Plasma Membrane Calcium ATPase-Neuroplastin Complexes Are Selectively Stabilized in GM1-Containing Lipid Rafts
Abstract
:1. Introduction
2. Results
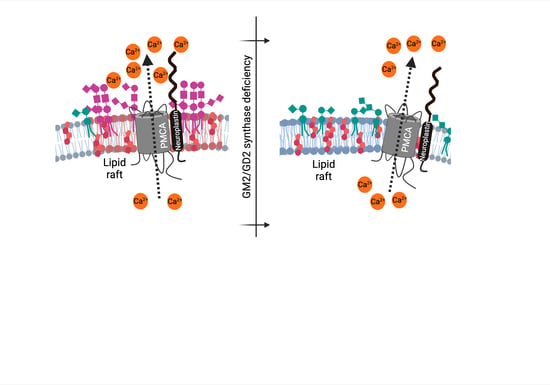
2.1. Content of Neuroplastin and PMCAs Is Altered in Lipid Rafts from GM2/GD2 Synthase-Deficient Mice
2.2. Neuroplastin 65 Colocalizes with GM1 Ganglioside in Cultured Hippocampal Neurons
2.3. Antibody Engagement of GM1 Ganglioside Results in Prolonged Calcium Level Restoration
2.4. Brain Ganglioside Content and Composition Are Not Significantly Affected by Neuroplastin Deficiency
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Lipid Raft Isolation
4.3. Western Blotting
4.4. Rat Neuronal Culture Preparation
4.5. Live Neuron Staining
4.6. Confocal Imaging and Image Analysis
4.7. Single Neuron Calcium Imaging Using Fluo4-AM
4.8. Ganglioside Purification
4.9. Ganglioside Analyses
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Sonnino, S.; Aureli, M.; Mauri, L.; Ciampa, M.G.; Prinetti, A. Membrane lipid domains in the nervous system. Front. Biosci. 2015, 20, 280–302. [Google Scholar] [CrossRef] [Green Version]
- Strehler, E.E.; Zacharias, D.A. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol. Rev. 2001, 81, 21–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamoto, E.M.; Vivar, C.; Camandola, S. Physiology and pathology of calcium signaling in the brain. Front. Pharmacol. 2012, 3, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, N.; Wilson, C.; Oceandy, D.; Neyses, L.; Cartwright, E.J. The plasma membrane calcium ATPases and their role as major new players in human disease. Physiol. Rev. 2017, 97, 1089–1125. [Google Scholar] [CrossRef] [Green Version]
- Gong, D.; Chi, X.; Ren, K.; Huang, G.; Zhou, G.; Yan, N.; Lei, J.; Zhou, Q. Structure of the human plasma membrane Ca2+-ATPase 1 in complex with its obligatory subunit neuroplastin. Nat. Commun. 2018, 9, 3623. [Google Scholar] [CrossRef]
- Schmidt, N.; Kollewe, A.; Constantin, C.E.; Henrich, S.; Ritzau-Jost, A.; Bildl, W.; Saalbach, A.; Hallermann, S.; Kulik, A.; Fakler, B.; et al. Neuroplastin and basigin are essential auxiliary subunits of plasma membrane Ca2+-ATPases and key regulators of Ca2+ clearance. Neuron 2017, 96, 827–838.e9. [Google Scholar] [CrossRef] [Green Version]
- Go, C.K.; Soboloff, J. Hold the door: hPMCA1/neuroplastin interactions regulate Ca2+-binding site accessibility. Cell Calcium 2018, 76, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Smalla, K.H.; Matthies, H.; Langnase, K.; Shabir, S.; Bockers, T.M.; Wyneken, U.; Staak, S.; Krug, M.; Beesley, P.W.; Gundelfinger, E.D. The synaptic glycoprotein neuroplastin is involved in long-term potentiation at hippocampal CA1 synapses. Proc. Natl. Acad. Sci. USA 2000, 97, 4327–4332. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Molina, R.; Sarto-Jackson, I.; Montenegro-Venegas, C.; Heine, M.; Smalla, K.H.; Seidenbecher, C.I.; Beesley, P.W.; Gundelfinger, E.D.; Montag, D. Structure of excitatory synapses and GABAA receptor localization at inhibitory synapses are regulated by neuroplastin-65. J. Biol. Chem. 2014, 289, 8973–8988. [Google Scholar] [CrossRef] [Green Version]
- Desrivieres, S.; Lourdusamy, A.; Tao, C.; Toro, R.; Jia, T.; Loth, E.; Medina, L.M.; Kepa, A.; Fernandes, A.; Ruggeri, B.; et al. Single nucleotide polymorphism in the neuroplastin locus associates with cortical thickness and intellectual ability in adolescents. Mol. Psychiatry 2015, 20, 263–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owczarek, S.; Berezin, V. Neuroplastin: Cell adhesion molecule and signaling receptor. Int. J. Biochem. Cell Biol. 2012, 44, 1–5. [Google Scholar] [CrossRef]
- Herrera-Molina, R.; Mlinac-Jerkovic, K.; Ilic, K.; Stober, F.; Vemula, S.K.; Sandoval, M.; Milosevic, N.J.; Simic, G.; Smalla, K.H.; Goldschmidt, J.; et al. Neuroplastin deletion in glutamatergic neurons impairs selective brain functions and calcium regulation: Implication for cognitive deterioration. Sci. Rep. 2017, 7, 7273. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zeng, J.; Huang, L.; Wu, D.; Liu, L.; Liu, Y.; Yuan, Q. Microarray analysis of gene expression changes in neuroplastin 65-knockout mice: Implications for abnormal cognition and emotional disorders. Neurosci. Bull. 2018, 34, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Vemula, S.K.; Malci, A.; Junge, L.; Lehmann, A.C.; Rama, R.; Hradsky, J.; Matute, R.A.; Weber, A.; Prigge, M.; Naumann, M.; et al. The interaction of TRAF6 with neuroplastin promotes spinogenesis during early neuronal development. Front. Cell Dev. Biol. 2020, 8, 579513. [Google Scholar] [CrossRef]
- Ilic, K.; Mlinac-Jerkovic, K.; Jovanov-Milosevic, N.; Simic, G.; Habek, N.; Bogdanovic, N.; Kalanj-Bognar, S. Hippocampal expression of cell-adhesion glycoprotein neuroplastin is altered in Alzheimer’s disease. J. Cell. Mol. Med. 2019, 23, 1602–1607. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Herrera-Molina, R.; Sabanov, V.; Ahmed, T.; Iscru, E.; Stober, F.; Richter, K.; Fischer, K.D.; Angenstein, F.; Goldschmidt, J.; et al. Genetically induced retrograde amnesia of associative memories after neuroplastin ablation. Biol. Psychiatry 2017, 81, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.H.; Wei, M.; Zhang, C.; Shi, Y.S. The amino-terminal domain of GluA1 mediates LTP maintenance via interaction with neuroplastin-65. Proc. Natl. Acad. Sci. USA 2021, 118, e2019194118. [Google Scholar] [CrossRef]
- Ilic, K.; Mlinac-Jerkovic, K.; Sedmak, G.; Rosenzweig, I.; Kalanj-Bognar, S. Neuroplastin in human cognition: Review of literature and future perspectives. Transl. Psychiatry 2021, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liang, Y.; Herrera-Molina, R.; Montag, D. Neuroplastin in neuropsychiatric diseases. Genes 2021, 12, 1507. [Google Scholar] [CrossRef] [PubMed]
- Mlinac, K.; Jovanov Milosevic, N.; Heffer, M.; Smalla, K.H.; Schnaar, R.L.; Kalanj Bognar, S. Neuroplastin expression in the hippocampus of mice lacking complex gangliosides. J. Mol. Neurosci. 2012, 48, 161–166. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [Green Version]
- Schnaar, R.L. The biology of gangliosides. Adv. Carbohydr. Chem. Biochem. 2019, 76, 113–148. [Google Scholar] [CrossRef]
- Padanyi, R.; Paszty, K.; Hegedus, L.; Varga, K.; Papp, B.; Penniston, J.T.; Enyedi, A. Multifaceted plasma membrane Ca2+ pumps: From structure to intracellular Ca2+ handling and cancer. Biochim. Biophys. Acta 2016, 1863, 1351–1363. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Fernandes, D.; Mehta, N.; Bean, J.L.; Michaelis, M.L.; Zaidi, A. Partitioning of the plasma membrane Ca2+-ATPase into lipid rafts in primary neurons: Effects of cholesterol depletion. J. Neurochem. 2007, 102, 378–388. [Google Scholar] [CrossRef]
- Sepulveda, M.R.; Berrocal-Carrillo, M.; Gasset, M.; Mata, A.M. The plasma membrane Ca2+-ATPase isoform 4 is localized in lipid rafts of cerebellum synaptic plasma membranes. J. Biol. Chem. 2006, 281, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Antalffy, G.; Enyedi, A.; Strehler, E.E. Apical localization of PMCA2w/b is lipid raft-dependent. Biochem. Biophys. Res. Commun. 2009, 384, 32–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlinac-Jerkovic, K.; Ilic, K.; Zjalić, M.; Mandic, D.; Debeljak, Z.; Balog, M.; Damjanovic, V.; Macek Hrvat, N.; Habek, N.; Kalanj-Bognar, S.; et al. Who’s in, who’s out? Re-evaluation of lipid raft residents. J. Neurochem. 2021, 158, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Puljko, B.; Stojanović, M.; Ilic, K.; Hrvat, N.; Zovko, A.; Damjanovic, V.; Mlinac-Jerkovic, K.; Kalanj-Bognar, S. Redistribution of gangliosides accompanies thermally induced Na+, K+-ATPase activity alternation and submembrane localisation in mouse brain. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183475. [Google Scholar] [CrossRef] [PubMed]
- Persaud-Sawin, D.A.; Lightcap, S.; Harry, G.J. Isolation of rafts from mouse brain tissue by a detergent-free method. J. Lipid Res. 2009, 50, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlinac, K.; Fabris, D.; Vukelic, Z.; Rozman, M.; Heffer, M.; Bognar, S.K. Structural analysis of brain ganglioside acetylation patterns in mice with altered ganglioside biosynthesis. Carbohydr. Res. 2013, 382, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, H.; Yoshida, S.; Sato, K.; Yamagata, T. Interaction of ganglioside with specific peptide sequences as a mechanism for the modulation of calmodulin-dependent enzymes. J. Biochem. 1996, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Higashi, H.; Omori, A.; Yamagata, T. Calmodulin, a ganglioside-binding protein. Binding of gangliosides to calmodulin in the presence of calcium. J. Biol. Chem. 1992, 267, 9831–9838. [Google Scholar] [CrossRef]
- Jiang, L.; Bechtel, M.D.; Bean, J.L.; Winefield, R.; Williams, T.D.; Zaidi, A.; Michaelis, E.K.; Michaelis, M.L. Effects of gangliosides on the activity of the plasma membrane Ca2+-ATPase. Biochim Biophys Acta 2014, 1838, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Zhang, J.; Zhao, Y.; Yang, F.; Zhang, X. Ganglioside GM2 modulates the erythrocyte Ca2+-ATPase through its binding to the calmodulin-binding domain and its ‘receptor’. Arch. Biochem. Biophys. 2006, 454, 155–159. [Google Scholar] [CrossRef]
- Huang, M.; Hu, M.; Song, Q.; Song, H.; Huang, J.; Gu, X.; Wang, X.; Chen, J.; Kang, T.; Feng, X.; et al. GM1-Modified Lipoprotein-like Nanoparticle: Multifunctional nanoplatform for the combination therapy of Alzheimer’s disease. ACS Nano 2015, 9, 10801–10816. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, K.; Yamamoto, A.; Furukawa, K.; Yamashiro, S.; Shin, M.; Okada, M.; Fukumoto, S.; Haraguchi, M.; Takeda, N.; Fujimura, K.; et al. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc. Natl. Acad. Sci. USA 1996, 93, 10662–10667. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.; Fromholt, S.E.; Hess, E.J.; Crawford, T.O.; Griffin, J.W.; Sheikh, K.A.; Schnaar, R.L. Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: Neuropathology and behavioral deficits in single- and double-null mice. Exp. Neurol. 2005, 195, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, K.A.; Sun, J.; Liu, Y.; Kawai, H.; Crawford, T.O.; Proia, R.; Griffin, J.W.; Schnaar, R.L. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc. Natl. Acad. Sci. USA 1999, 96, 7532–7537. [Google Scholar] [CrossRef] [Green Version]
- Chiavegatto, S.; Sun, J.; Nelson, R.J.; Schnaar, R. A functional role for complex gangliosides: Motor deficits in GM2/GD2 synthase knockout mice. Exp. Neurol. 2000, 166, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, K.; Takamiya, K.; Furukawa, K. Beta1,4-N-acetylgalactosaminyltransferase—GM2/GD2 synthase: A key enzyme to control the synthesis of brain-enriched complex gangliosides. Biochim. Biophys. Acta 2002, 1573, 356–362. [Google Scholar] [CrossRef]
- Sha, S.; Zhou, L.; Yin, J.; Takamiya, K.; Furukawa, K.; Furukawa, K.; Sokabe, M.; Chen, L. Deficits in cognitive function and hippocampal plasticity in GM2/GD2 synthase knockout mice. Hippocampus 2014, 24, 369–382. [Google Scholar] [CrossRef]
- Harlalka, G.V.; Lehman, A.; Chioza, B.; Baple, E.L.; Maroofian, R.; Cross, H.; Sreekantan-Nair, A.; Priestman, D.A.; Al-Turki, S.; McEntagart, M.E.; et al. Mutations in B4GALNT1 (GM2 synthase) underlie a new disorder of ganglioside biosynthesis. Brain 2013, 136, 3618–3624. [Google Scholar] [CrossRef] [Green Version]
- Boukhris, A.; Schüle-Freyer, R.; Loureiro, J.L.; Lourenço, C.M.; Mundwiller, E.; Gonzalez, M.A.; Charles, P.; Gauthier, J.; Rekik, I.; Lebrigio, R.F.A.; et al. Alteration of ganglioside biosynthesis responsible for complex hereditary spastic paraplegia. Am. J. Hum. Genet. 2013, 93, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakil, S.M.; Monies, D.M.; Ramzan, K.; Hagos, S.; Bastaki, L.; Meyer, B.F.; Bohlega, S. Novel B4GALNT1mutations in a complicated form of hereditary spastic paraplegia. Clin. Genet. 2014, 86, 500–501. [Google Scholar] [CrossRef]
- Rose, L.; Hall, K.; Tang, S.; Hasadsri, L.; Kimonis, V. Homozygous B4GALNT1 mutation and biochemical glutaric acidemia type II: A case report. Clin. Neurol. Neurosurg. 2020, 189, 105553. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.J.; Xu, C.H.; Li, D.; Liu, Z.J.; Wu, Y. The investigation of genetic and clinical features in patients with hereditary spastic paraplegia in central-Southern China. Mol. Genet. Genom. Med. 2021, 9, e1627. [Google Scholar] [CrossRef]
- Li, M.; Ho, P.W.; Pang, S.Y.; Tse, Z.H.; Kung, M.H.; Sham, P.C.; Ho, S.L. PMCA4 (ATP2B4) mutation in familial spastic paraplegia. PLoS ONE 2014, 9, e104790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brini, M.; Carafoli, E.; Cali, T. The plasma membrane calcium pumps: Focus on the role in (neuro)pathology. Biochem. Biophys. Res. Commun. 2017, 483, 1116–1124. [Google Scholar] [CrossRef]
- Rawal, P.; Zhao, L. Sialometabolism in brain health and Alzheimer’s disease. Front. Neurosci. 2021, 15, 648617. [Google Scholar] [CrossRef] [PubMed]
- Rahmann, H. Brain gangliosides and memory formation. Behav. Brain Res. 1995, 66, 105–116. [Google Scholar] [CrossRef]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the brain: Physiology, pathophysiology and therapeutic applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Carafoli, E. Calcium pumps in health and disease. Physiol. Rev. 2009, 89, 1341–1378. [Google Scholar] [CrossRef] [Green Version]
- Empson, R.M.; Garside, M.L.; Knöpfel, T. Plasma Membrane Ca2+ ATPase 2 contributes to short-term synapse plasticity at the parallel fiber to Purkinje neuron synapse. J. Neurosci. 2007, 27, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.A.; Patel, H.V.; Vyas, A.A.; Schnaar, R.L. Segregation of gangliosides GM1 and GD3 on cell membranes, isolated membrane rafts, and defined supported lipid monolayers. Biol. Chem. 2001, 382, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, G.; Fazzari, M.; Di Biase, E.; Mauri, L.; Sonnino, S.; Chiricozzi, E. Modulation of calcium signaling depends on the oligosaccharide of GM1 in Neuro2a mouse neuroblastoma cells. Glycoconj. J. 2020, 37, 713–727. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Lunghi, G.; Di Biase, E.; Fazzari, M.; Sonnino, S.; Mauri, L. GM1 ganglioside is a key factor in maintaining the mammalian neuronal functions avoiding neurodegeneration. Int. J. Mol. Sci. 2020, 21, 868. [Google Scholar] [CrossRef] [Green Version]
- Aureli, M.; Mauri, L.; Ciampa, M.G.; Prinetti, A.; Toffano, G.; Secchieri, C.; Sonnino, S. GM1 ganglioside: Past studies and future potential. Mol. Neurobiol. 2016, 53, 1824–1842. [Google Scholar] [CrossRef]
- Wu, G.; Xie, X.; Lu, Z.H.; Ledeen, R.W. Cerebellar neurons lacking complex gangliosides degenerate in the presence of depolarizing levels of potassium. Proc. Natl. Acad. Sci. USA 2001, 98, 307–312. [Google Scholar] [CrossRef]
- Balog, M.; Blazetic, S.; Ivic, V.; Labak, I.; Krajnik, B.; Marin, R.; Canerina-Amaro, A.; de Pablo, D.P.; Bardak, A.; Gaspar, R.; et al. Disarranged neuroplastin environment upon aging and chronic stress recovery in female Sprague Dawley rats. Eur. J. Neurosci. 2021. [Google Scholar] [CrossRef]
- Grassi, S.; Giussani, P.; Mauri, L.; Prioni, S.; Sonnino, S.; Prinetti, A. Lipid rafts and neurodegeneration: Structural and functional roles in physiologic aging and neurodegenerative diseases. J. Lipid Res. 2020, 61, 636–654. [Google Scholar] [CrossRef] [Green Version]
- Mlinac, K.; Fon Tacer, K.; Heffer, M.; Rozman, D.; Bognar, S.K. Cholesterogenic genes expression in brain and liver of ganglioside-deficient mice. Mol. Cell. Biochem. 2012, 369, 127–133. [Google Scholar] [CrossRef]
- Pang, Y.; Zhu, H.; Wu, P.; Chen, J. The characterization of plasma membrane Ca2+-ATPase in rich sphingomyelin-cholesterol domains. FEBS Lett. 2005, 579, 2397–2403. [Google Scholar] [CrossRef] [Green Version]
- Ilic, K.; Auer, B.; Mlinac-Jerkovic, K.; Herrera-Molina, R. Neuronal signaling by Thy-1 in nanodomains with specific ganglioside composition: Shall we open the door to a new complexity? Front. Cell Dev. Biol. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Rivero-Gutierrez, B.; Anzola, A.; Martinez-Augustin, O.; de Medina, F.S. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal. Biochem. 2014, 467, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Schnaar, R.L.; Fromholt, S.E.; Gong, Y.; Vyas, A.A.; Laroy, W.; Wayman, D.M.; Heffer-Lauc, M.; Ito, H.; Ishida, H.; Kiso, M.; et al. Immunoglobulin G-class mouse monoclonal antibodies to major brain gangliosides. Anal. Biochem. 2002, 302, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolte, S.; Cordelieres, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]
- Zhang, G.; Lopez, P.H.; Li, C.Y.; Mehta, N.R.; Griffin, J.W.; Schnaar, R.L.; Sheikh, K.A. Anti-ganglioside antibody-mediated neuronal cytotoxicity and its protection by intravenous immunoglobulin: Implications for immune neuropathies. Brain 2004, 127, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L.; Fredman, P. A procedure for the quantitative isolation of brain gangliosides. Biochim. Biophys. Acta 1980, 617, 97–109. [Google Scholar] [CrossRef]
- Schnaar, R.L. Isolation of glycosphingolipids. Methods Enzymol. 1994, 230, 348–370. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.A.; Dittmer, J.C. The use of sephadex for the removal of nonlipid contaminants from lipid extracts. Biochemistry 1963, 2, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L. Quantitive estimation of sialic acids: II. A colorimetric resorcinol-hydrochloric acid method. Biochim. Biophys. Acta 1957, 24, 604–611. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Needham, L.K. Thin-layer chromatography of glycosphingolipids. Methods Enzymol. 1994, 230, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, P.; Fredman, P.; Mansson, J.E.; Svennerholm, L. Determination of gangliosides and sulfatide in human cerebrospinal fluid with a microimmunoaffinity technique. Clin. Chim. Acta 1991, 197, 105–115. [Google Scholar] [CrossRef]





| Antibody | Host Species | Supplier | Cat. Number | Dilution |
|---|---|---|---|---|
| Primary Antibodies | ||||
| Anti-Transferrin receptor | Mouse | Thermo Fisher, Life Technologies Corporation, Carlsbad, CA, USA | 136800 | 1:2000 |
| Anti-Flotillin-1 | BD Biosciences, Franklin Lakes, NJ, USA | 610821 | 1:1000 | |
| Anti-Neuroplastin 65 | Goat | R&D Systems, Minneapolis, MN, USA | AF5360 | 1:200 LNS 1:1000 WB |
| Anti-pan Neuroplastin | Sheep | R&D Systems, Minneapolis, MN, USA | AF7818 | 1:200 LNS 1:1000 WB |
| Anti-pan PMCA | Mouse | Abcam, Cambridge, UK | ab2825 | 1:500 |
| Anti-PMCA4 | ab2783 | 1:1000 | ||
| Anti-PMCA1 | Rabbit | ab190355 | 1:1000 | |
| Anti-PMCA2 | ab3529 | 1:1000 | ||
| Anti-PMCA3 | Novus Biologicals, Bio-Techne Ltd., Abingdon, UK | NBP1-59465 | 1:1000 | |
| Anti-GM1 ganglioside | Mouse | Monoclonal antibodies prepared and validated as reported [66] | 4.3 μg/mL | |
| Anti-GD1a ganglioside | 0.64 μg/mL | |||
| Anti-GD1b ganglioside | 2 μg/mL | |||
| Anti-GT1b ganglioside | 1.84 μg/mL | |||
| Secondary Antibodies | ||||
| Anti-goat Cy5 | Donkey | Jackson ImmunoResearch Europe Ltd., Ely, UK | 705-175-147 | 1:1000 |
| Anti-sheep Cy3 | 713-165-003 | 1:1000 | ||
| Anti-mouse 488 | 715-545-150 | 1:1000 | ||
| Anti-mouse HRP | 715-035-150 | 1:50,000 | ||
| Anti-goat HRP | 705-035-003 | |||
| Anti-sheep HRP | 713-035-147 | |||
| Anti-rabbit HRP | 711-035-152 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilic, K.; Lin, X.; Malci, A.; Stojanović, M.; Puljko, B.; Rožman, M.; Vukelić, Ž.; Heffer, M.; Montag, D.; Schnaar, R.L.; et al. Plasma Membrane Calcium ATPase-Neuroplastin Complexes Are Selectively Stabilized in GM1-Containing Lipid Rafts. Int. J. Mol. Sci. 2021, 22, 13590. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222413590
Ilic K, Lin X, Malci A, Stojanović M, Puljko B, Rožman M, Vukelić Ž, Heffer M, Montag D, Schnaar RL, et al. Plasma Membrane Calcium ATPase-Neuroplastin Complexes Are Selectively Stabilized in GM1-Containing Lipid Rafts. International Journal of Molecular Sciences. 2021; 22(24):13590. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222413590
Chicago/Turabian StyleIlic, Katarina, Xiao Lin, Ayse Malci, Mario Stojanović, Borna Puljko, Marko Rožman, Željka Vukelić, Marija Heffer, Dirk Montag, Ronald L. Schnaar, and et al. 2021. "Plasma Membrane Calcium ATPase-Neuroplastin Complexes Are Selectively Stabilized in GM1-Containing Lipid Rafts" International Journal of Molecular Sciences 22, no. 24: 13590. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222413590