] [CrossRef]
- Pandey, P.; Aggarwal, H.; Tikku, A.; Singh, A.; Bains, R.; Mishra, S. Comparative evaluation of sealing ability of gutta percha and resilon as root canal filling materials—A systematic review. J. Oral Biol. Craniofacial Res. 2019, 10, 220–226. [Google Scholar] [CrossRef]
- Bromberg, C.R.; Alves, C.B.; Stona, D.; Spohr, A.M.; Rodrigues-Junior, S.A.; Melara, R.; Burnett, L.H. Fracture resistance of endodontically treated molars restored with horizontal fiberglass posts or indirect techniques. J. Am. Dent. Assoc. 2016, 147, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Zayed, M. Pulp Regeneration: Current Approaches, Challenges, and Novel Rejuvenating Strategies for an Aging Population. J. Endod. 2020, 46, S135–S142. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Rossi-Fedele, G. A Review of Tooth Discoloration after Regenerative Endodontic Therapy. J. Endod. 2016, 42, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.M.; Huang, G.T.; Sigurdsson, A.; Kahler, B. Clinical cell-based versus cell-free regenerative endodontics: Clarification of concept and term. Int. Endod. J. 2021, 54, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zeng, Q.; Wei, X.; Zhao, W.; Cui, M.; Gu, J.; Lu, J.; Yang, M.; Ling, J. Regenerative Endodontics Versus Apexification in Immature Permanent Teeth with Apical Periodontitis: A Prospective Randomized Controlled Study. J. Endod. 2017, 43, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Mistry, S.; Moule, A.; Ringsmuth, A.; Case, P.; Thomson, A.; Holcombe, T. Revascularization Outcomes: A Prospective Analysis of 16 Consecutive Cases. J. Endod. 2013, 40, 333–338. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, L.; Arbildo-Vega, H.I.; Lapinska, B.; Lukomska-Szymanska, M.; Khijmatgar, S.; Parolia, A.; Bucchi, C.; Del Fabbro, M. Effectiveness of Autologous Platelet Concentrates in Management of Young Immature Necrotic Permanent Teeth—A Systematic Review and Meta-Analysis. Cells 2020, 9, 2241. [Google Scholar] [CrossRef]
- Kuang, R.; Zhang, Z.; Jin, X.; Hu, J.; Shi, S.; Ni, L.; Ma, P.X. Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta Biomater. 2016, 33, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, R.; Zhang, Z.; Jin, X.; Hu, J.; Gupte, M.J.; Ni, L.; Ma, P.X. Nanofibrous Spongy Microspheres Enhance Odontogenic Differentiation of Human Dental Pulp Stem Cells. Adv. Health Mater. 2015, 4, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Horibe, H.; Iohara, K.; Hayashi, Y.; Osako, Y.; Takei, Y.; Nakata, K.; Motoyama, N.; Kurita, K.; Nakashima, M. The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials 2013, 34, 9036–9047. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, M.; Iohara, K.; Ishikawa, M.; Ito, M.; Tomokiyo, A.; Tanaka, T.; Akamine, A. Stimulation of Reparative Dentin Formation by Ex Vivo Gene Therapy Using Dental Pulp Stem Cells Electrotransfected with Growth/differentiation factor 11 (Gdf11). Hum. Gene Ther. 2004. [Google Scholar] [CrossRef]
- Yan, X.; Qin, H.; Qu, C.; Tuan, R.S.; Shi, S.; Huang, G.T.-J. iPS Cells Reprogrammed from Human Mesenchymal-Like Stem/Progenitor Cells of Dental Tissue Origin. Stem Cells Dev. 2010, 19, 469–480. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wu, C.-T.; Wang, S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res. Ther. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Zhou, J.; Chen, M.; Lin, C.-S.; Kim, S.G.; Zhou, Y.; Xiang, L.; Xie, M.; Bai, H.; Yao, H.; et al. Parenchymal and stromal tissue regeneration of tooth organ by pivotal signals reinstated in decellularized matrix. Nat. Mater. 2019, 18, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. Shed: Stem Cells from Human Exfoliated Deciduous Teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Sugimura-Wakayama, Y.; Katagiri, W.; Osugi, M.; Kawai, T.; Ogata, K.; Sakaguchi, K.; Hibi, H. Peripheral Nerve Regeneration by Secretomes of Stem Cells from Human Exfoliated Deciduous Teeth. Stem Cells Dev. 2015, 24, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Bronckaers, A.; Dillen, Y.; Gervois, P.; Vangansewinkel, T.; Driesen, R.B.; Wolfs, E.; Lambrichts, I.; Hilkens, P. The Neurovascular Properties of Dental Stem Cells and Their Importance in Dental Tissue Engineering. Stem Cells Int. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zhao, B.; Gao, Z.; Xu, J.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Regeneration characteristics of different dental derived stem cell sheets. J. Oral Rehabil. 2019, 47, 66–72. [Google Scholar] [CrossRef]
- Gonçalves, S.B.; Dong, Z.; Bramante, C.M.; Holland, G.R.; Smith, A.J.; Nör, J.E. Tooth Slice–Based Models for the Study of Human Dental Pulp Angiogenesis. J. Endod. 2007, 33, 811–814. [Google Scholar] [CrossRef]
- Huang, G.T.-J.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/Progenitor Cell–Mediated De Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an In Vivo Model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Ishizaka, R.; Iohara, K.; Murakami, M.; Fukuta, O.; Nakashima, M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 2011, 33, 2109–2118. [Google Scholar] [CrossRef]
- Murakami, M.; Hayashi, Y.; Iohara, K.; Osako, Y.; Hirose, Y.; Nakashima, M. Trophic Effects and Regenerative Potential of Mobilized Mesenchymal Stem Cells from Bone Marrow and Adipose Tissue as Alternative Cell Sources for Pulp/Dentin Regeneration. Cell Transplant. 2015, 24, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.G.; Cooper, P.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J. Bone Miner. Metab. 2014, 33, 371–382. [Google Scholar] [CrossRef]
- Sabbagh, J.; Ghassibe-Sabbagh, M.; Fayyad-Kazan, M.; Al-Nemer, F.; Fahed, J.C.; Berberi, A.; Badran, B. Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. J. Dent. 2020, 101, 103413. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, W.; Liu, A.; Wu, M.; Shuai, Y.; Li, B.; Huang, X.; Liu, X.; Yang, X.; Guo, X.; et al. SHED promote angiogenesis in stem cell-mediated dental pulp regeneration. Biochem. Biophys. Res. Commun. 2020, 529, 1158–1164. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Nakajima, K.; Awada, T.; Tsuka, Y.; Abe, T.; Ando, K.; Hiraki, T.; Kimura, A.; Tanimoto, K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow–derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 501, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; He, Y.; Zhang, X.; Lu, W.; Wang, C.; Yu, H.; Liu, Y.; Zhou, Y.; Zhou, J.; Zhang, M.; et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials 2009, 30, 6708–6723. [Google Scholar] [CrossRef]
- Sowmya, S.; Chennazhi, K.P.; Arzate, H.; Jayachandran, P.; Nair, S.V.; Jayakumar, R.; Srinivasan, S.; Rangasamy, J. Periodontal Specific Differentiation of Dental Follicle Stem Cells into Osteoblast, Fibroblast, and Cementoblast. Tissue Eng. Part C Methods 2015, 21, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Nada, O.; El Backly, R.M. Stem Cells From the Apical Papilla (SCAP) as a Tool for Endogenous Tissue Regeneration. Front. Bioeng. Biotechnol. 2018, 6, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, S.; Liu, X.-M.; Liu, Y.; Bi, J.; Zhu, S.; Chen, X. Lipopolysaccharide Downregulates the Osteo-/Odontogenic Differentiation of Stem Cells from Apical Papilla by Inducing Autophagy. J. Endod. 2020, 46, 502–508. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Zhang, Z.; Guo, W.; Chen, G.; Tian, W. Development of immortalized Hertwig’s epithelial root sheath cell lines for cementum and dentin regeneration. Stem Cell Res. Ther. 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, X.; Zhang, S.; Wang, S.; Wang, T.; Chen, H.; Yang, Y.; Jia, S.; Chen, G.; Tian, W. Therapeutic potential of HERS spheroids in tooth regeneration. Theranostics 2020, 10, 7409–7421. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, S.P.; He, W.; Monteiro, N.; Athirasala, A.; França, C.M.; Bertassoni, L.E. Engineering pericyte-supported microvascular capillaries in cell-laden hydrogels using stem cells from the bone marrow, dental pulp and dental apical papilla. Sci. Rep. 2020, 10, 21579. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Iohara, K.; Zheng, L.; Ito, M.; Tomokiyo, A.; Matsushita, K.; Nakashima, M. Side Population Cells Isolated from Porcine Dental Pulp Tissue with Self-Renewal and Multipotency for Dentinogenesis, Chondrogenesis, Adipogenesis, and Neurogenesis. Stem Cells 2006, 24, 2493–2503. [Google Scholar] [CrossRef]
- D’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007, 14, 1162–1171. [Google Scholar] [CrossRef] [Green Version]
- Iohara, K.; Zheng, L.; Ito, M.; Ishizaka, R.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. Regeneration of dental pulp after pulpotomy by transplantation of CD31-/CD146-side population cells from a canine tooth. Regen. Med. 2009, 4, 377–385. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Sugiyama, M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009, 20, 435–440. [Google Scholar] [CrossRef]
- Yu, J.; He, H.; Tang, C.; Zhang, G.; Li, Y.; Wang, R.; Shi, J.; Jin, Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of Shh by a Neurovascular Bundle Niche Supports Mesenchymal Stem Cell Homeostasis in the Adult Mouse Incisor. Cell Stem Cell 2014, 14, 160–173. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, R.; Lee, H.-L.; Hong, C.; Wang, C.-Y. Single CD271 marker isolates mesenchymal stem cells from human dental pulp. Int. J. Oral Sci. 2015, 7, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Mabuchi, Y.; Toriumi, H.; Ebine, T.; Niibe, K.; Houlihan, D.; Morikawa, S.; Onizawa, K.; Kawana, H.; Akazawa, C.; et al. Purified Human Dental Pulp Stem Cells Promote Osteogenic Regeneration. J. Dent. Res. 2015, 95, 206–214. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Sabalic, M.; Bloomquist, R.; Fowler, T.E.; Streelman, T.; Sharpe, P.T. A quiescent cell population replenishes mesenchymal stem cells to drive accelerated growth in mouse incisors. Nat. Commun. 2018, 9, 378. [Google Scholar] [CrossRef] [Green Version]
- Vidovic, I.; Banerjee, A.; Fatahi, R.; Matthews, B.G.; Dyment, N.A.; Kalajzic, I.; Mina, M. Asma-Expressing Perivascular Cells Represent Dental Pulp Progenitors in Vivo. J. Dent. Res. 2017, 96, 323–330. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, H.; Kou, X.; Chen, C.; Liu, D.; Jin, Y.; Lu, L.; Shi, S. PD-1 is required to maintain stem cell properties in human dental pulp stem cells. Cell Death Differ. 2018, 25, 1350–1360. [Google Scholar] [CrossRef]
- Chen, H.; Fu, H.; Wu, X.; Duan, Y.; Zhang, S.; Hu, H.; Liao, Y.; Wang, T.; Yang, Y.; Chen, G.; et al. Regeneration of pulpo-dentinal–like complex by a group of unique multipotent CD24a+ stem cells. Sci. Adv. 2020, 6, eaay1514. [Google Scholar] [CrossRef] [Green Version]
- Duncan, H.F.; Smith, A.J.; Fleming, G.; Cooper, P. Epigenetic modulation of dental pulp stem cells: Implications for regenerative endodontics. Int. Endod. J. 2015, 49, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Xu, L.; Liu, H.; Sun, Q.; Chen, Z.; Yuan, G.; Chen, Z. KLF4 Promotes the Odontoblastic Differentiation of Human Dental Pulp Cells. J. Endod. 2011, 37, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, H.; Sun, Q.; Yuan, G.; Zhang, L.; Chen, Z. KLF4 promoted odontoblastic differentiation of mouse dental papilla cells via regulation of DMP1. J. Cell. Physiol. 2013, 228, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Lee, D.-S.; Park, S.-J.; Cho, K.-H.; Bae, H.-S.; Park, J.-C. Nuclear Factor I-C (NFIC) Regulates Dentin Sialophosphoprotein (DSPP) and E-cadherin via Control of Krüppel-like Factor 4 (KLF4) During Dentinogenesis. J. Biol. Chem. 2014, 289, 28225–28236. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Yu, S.; Chen, S.; Liu, H.; Chen, Z. SP1 regulates KLF4 via SP1 binding motif governed by DNA methylation during odontoblastic differentiation of human dental pulp cells. J. Cell. Biochem. 2019, 120, 14688–14699. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Lin, H.; Li, S.; Tao, H.; Zhang, L.; Yuan, G.; Chen, Z. Sp1 is a competitive endogenous RNA of Klf4 during odontoblast differentiation. Int. J. Biochem. Cell Biol. 2017, 85, 159–165. [Google Scholar] [CrossRef]
- Tao, H.; Lin, H.; Sun, Z.; Pei, F.; Zhang, J.; Chen, S.; Liu, H.; Chen, Z. Klf4 Promotes Dentinogenesis and Odontoblastic Differentiation via Modulation of TGF-β Signaling Pathway and Interaction with Histone Acetylation. J. Bone Miner. Res. 2019, 34, 1502–1516. [Google Scholar] [CrossRef]
- Li, Q.; Yi, B.; Feng, Z.; Meng, R.; Tian, C.; Xu, Q. FAM20C could be targeted by TET1 to promote odontoblastic differentiation potential of human dental pulp cells. Cell Prolif. 2017, 51, e12426. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Nakamura-Yamada, S.; Kusano, K.; Baba, S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int. J. Mol. Sci. 2019, 20, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jazayeri, H.E.; Lee, S.-M.; Kuhn, L.; Fahimipour, F.; Tahriri, M.; Tayebi, L. Polymeric scaffolds for dental pulp tissue engineering: A review. Dent. Mater. 2019, 36, e47–e58. [Google Scholar] [CrossRef]
- Matoug-Elwerfelli, M.; Duggal, M.S.; Nazzal, H.; Esteves, F.; Raïf, E. A biocompatible decellularized pulp scaffold for regenerative endodontics. Int. Endod. J. 2017, 51, 663–673. [Google Scholar] [CrossRef]
- Song, J.S.; Takimoto, K.; Jeon, M.; Vadakekalam, J.; Ruparel, N.; Diogenes, A. Decellularized Human Dental Pulp as a Scaffold for Regenerative Endodontics. J. Dent. Res. 2017, 96, 640–646. [Google Scholar] [CrossRef]
- Li, J.; Rao, Z.; Zhao, Y.; Xu, Y.; Chen, L.; Shen, Z.; Bai, Y.; Lin, Z.; Huang, Q. A Decellularized Matrix Hydrogel Derived from Human Dental Pulp Promotes Dental Pulp Stem Cell Proliferation, Migration, and Induced Multidirectional Differentiation In Vitro. J. Endod. 2020, 46, 1438–1447.e5. [Google Scholar] [CrossRef] [PubMed]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; El Moshy, S.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; El-Sayed, K.M.F. Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef]
- Alqahtani, Q.; Zaky, S.; Patil, A.; Beniash, E.; Ray, H.; Sfeir, C. Decellularized Swine Dental Pulp Tissue for Regenerative Root Canal Therapy. J. Dent. Res. 2018, 97, 1460–1467. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Pezeshki-Modaress, M.; Kiaipour, Z.; Shafiee, M.; Ellini, M.R.; Mazidi, A.; Rajabi, S.; Benisi, S.Z.; Ostad, S.N.; Galler, K.; et al. Pulp ECM-derived macroporous scaffolds for stimulation of dental-pulp regeneration process. Dent. Mater. 2019, 36, 76–87. [Google Scholar] [CrossRef]
- Matoug-Elwerfelli, M.; Nazzal, H.; Raif, E.M.; Wilshaw, S.-P.; Esteves, F.; Duggal, M. Ex-vivo recellularisation and stem cell differentiation of a decellularised rat dental pulp matrix. Sci. Rep. 2020, 10, 21553. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Lu, Q.; Chen, D.; Zhou, M.; Kuang, Y.; Ying, S.; Song, J. Proteomics and N-glycoproteomics analysis of an extracellular matrix-based scaffold-human treated dentin matrix. J. Tissue Eng. Regen. Med. 2019, 13, 1164–1177. [Google Scholar] [CrossRef]
- Li, R.; Guo, W.; Yang, B.; Guo, L.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011, 32, 4525–4538. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Colombo, J.S.; Ayre, W.N.; Sloan, A.; Waddington, R. Elucidating the cellular actions of demineralised dentine matrix extract on a clonal dental pulp stem cell population in orchestrating dental tissue repair. J. Tissue Eng. 2015, 6. [Google Scholar] [CrossRef]
- Salehi, S.; Cooper, P.; Smith, A.; Ferracane, J. Dentin matrix components extracted with phosphoric acid enhance cell proliferation and mineralization. Dent. Mater. 2016, 32, 334–342. [Google Scholar] [CrossRef]
- Chen, J.; Cui, C.; Qiao, X.; Yang, B.; Yu, M.; Guo, W.; Tian, W. Treated dentin matrix paste as a novel pulp capping agent for dentin regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 3428–3436. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Y.; Feng, K.-C.; Chuang, Y.-C.; Zuo, X.; Zhou, Y.; Chang, C.-C.; Simon, M.; Rafailovich, M. Templated dentin formation by dental pulp stem cells on banded collagen bundles nucleated on electrospun poly (4-vinyl pyridine) fibers in vitro. Acta Biomater. 2018, 76, 80–88. [Google Scholar] [CrossRef]
- Khoroushi, M.; Foroughi, M.R.; Karbasi, S.; Hashemibeni, B.; Khademi, A.A. Effect of Polyhydroxybutyrate/Chitosan/Bioglass nanofiber scaffold on proliferation and differentiation of stem cells from human exfoliated deciduous teeth into odontoblast-like cells. Mater. Sci. Eng. C 2018, 89, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.S.; Enderami, S.E.; Hadian, A.; Abazari, M.F.; Ardeshirylajimi, A.; Saburi, E.; Soleimanifar, F.; Nazemisalman, B. Efficient osteogenic differentiation of the dental pulp stem cells on β-glycerophosphate loaded polycaprolactone/polyethylene oxide blend nanofibers. J. Cell. Physiol. 2019, 234, 13951–13958. [Google Scholar] [CrossRef]
- Iohara, K.; Murakami, M.; Takeuchi, N.; Osako, Y.; Ito, M.; Ishizaka, R.; Utunomiya, S.; Nakamura, H.; Matsushita, K.; Nakashima, M. A Novel Combinatorial Therapy with Pulp Stem Cells and Granulocyte Colony-Stimulating Factor for Total Pulp Regeneration. Stem Cells Transl. Med. 2013, 2, 521–533. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K. Mobilized Dental Pulp Stem Cells for Pulp Regeneration: Initiation of Clinical Trial. J. Endod. 2014, 40, S26–S32. [Google Scholar] [CrossRef]
- Wu, R.-X.; Xu, X.-Y.; Wang, J.; He, X.; Sun, H.-H.; Chen, F.-M. Biomaterials for endogenous regenerative medicine: Coaxing stem cell homing and beyond. Appl. Mater. Today 2018, 11, 144–165. [Google Scholar] [CrossRef]
- Eramo, S.; Natali, A.; Pinna, R.; Milia, E. Dental pulp regeneration via cell homing. Int. Endod. J. 2017, 51, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, M.; Reddi, A.H. The application of bone morphogenetic proteins to dental tissue engineering. Nat. Biotechnol. 2003, 21, 1025–1032. [Google Scholar] [CrossRef]
- Yang, W.; Harris, M.; Cui, Y.; Mishina, Y.; Harris, S.; Gluhak-Heinrich, J. Bmp2 Is Required for Odontoblast Differentiation and Pulp Vasculogenesis. J. Dent. Res. 2011, 91, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Lee, C.; Chen, M.; Zhao, W.; Fu, S.; Qi, J.; Chotkowski, G.; Eisig, S.; Wong, A.; Mao, J. Induced Migration of Dental Pulp Stem Cells for in vivo Pulp Regeneration. J. Dent. Res. 2011, 90, 1013–1018. [Google Scholar] [CrossRef]
- Yang, J.W.; Zhang, Y.F.; Sun, Z.Y.; Song, G.T.; Chen, Z. Dental Pulp Tissue Engineering with Bfgf-Incorporated Silk Fibroin Scaffolds. J. Biomater. Appl. 2015, 30, 221–229. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chang, M.-C.; Chen, Y.-J.; Liou, J.-U.; Chang, H.-H.; Huang, W.-L.; Liao, W.-C.; Chan, C.-P.; Jeng, P.-Y.; Jeng, J.-H. Basic Fibroblast Growth Factor Regulates Gene and Protein Expression Related to Proliferation, Differentiation, and Matrix Production of Human Dental Pulp Cells. J. Endod. 2017, 43, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Dangaria, S.; Gopinathan, G.; Yan, X.; Lu, X.; Kolokythas, A.; Niu, Y.; Luan, X. SCF Promotes Dental Pulp Progenitor Migration, Neovascularization, and Collagen Remodeling—Potential Applications as a Homing Factor in Dental Pulp Regeneration. Stem Cell Rev. Rep. 2013, 9, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruangsawasdi, N.; Zehnder, M.; Patcas, R.; Ghayor, C.; Siegenthaler, B.; Gjoksi, B.; Weber, F.E. Effects of Stem Cell Factor on Cell Homing During Functional Pulp Regeneration in Human Immature Teeth. Tissue Eng. Part A 2017, 23, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-Y.; Chen, X.; Yue, L.; Huang, G.T.-J.; Zou, X.-Y. CXC Chemokine Receptor 4 Is Expressed Paravascularly in Apical Papilla and Coordinates with Stromal Cell–derived Factor-1α during Transmigration of Stem Cells from Apical Papilla. J. Endod. 2015, 41, 1430–1436. [Google Scholar] [CrossRef]
- Yang, J.-W.; Zhang, Y.; Wan, C.-Y.; Sun, Z.-Y.; Nie, S.; Jian, S.-J.; Zhang, L.; Song, G.-T.; Chen, Z. Autophagy in SDF-1α-mediated DPSC migration and pulp regeneration. Biomaterials 2015, 44, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Kim, G.-H.; Bae, Y.-K.; Jeong, D.-E.; Joo, K.-M.; Lee, K.; Lee, S.-H. Angiogenic Capacity of Dental Pulp Stem Cell Regulated by SDF-1α-CXCR4 Axis. Stem Cells Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Nor, F.; Oh, M.; Cucco, C.; Shi, S.; Nör, J.E. Wnt/β-Catenin Signaling Determines the Vasculogenic Fate of Postnatal Mesenchymal Stem Cells. Stem Cells 2016, 34, 1576–1587. [Google Scholar] [CrossRef] [Green Version]
- Bae, W.-J.; Yi, J.-K.; Park, J.; Kang, S.-K.; Jang, J.-H.; Kim, E.-C. Lysyl oxidase-mediated VEGF-induced differentiation and angiogenesis in human dental pulp cells. Int. Endod. J. 2017, 51, 335–346. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, F.; Zhang, X.; Wang, S.; Jin, Y.; Zhang, W.; Jiang, X. The Effects of Platelet-Derived Growth Factor-BB on Human Dental Pulp Stem Cells Mediated Dentin-Pulp Complex Regeneration. Stem Cells Transl. Med. 2017, 6, 2126–2134. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z. PDGF-BB, NGF and BDNF enhance pulp-like tissue regeneration via cell homing. RSC Adv. 2016, 6, 109519–109527. [Google Scholar] [CrossRef]
- Dissanayaka, W.; Zhang, C. The Role of Vasculature Engineering in Dental Pulp Regeneration. J. Endod. 2017, 43, S102–S106. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Lachaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Liu, B.; Tulu, U.; Helms, J. Wnt-Responsive Odontoblasts Secrete New Dentin after Superficial Tooth Injury. J. Dent. Res. 2018, 97, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, G.; Wu, J.; Zhou, X.; Zhao, Y.; Chen, Z.; Lin, Z.; Xiao, Y. Multi-faceted effects of mesenchymal stem cells (MSCs) determined by immune microenvironment and their implications on MSC/biomaterial-based inflammatory disease therapy. Appl. Mater. Today 2019, 18, 100485. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Bottino, M.C.; Fouad, A.F.; Nör, J.E.; Huang, G.T.-J. Animal Models for Stem Cell-Based Pulp Regeneration: Foundation for Human Clinical Applications. Tissue Eng. Part B Rev. 2019, 25, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Kawamura, R.; Nishimatsu, S.-I.; Fukuta, O.; Nakashima, M. Stem Cell-Induced Pulp Regeneration Can Be Enhanced by Administration of CCL11-Neutralizing Antibody in the Ectopic Tooth Transplantation Model in the Aged Mice. Rejuvenation Res. 2019, 22, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Alexander, A.; Vahdati, S.A.; Grandhi, A.; Baylink, D.; Shabahang, S. Effect of Residual Dental Pulp Tissue on Regeneration of Dentin-pulp Complex: An In Vivo Investigation. J. Endod. 2018, 44, 1796–1801. [Google Scholar] [CrossRef]
- Alexander, A.; Torabinejad, M.; Vahdati, S.A.; Nosrat, A.; Verma, P.; Grandhi, A.; Shabahang, S. Regenerative Endodontic Treatment in Immature Noninfected Ferret Teeth Using Blood Clot or SynOss Putty as Scaffolds. J. Endod. 2019, 46, 209–215. [Google Scholar] [CrossRef]
- Zayed, M.; Iohara, K.; Watanabe, H.; Nakashima, M. CCR3 antagonist protects against induced cellular senescence and promotes rejuvenation in periodontal ligament cells for stimulating pulp regeneration in the aged dog. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Iohara, K.; Utsunomiya, S.; Kohara, S.; Nakashima, M. Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Res. Ther. 2018, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Liu, J.; Yu, Z.; Chen, C.-A.; Aksel, H.; Azim, A.; Huang, G.T.-J. A Miniature Swine Model for Stem Cell-Based De Novo Regeneration of Dental Pulp and Dentin-Like Tissue. Tissue Eng. Part C Methods 2018, 24, 108–120. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Yang, B.; Li, L.; Luo, X.; Zhang, X.; Feng, L.; Jiang, Z.; Yu, M.; Guo, W.; et al. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials 2015, 52, 56–70. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, Q.; Karabucak, B.; Le, A. DPSCs from Inflamed Pulp Modulate Macrophage Function via the TNF-α/IDO Axis. J. Dent. Res. 2016, 95, 1274–1281. [Google Scholar] [CrossRef] [Green Version]
- Fawzy El-Sayed, K.M.; Elsalawy, R.; Ibrahim, N.; Gadalla, M.; Albargasy, H.; Zahra, N.; Mokhtar, S.; El Nahhas, N.; El Kaliouby, Y.; Dörfer, C.E. The Dental Pulp Stem/Progenitor Cells-Mediated Inflammatory-Regenerative Axis. Tissue Eng. Part B Rev. 2019, 25, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-L.; Liu, W.; Wu, Y.-M.; Sun, W.-L.; Dörfer, C.E.; El-Sayed, K.M.F. Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters. Stem Cells Int. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collart-Dutilleul, P.-Y.; Chaubron, F.; De Vos, J.; Cuisinier, F.J. Allogenic banking of dental pulp stem cells for innovative therapeutics. World J. Stem Cells 2015, 7, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Urraca, N.; Skobowiat, C.; Hope, K.A.; Miravalle, L.; Chamberlin, R.; Donaldson, M.; Seagroves, T.N.; Reiter, L.T. Assessment of the Tumorigenic Potential of Spontaneously Immortalized and Htert-Immortalized Cultured Dental Pulp Stem Cells. Stem Cells Transl. Med. 2015, 4, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-C.; Lai, Y.-C.; Li, L.-H.; Liao, K.; Lai, H.-C.; Kao, S.-Y.; Wang, J.; Chuong, C.-M.; Hung, S.-C. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat. Commun. 2019, 10, 2226. [Google Scholar] [CrossRef]
- Wang, W.; Dang, M.; Zhang, Z.; Hu, J.; Eyster, T.W.; Ni, L.; Ma, P.X. Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomater. 2016, 36, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ma, C.; Xie, X.; Sun, H.; Liu, X. Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system. Acta Biomater. 2016, 35, 57–67. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Q.; Xie, L.; Zhang, R.; Qian, R.; Tian, Y.; Chen, G.; Tian, W. Hdpsc-Laden Gelma Microspheres Fabricated Using Electrostatic Microdroplet Method for Endodontic Regeneration. Mater. Sci. Eng. C 2021, 121, 111850. [Google Scholar] [CrossRef]
- Xing, X.; Han, S.; Li, Z.; Li, Z. Emerging Role of Exosomes in Craniofacial and Dental Applications. Theranostics 2020, 10, 8648–8664. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F.; Ravindran, S.; Huang, C.-C.; Kang, M. A Role for Exosomes in Craniofacial Tissue Engineering and Regeneration. Front. Physiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhong, Y.; Kong, Y.; Chen, Y.; Feng, J.; Zheng, J. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res. Ther. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xian, X.; Gong, Q.; Li, C.; Guo, B.; Jiang, H. Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J. Endod. 2018, 44, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Kumar, S.; Padmanabhan, P.; Gulyas, B.; Wan, A.C.; Rajendran, V.M. Lineage-specific exosomes could override extracellular matrix mediated human mesenchymal stem cell differentiation. Biomaterials 2018, 182, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Jia, S.; Chen, H.; Duan, Y.; Li, X.; Wang, S.; Wang, T.; Lyu, Y.; Chen, G.; et al. Exosome-Like Vesicles Derived from Hertwigss Epithelial Root Sheath Cells Promote the Regeneration of Dentin-Pulp Tissue. Theranostics 2020, 10, 5914–5931. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Ye, Y.; He, S.; Song, J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation 2019, 111, 1–11. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, J.-Y.; Zhou, G. Emerging functions and clinical applications of exosomes in human oral diseases. Cell Biosci. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Shi, X.; Mao, J.; Liu, Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Vijaykumar, A.; Root, S.; Mina, M. Wnt/β-Catenin Signaling Promotes the Formation of Preodontoblasts In Vitro. J. Dent. Res. 2020, 100, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Etxebarria, V.; Agliano, A.; Unda, F.; Ibarretxe, G. Wnt signaling reprograms metabolism in dental pulp stem cells. J. Cell. Physiol. 2018, 234, 13068–13082. [Google Scholar] [CrossRef] [Green Version]
- Uribe-Etxebarria, V.; García-Gallastegui, P.; Pérez-Garrastachu, M.; Andres, M.D.R.C.; Irastorza, I.; Unda, F.; Ibarretxe, G.; Subirán, N. Wnt-3a Induces Epigenetic Remodeling in Human Dental Pulp Stem Cells. Cells 2020, 9, 652. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Meza, G.; Urrejola, D.; Jean, N.S.; Inostroza, C.; López, V.; Khoury, M.; Brizuela, C. Personalized Cell Therapy for Pulpitis Using Autologous Dental Pulp Stem Cells and Leukocyte Platelet-rich Fibrin: A Case Report. J. Endod. 2019, 45, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, V.P.; Mota, M.N.G.; Vieira, L.V.; de Paula, D.M.; Gomes, L.L.R.; Solheiro, L.K.R.; Aguiar Neto, M.A.; Carvalho, D.A.L.; Silvestre, F.A. Dental Pulp Autotransplantation: A New Modality of Endodontic Regenerative Therapy-Follow-up of 3 Clinical Cases. J. Endod. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shawli, H.; Iohara, K.; Tarrosh, M.; Huang, G.T.-J.; Nakashima, M.; Azim, A.A. Nanobubble-Enhanced Antimicrobial Agents: A Promising Approach for Regenerative Endodontics. J. Endod. 2020, 46, 1248–1255. [Google Scholar] [CrossRef]




| Approaches | Root Canal Therapy (RCT) | Pulp Revascularisation | Pulp Regeneration |
|---|---|---|---|
| Treatment procedures | Root canal preparation, disinfection and filling [4] | Sufficient chemical disinfection, induction of bleeding into the canal and careful sealing [9] | Based on tissue engineering strategies, such as exogenous cell transplantation and endogenous cell homing [7,8] |
| Root canal fillings | Inert materials such as gutta-percha | Periodontal-like tissue [11] | Pulp/dentin-like tissue |
| Outcomes | Nonvital teeth | Uncertainty (new dentin formation and continued root development) | Vital teeth (restored homeostasis and natural defence that promote tooth survival) |
| Complications and limitations |
|
| Involved Strategies | Exogenous Stem Cells | Biomaterials | Growth Factors | Examples | Situations |
|---|---|---|---|---|---|
| Cell transplantation | Yes | Yes | Yes | Pulp CD105+ cells with SDF-1 and collagen scaffold [13] | Inferior vitality and limited sources of donor cells (elderly individuals, people with systemic diseases) |
| Yes | Yes | None | DPSCs within nanofibrous spongy microspheres [26,27] | ||
| Yes | None | Yes | DPSCs mobilised by G-CSF [28] | ||
| Yes | None | None | DPSCs electrotransfected with Gdf11 [29], iPS cells [30], SHED aggregates [31], and DPSC injection solution [32] | ||
| Stem cell-derived extracellular vesicles | None | Yes | Yes | DPSC-derived exosomes within collagen sponges [33] | Avoiding the dosage, delivery and safety concerns of exogenous stem cells and growth factors |
| Cell homing | None | None | None | Pulp revascularisation [9] | Immature permanent teeth |
| None | None | Yes | Autologous platelet concentrates applied in pulp revascularisation [25] | Immature and mature permanent teeth | |
| None | Yes | Yes | A combination of Wnt3a, BMP7 and collagen gel [34] | Inferior vitality and limited sources of donor cells (elderly individuals, people with systemic diseases) |
| Timeline | Clinical Significance | Methods | Species/Implantation Sites |
|---|---|---|---|
| 2000 [35] | Discovery of DPSCs | DPSC transplantation with HA/TCP powder | Mice/subcutaneous space |
| 2003 [36] | Discovery of SHEDs | SHED transplantation with HA/TCP powder | Mice/subcutaneous space |
| 2004 [29] | The first DPSC transplantation in a dog model | Gdf11-transfected DPSCs cultured as a pellet | Dogs/root canals after partial pulpectomy |
| 2007 [41] | Successful revascularisation after DPSC transplantation | DPSCs seeded in human tooth slices | Mice/subcutaneous space |
| 2010 [42] | The goal of functional pulp/dentin regeneration and major concerns, such as less organised dentinal tubules and vascularity, are addressed | DPSC transplantation with PLG scaffolds and human tooth root fragments | Mice/subcutaneous space |
| 2010 [15] | Cell homing approach for pulp regeneration | Endodontic treatment of human teeth with a cytokine-adsorbed collagen gel, such as that containing bFGF, VEGF, or PDGF | Mice/subcutaneous space |
| 2011 [13] | The first complete orthotopic pulp regeneration replete with angiogenesis and neurogenesis | Pulp CD105+ cells/SDF-1/collagen scaffold | Dogs/root canals after pulpectomy |
| 2018 [31] | The first randomised clinical trial using autologous SHEDs for pulp regeneration | Autologous SHED aggregates containing cells and ECM | Immature permanently injured incisors |
| 2020 [32] | Registration of DPSC injection solution for clinical applications | DPSC injection solution | Patients |
| Stem Cells | Advantages | Disadvantages | References |
|---|---|---|---|
| DPSCs |
|
| [40,43,44,45] |
| SHEDs |
|
| [46,47,48] |
| PDLSCs |
|
| [37,49] |
| DFPCs |
|
| [50,51] |
| SCAPs |
|
| [52,53] |
| HERS |
|
| [54,55] |
| BMSCs |
|
| [43,44,45,56] |
| ADSCs |
|
| [43,44,45] |
| Surface Markers | Authors and Year | Events and Results | Specific Functions |
|---|---|---|---|
| Flk-1+ (VEGF-R2+) | Aquino et al. (2007) [59] | Giving rise to the regeneration of vascularised tissues by synergistically differentiating into osteoblasts and endothelial cells. | Angiogenesis |
| CD31−/CD146− | Iohara et al. (2009) [58,60] | Total pulp regeneration with capillaries, nerve cells, and expression of pro-angiogenic factors. | Dentinogenesis, angiogenesis and neurogenesis |
| CD105+ | Iohara et al. (2009) [13,61] | Larger regenerated pulp tissues including nerves, vasculature and dentin formation. | Potent trophic effects on neovascularization |
| STRO-1+ | Yu et al. (2010) [62] | Spontaneous differentiation into odontoblasts, osteoblasts, and chondrocytes. | Dentinogenesis, osteogenesis and chondrogenesis |
| NG2+ | Zhao et al. (2014) [63] | Participation in emergency responses such as injury repair of pulp tissues rather than physiological homeostasis. | Actively involved in reparative dentin formation |
| CD271+ (LNGFR+) | Alvarez et al. (2015) [64] | The isolation of a relatively large population of DPSCs (10.6%) with the strongest odontogenic and chondrogenic potential. | Dentinogenesis and chondrogenesis |
| CD271Low+CD90High+ | Yasui et al. (2016) [65] | The most clonogenic population in dental pulp capable of adipogenic, osteogenic, and chondrogenic differentiation in vitro and promotion of new bone formation in vivo. | Long-term viability, clonogenicity and osteogenicity |
| CD90+ (Thy-1+) | An et al. (2018) [66] | Contributes 30% of the odontoblasts and pulp cells during early postnatal development, as well as when the tips of the incisors are clipped. | Corresponding to a rapid growth rate increase in both established and re-established tooth length |
| αSMA | Vidovic et al. (2017) [67] | A second generation of odontoblasts during reparative dentinogenesis, also a small contribution to odontoblasts during primary dentinogenesis. | Dentinogenesis |
| PD-1 | Liu et al. (2018) [68] | Controlling cell proliferation and multipotential differentiation of DPSCs. | Stemness maintenance |
| CD24a+ | Chen et al. (2020) [69] | Enhanced osteogenic/odontogenic differentiation capabilities, regenerative dentin and neurovascular-like structures formation in vivo. | High proliferative and self-renewal capacity, highly efficient regeneration of pulpodentinal complex-like tissues |
| Growth Factors | Authors/Years | Employed Cells | Mechanism | Test Model | Functions |
|---|---|---|---|---|---|
| BMP2, 4, 7 * | Nakashima et al. (2003) [99] | DPSCs | BMP/Smad pathway | In vitro | Pulpodentinal complex, periodontal and craniofacial regeneration |
| Yang et al. (2012) [100] | DPSCs | VEGFA/VEGFR2 pathway | In vivo | Dentinogenesis and angiogenesis | |
| bFGF | Suzuki et al. (2011) [101] | None | None | In vivo | Induction of recellularisation and revascularisation |
| Yang et al. (2015) [102] | DPSCs | None | In vivo | Dentinogenesis, angiogenesis and neurogenesis | |
| Chang et al. (2017) [103] | DPSCs | MEK/ERK pathway | In vitro | Promotion of proliferation, differentiation, and matrix production of DPSCs | |
| VEGF | Silvana et al. (2007) [41] | DPSCs | SDF-1α activation and angiogenic cascade initiation | In vivo | Dentinogenesis and angiogenesis |
| Zhang et al. (2016) [109] | DPSCs | Wnt/β-catenin pathway | In vivo | Vasculogenesis and angiogenesis | |
| Bae et al. (2018) [110] | DPSCs | LOX activation | In vitro | Odontogenesis and angiogenesis | |
| SCF | Pan et al. (2013) [104]; Ruangsawasdi et al. (2017) [105] | DPSCs | PI3K/Akt and MEK/ERK pathway | In vivo | Promotion of DPSC migration, neovascularisation, and collagen remodelling |
| G-CSF * | Nakashima et al. (2013) [28,95,96] | MDPSCs | G-CSF/G-CSFR | In situ | Inhibition of apoptosis, promotion of cell survival, suppression of inflammation, and induction of angiogenesis and neurogenesis |
| SDF-1α | Li et al. (2015) [106] | SCAPs | SDF-1α/CXCR4 axis | In vitro | Chemotactic function |
| Yang et al. (2015)107 | DPSCs | Autophagy activation | In situ | Mineralisation, neovascularisation and chemotactic function; probably innervation | |
| Nam et al. (2017) [108] | DPSCs | SDF-1α/CXCR4 axis | In vivo | Angiogenesis | |
| PDGF-BB * | Zhang et al. (2017) [111] | DPSCs | PI3K/Akt pathway | In vivo | Enhancing hDPSC proliferation, migration, angiogenesis, and odontogenic differentiation |
| L-WNT3A | Zhao et al. (2018) [115]; Chen et al. (2020) [116] | DPSCs and odontoblasts | Wnt/β-catenin pathway | In vivo | Prosurvival and antiapoptic effects, as well as induction of more tertiary dentin formation |
| Research Patterns | Animals | Scaffold /Implantation Sites | Advantages | Limitations | Application Conditions |
|---|---|---|---|---|---|
| Ectopic model | Mice/rats [35,36] | HA/TCP; the dorsum subcutaneous space |
|
|
|
| Semiorthotopic model | Mice/rats [41,42,118] | Tooth slices/fragments; the dorsum subcutaneous space |
|
|
|
| Orthotopic model | Ferrets [117,119,120] | Various biomaterials; root canals after pulpotomy or pulpectomy |
|
|
|
| Dogs [121,122] |
|
|
| ||
| Swine [123,124] |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. Int. J. Mol. Sci. 2021, 22, 8991. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168991
Xie Z, Shen Z, Zhan P, Yang J, Huang Q, Huang S, Chen L, Lin Z. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. International Journal of Molecular Sciences. 2021; 22(16):8991. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168991
Chicago/Turabian StyleXie, Zhuo, Zongshan Shen, Peimeng Zhan, Jiayu Yang, Qiting Huang, Shuheng Huang, Lingling Chen, and Zhengmei Lin. 2021. "Functional Dental Pulp Regeneration: Basic Research and Clinical Translation" International Journal of Molecular Sciences 22, no. 16: 8991. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168991