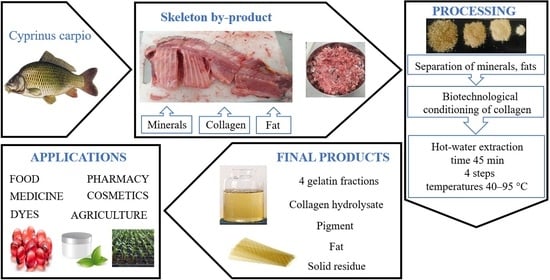
Cyprinus carpio Skeleton Byproduct as a Source of Collagen for Gelatin Preparation
Abstract
:1. Introduction
2. Results
2.1. Mass Balance of the Process
2.2. Gel-Forming Properties of Gelatins
2.2.1. Gel Strength
2.2.2. Melting Point and Gelling Point
2.2.3. Water Holding Capacity and Fat Binding Capacity
2.3. Surface Properties of Gelatins and Viscosity
2.3.1. Foaming Capacity and Stability
2.3.2. Emulsifying Capacity and Stability
2.3.3. Viscosity
2.4. Other Properties of Gelatins
3. Discussion
3.1. Mass Balance of the Process
3.2. Gel-Forming Properties of Gelatins
3.2.1. Gel Strength
3.2.2. Melting Point and Gelling Point
3.2.3. Water Holding Capacity and Fat Binding Capacity
3.3. Surface Properties of Gelatins and Viscosity
3.3.1. Foaming Capacity and Stability
3.3.2. Emulsifying Capacity and Stability
3.3.3. Viscosity
3.4. Other Properties of Gelatins
3.5. Discussion Summary
3.6. The Importance of Work Results for Pratice
4. Materials and Methods
4.1. Materials, Appliances and Chemicals
4.2. Design of Eperiments and Statistical Analysis
4.3. Processing of Cyprinus carpio Skeletons into Gelatins
- Separation of organic matter. The thawed raw material was first washed with cold H2O. It was mixed with 0.2 mol/L NaCl in a ratio of 1:6 and shaken at room temperature (23.0 ± 1.0 °C) for 90 min, then washed with cold H2O. It was then mixed with 0.03 mol/L NaOH in a ratio of 1:6 and shaken at room temperature for 45 min and, after filtration, washed with cold H2O; this procedure was repeated three more times. Finally, the raw material was washed with cold H2O and dried at 35 °C for 24 h. This was followed by a defatting step; the raw material was mixed in a ratio of 1:9 (w/v) with petroleum ether and ethanol (mixed in a ratio of 1:1, v/v) and shaken for 48 h at room temperature, then after 12 h the solvent was changed.
- Separation of inorganic matter. The raw material was mixed in a ratio of 1:10 with HCl (concentration according to Factor A) and demineralized with gentle shaking at room temperature for 48 h; after 24 h, the acid was replaced with new acid. After filtration, the demineralized collagen was washed thoroughly with cold H2O and dried for 24 h at 35 °C.
- The purified collagen was mixed with H2O in a ratio of 1:15 and after shaking for 20 min, the pH was adjusted to 6.5–7.0 (by adding 5 wt% NaOH solution). The proteolytic enzyme was then added in an amount according to Factor B and the mixture was shaken at room temperature for 4 h; at 30-min intervals, the pH was checked (and adjusted) to the prescribed range. After filtering off the liquid product (collagen hydrolysate), the collagen was thoroughly washed with cold H2O. Collagen hydrolysate was dried in a thin layer (4 mm) in a circulating air drier at 50.0 ± 0.5 °C for 20 h.
- Multistage extraction of gelatins. Biotechnologically treated collagen was subjected to four separate sequential extraction cycles using a batch process extractor. In the first extraction stage, collagen was mixed with H2O in a ratio of 1:15 and the mixture was heated while stirring at a rate of dt/dτ = 10 °C/min to a temperature of 40.0 ± 0.5 °C, at which point the extraction lasted 45 min. After filtration, the solution of the first gelatin fraction was immediately heated to a temperature of 95.0 ± 0.5 °C (dt/dτ = 15 °C/min) and maintained at this temperature for 5 min; the residual enzyme was inactivated in this way. After cooling to room temperature, the gelatin solution was centrifuged at 4000 rpm for 4 min; the pigment (bottom layer) and residual fat (top layer) were separated from the gelatin solution. The gelatin solution was poured into a thin film (4 mm) and cooled into a gel in a refrigerator at 5.0 ± 0.5 °C for 30 min and then dried in a circulating air drier, first at 40.0 ± 0.5 °C for 12 h, then at 65.0 ± 0.5 °C for 8 h. The resulting gelatin film was scraped off, weighed and ground to a powder. In the second, third, and fourth extraction stages, the same procedure was followed at extraction temperatures of 50.0 ± 0.5 °C, 70.0 ± 0.5 °C and 95.0 ± 0.5 °C. The second and third gelatin fractions were inactivated in the same way as the first gelatin fraction. After centrifugation, less pigment and residual fat were separated from each subsequent extraction. The undissolved residue remaining after the fourth extraction cycle was dried at 103.0 ± 1.0 °C to a constant weight and then weighed; the pigment was dried overnight at 40.0 ± 1.0 °C and then weighed. The prepared gelatins were then subjected to further analyses.
4.4. Analytical Part
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ockerman, H.W.; Hansen, C.I. Animal By-Product Processing and Utilization, 1st ed.; CRC Press: London, UK, 2000; pp. 23–83. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of by products and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [Green Version]
- Komeroski, M.R.; Homem, R.V.; Schmidt, H.O.; Rockett, F.C.; Lira, L.; Farias, D.V.; Kist, T.L.; Doneda, D.; Rios, A.O.; Oliveira, V.R. Effect of whey protein and mixed flours on the quality parameters of gluten-free breads. Int. J. Gastron. Food Sci. 2021, 24, 100361. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, H. Microbial production of value-added bioproducts and enzymes from molasses, a by-product of sugar industry. Food Chem. 2021, 346, 128860. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 Laying Down Health Rules as Regards Animal By-Products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 (Animal By-Products Regulation). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:300:0001:0033:EN:PDF (accessed on 6 May 2021).
- Food and Agriculture Organization of the United Nation. GLOBEFISH–Information and Analysis on World Fish Trade, 2021. Available online: http://www.fao.org/in-action/globefish/fishery-information/resource-detail/es/c/338772/ (accessed on 7 May 2021).
- ECD-FAO. Agricultural Outlook 2020–2029. Chapter 8. Fish. Available online: http://0-www-oecd--ilibrary-org.brum.beds.ac.uk/sites/4dd9b3d0-en/index.html?itemId=/content/component/4dd9b3d0-en#section-d1e19913 (accessed on 15 December 2021).
- Monteiro, A.; Paquincha, D.; Martins, F.; Queirós, R.P.; Saraiva, J.A.; Švarc-Gajić, J.; Nastić, N.; Delerue-Matos, C.; Carvalho, A.P. Liquid by-products from fish canning industry as sustainable sources of ω3 lipids. J. Environ. Manag. 2018, 219, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamorano-Apodaca, J.C.; García-Sifuentes, C.O.; Carvajal-Millan, E.; Vallejo-Galland, B.; Scheuren-Acevedo, S.M.; Lugo-Sánchez, M.E. Biological and functional properties of peptide fractions obtained from collagen hydrolysate derived from mixed by-products of different fish species. Food Chem. 2020, 331, 127350. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.W. Fish Processing by-products and their reclamation. In Inedible Meat By-Products, 1st ed.; Pearson, A.M., Dutson, T.R., Eds.; Advances in Meat Research Series; Springer: Dordrecht, The Netherland, 1992; Volume 8, pp. 199–216. [Google Scholar]
- Araújo, C.S.; Rodrigues, A.M.C.; Peixoto Joele, M.R.S.; Araújo, E.A.F.; Lourenço, L.F.H. Optimizing process parameters to obtain a bioplastic using proteins from fish byproducts through the response surface methodology. Food Packag. Shelf Life 2018, 16, 23–30. [Google Scholar] [CrossRef]
- Tuomisto, J.T.; Asikainen, A.; Meriläinen, P.; Haapasaari, P. Health effects of nutrients and environmental pollutants in Baltic herring and salmon: A quantitative benefit-risk assessment. BMC Public Health 2020, 20, 64. [Google Scholar] [CrossRef] [Green Version]
- Cal, L.; Suarez-Bregua, P.; Cerdá-Reverter, J.M.; Braasch, I.; Rotllant, J. Fish pigmentation and the melanocortin system. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 211, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Senevirathne, M.; Kim, S.K. Utilization of seafood processing by-products: Medicinal applications. Adv. Food Nutr. Res. 2012, 65, 495–512. [Google Scholar] [CrossRef]
- Yang, X.R.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Preparation and characterization of gelatin and antioxidant peptides from gelatin hydrolysate of skipjack tuna (Katsuwonus pelamis) bone stimulated by in vitro gastrointestinal digestion. Mar. Drugs 2019, 17, 78. [Google Scholar] [CrossRef] [Green Version]
- Nurilmala, M.; Hizbullah, H.H.; Karnia, E.; Kusumaningtyas, E.; Ochiai, Y. Characterization and antioxidant activity of collagen, gelatin, and the derived peptides from yellowfin tuna (Thunnus albacares) skin. Mar. Drugs 2020, 18, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Sultana, S.; Ali, M.E.; Uddin Ahamad, M.N. Gelatine, collagen, and single cell proteins as a natural and newly emerging food ingredients. In Preparation and Processing of Religious and Cultural Foods, 1st ed.; Ali, M.E., Ahmad Nizar, N.N., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Duxford, UK, 2018; Volume 11, pp. 215–239. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Gelatin alternatives for the food industry: Recent developments, challenges and prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Boran, G.; Regenstein, J.M. Fish gelatin. Adv. Food Nutr. Res. 2010, 60, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialization. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Nazmi, N.N.M.; Isa, M.I.; Sarbon, N.M. Characterisation of biodegradable protein based films from gelatin alternative: A review. Int. Food Res. J. 2020, 27, 971–987. [Google Scholar]
- Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries, review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef]
- Barbooti, M.M.; Raouf, S.R.; Al-Hamdani, F.H.K. Optimization of production of food grade gelatin from bovine hide wastes. Eng. Technol. J. 2008, 26, 240–253. [Google Scholar]
- Schrieber, R.; Gareis, H. Gelatine Handbook–Theory and Industrial Practice, 1st ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 45–117. [Google Scholar] [CrossRef]
- Chakka, A.K.; Muhammed, A.; Sakhare, P.Z.; Bhaskar, N. Poultry processing waste as an alternative source for mammalian gelatin: Extraction and characterization of gelatin from chicken feet using food grade acids. Waste Biomass Valor. 2017, 8, 2583–2593. [Google Scholar] [CrossRef]
- Almeida, P.F.; da Silva Lannes, S.C. Extraction and physicochemical characterization of gelatin from chicken by-product. J. Food Process Eng. 2013, 36, 824–833. [Google Scholar] [CrossRef]
- Montero, M.; Acosta, Ó.G. Tuna skin gelatin production: Optimization of extraction steps and process scale-up. CyTA—J. Food 2020, 18, 580–590. [Google Scholar] [CrossRef]
- Norziah, M.H.; Al-Hassan, A.; Khairulnizam, A.B.; Mordi, M.N.; Norita, M. Characterization of fish gelatin from surimi processing wastes: Thermal analysis and effect of transglutaminase on gel properties. Food Hydrocoll. 2009, 23, 1610–1616. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 2014, 152, 276–284. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocoll. 2011, 25, 381–388. [Google Scholar] [CrossRef]
- Pradarameswari, K.A.; Zaelani, K.; Waluyo, E.; Nurdiani, R. The physico-chemical properties of pangas catfish (Pangasius pangasius) skin gelatin. In IOP Conference Series: Earth and Environmental Science, Proceedings of the Asean-Fen International Fisheries Symposium, Batu City, East Java, Indonesia, 7–9 November 2017; IOP Publishing Ltd.: Bristol, UK, 2018; Volume 137, p. 012067. [Google Scholar] [CrossRef]
- Irwandi, J.; Faridayanti, S.; Mohamed, E.S.M.; Hamzah, M.S.; Torla, H.H.; Che Man, Y.B. Extraction and characterization of gelatin from different marine fish species in Malaysia. Int. Food Res. J. 2009, 16, 381–389. [Google Scholar]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ponnan Ettiyappan, J.B. Extraction, optimization and characterization of collagen sole fish skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- Gómez-Guillœn, M.C.; Montero, M.V.P. Extraction of gelatin from megrim (Lepidorhombus boscii) skins with several organic acids. J. Food Sci. 2001, 66, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Nalinanon, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H. Improvement of gelatin extraction from bigeye snapper skin using pepsin-aided process in combination with protease inhibitor. Food Hydrocoll. 2008, 22, 615–622. [Google Scholar] [CrossRef]
- Kasankala, L.M.; Xue, Y.; Weilong, Y.; Hong, S.D.; He, Q. Optimization of gelatine extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Bioresour. Technol. 2007, 98, 3338–3343. [Google Scholar] [CrossRef]
- Da Trindade Alfaro, A.; Fonseca, G.; Prentice, C. Enhancement of functional properties of wami tilapia (Oreochromis urolepis hornorum) skin gelatin at different pH values. Food Bioproc. Technol. 2013, 6, 2118–2127. [Google Scholar] [CrossRef]
- Shahiri Tabarestani, H.; Maghsoudlou, Y.; Motamedzadegan, A.; Sadeghi Mahoonak, A.R. Optimization of physico-chemical properties of gelatin extracted from fish skin of rainbow trout (Onchorhynchus mykiss). Bioresour. Technol. 2010, 101, 6207–6214. [Google Scholar] [CrossRef] [PubMed]
- Ninan, G.; Jose, J.; Abubacker, Z. Preparation and characterization of gelatin extracted from the skins of rohu (Labeo Rohita) and common carp (Cyprinus carpio). J. Food Process. Preserv. 2010, 35, 143–162. [Google Scholar] [CrossRef]
- Mokrejš, P.; Mrázek, P.; Gál, R.; Pavlačková, J. Biotechnological preparation of gelatines from chicken paws. Polymers 2019, 11, 1060. [Google Scholar] [CrossRef] [Green Version]
- Gál, R.; Mokrejš, P.; Mrázek, P.; Pavlačková, J.; Janáčová, D.; Orsavová, J. Chicken heads as a promising by-product for preparation of food gelatins. Molecules 2020, 25, 494. [Google Scholar] [CrossRef] [Green Version]
- Jridi, M.; Nasri, R.; Lassoued, I.; Souissi, N.; Mbarek, A.; Barkia, A.; Nasri, M. Chemical and biophysical properties of gelatins extracted from alkali-pretreated skin of cuttlefish (Sepia officinalis) using pepsin. Food Res. Int. 2013, 54, 1680–1687. [Google Scholar] [CrossRef]
- Badway, H.M.R.; Abd El-Moniem, S.M.; Soliman, A.M.; Rabie, M.A. Physicochemical properties of gelatin extracted from Nile tilapia (Oreochromis niloticus) and Nile perch (Lates niloticus) fish skins. Zagazig J. Agric. Res. 2019, 46, 1529–1537. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Rawdkuen, S.; Chaijan, M.; Benjakul, S.; Osako, K.; Tanaka, M. Chemical compositions and characterisation of skin gelatin from farmed giant catfish (Pangasianodon gigas). LWT—Food Sci. Technol. 2010, 43, 161–165. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P.; Kishimura, H. Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocoll. 2012, 29, 389–397. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Q.; Xiao, Y.; Li, C.; Bi, H.; Zhang, M.; Wei, L.; Du, Y. Effect of preparation method on physicochemical, scavenging, and proliferative properties of gelatin from yak skin. J. Food Process. Preserv. 2020, 44, e14884. [Google Scholar] [CrossRef]
- Mrázek, P.; Mokrejš, P.; Gál, R.; Orsavová, J. Chicken skin gelatine as an alternative to pork and beef gelatins. Potravin. Slovak J. Food Sci. 2019, 13, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, D.; Koomsap, P.; Lamichhane, A.; Sadiq, M.B.; Anal, A.K. Optimization of collagen extraction from chicken feet by papain hydrolysis and synthesis of chicken feet collagen based biopolymeric fibres. Food Biosci. 2018, 23, 23–30. [Google Scholar] [CrossRef]
- Surangna, J.; Anal, A.K. Optimization of extraction of functional protein hydrolysates from chicken egg shell membrane (ESM) by ultrasonic assisted extraction (UAE) and enzymatic hydrolysis. Food Sci. Technol. 2016, 69, 295–302. [Google Scholar] [CrossRef]
- Li, F.; Jia, D.; Yao, K. Amino acid composition and functional properties of collagen polypeptide from yak (Bos grunniens) bone. LWT—Food Sci. Technol. 2009, 42, 945–949. [Google Scholar] [CrossRef]
- Shahidi, F.; Han, X.Q.; Synowiecki, J. Production and characteristics of protein hydrolysates from capelin (Mallotus-villosus). Food Chem. 1995, 53, 285–293. [Google Scholar] [CrossRef]
- Jamilah, B.; Harvinder, K.G. Properties of gelatins from skins of fish—Black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chem. 2002, 77, 81–84. [Google Scholar] [CrossRef]
- European Pharmacopoeia 9.0. European Directorate for the Quality of Medicines & Health Care. 2017. Available online: https://www.edqm.eu/en/news/shutdown-european-pharmacopoeia-9th-edition (accessed on 8 January 2021).
- Food Chemical Codex 12. Available online: https://www.foodchemicalscodex.org/ (accessed on 8 January 2021).
- Djabourov, M.; Leblond, J.; Papon, P. Gelation of aqueous gelatin solutions. I. Structural investigation. J. Phys. 1988, 49, 319–332. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Alfaro, A.T.; Biluca, F.C.; Marquetti, C.; Tonial, I.B.; Nilson, E.; de Souza, N.E. African catfish (Clarias gariepinus) skin gelatin: Extraction optimization and physical–chemical properties. Food Res. Int. 2014, 65, 416–422. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Sultana, S.; Hossain, M.A.M.; Zaidul, I.S.M.; Ali, M.E. Multiplex PCR to discriminate bovine, porcine, and fish DNA in gelatin and confectionery products. LWT—Food Sci. Technol. 2018, 92, 169–176. [Google Scholar] [CrossRef]
- Mahmood, K.; Muhammad, L.; Arin, F.; Kamilah, H.; Razak, A.; Sulaiman, S. Review of fish gelatin extraction, properties and packaging applications. Food Sci. Qual. Manag. 2016, 56, 47–59. [Google Scholar]
- Eysturskard, J.; Haug, I.J.; Elharfaoui, N.; Djabourov, M.; Draget, K.I. Structural and mechanical properties of fish gelatin as a function of extraction conditions. Food Hydrocoll. 2009, 23, 1702–1711. [Google Scholar] [CrossRef]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kee, L.T.; Awad, E.A.; Sazili, A.Q. Extraction, characterization and molecular structure of bovine skin gelatin extracted with plant enzymes bromelain and zingibain. J. Food Sci. Technol. 2020, 57, 3772–3781. [Google Scholar] [CrossRef]
- Elharfaoui, N.; Djabourov, M.; Babel, W. Molecular weight influence on gelatin gels: Structure, enthalpy and rheology. Macromol. Symp. 2007, 256, 149–157. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Structure and rheology of gelatin gels: Recent progress. Polymer 1992, 33, 2622–2627. [Google Scholar] [CrossRef]
- Díaz-Calderón, P.; Flores, E.; González-Muñoz, A.; Pepczynska, M.; Quero, F.; Enrione, J. Influence of extraction variables on the structure and physical properties of salmon gelatin. Food Hydrocoll. 2017, 71, 118–128. [Google Scholar] [CrossRef]
- Sila, A.; Martinez-Alvarez, O.; Krichen, F.; Gomez-Guillen, M.C.; Bougatef, A. Gelatin prepared from European eel (Anguilla anguilla) skin: Physicochemical, textural, viscoelastic and surface properties. Colloid Surf. A 2017, 529, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Gilsenan, P.M.; Ross-Murphy, S.B. Rheological characterization of gelatins from mammalian and marine sources. Food Hydrocoll. 2000, 14, 191–195. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring cod gelatin structure and physical properties with acid and alkaline extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Khiari, Z.; Rico, D.; Martin-Diana, B.A.; Barry-Ryana, C. Comparison between gelatines extracted from mackerel and blue whiting bones after pre-treatments. Food Chem. 2013, 139, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Cheow, C.S.; Norizah, M.S.; Kyaw, Z.Y.; Howell, N.K. Preparation and characterisation of gelatins from the skins of sin croaker (Johnius dussumieri) and shortfin scad (Decapterus macrosoma). Food Chem. 2007, 101, 386–391. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Extraction of muscle proteins and gelatine from cod head. Process Biochem. 2006, 41, 697–700. [Google Scholar] [CrossRef]
- Kolodziejska, I.; Skierka, E.; Sadowska, M.; Kolodziejski, W.; Niecikowska, C. Effect of extracting time and temperature on yield of gelatin from different fish offal. Food Chem. 2008, 107, 700–706. [Google Scholar] [CrossRef]
- Widyasari, R.; Rawdkuen, S. Extraction and characterization of gelatin from chicken feet by acid and ultrasound assisted extraction. Food Appl. Biosci. J. 2014, 2, 83–95. [Google Scholar] [CrossRef]
- Taufik, M.; Triatmojo, S.; Erwanto, Y.; Santoso, U. Effect of broiler age and extraction temperature on characteristic chicken feet skin gelatin. In Proceedings of the 5th International Seminar on Tropical Animal Production, Yogyakarta, Indonesia, 19–22 October 2010; pp. 649–656. [Google Scholar]
- Haug, I.J.; Draget, K.I. Gelatin. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G., Williams, P., Eds.; Woodhead Publishing: Cambridge, UK, 2009; Volume 6, pp. 142–163. [Google Scholar]
- Nollet, L.M.L.; Toldrá, F. Handbook of Food Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 357–754. [Google Scholar] [CrossRef]
- ISO 3496:1994; Meat and Meat Products–Determination of Hydroxyproline Content. ISO: Geneva, Switzerland. Available online: https://cdn.standards.iteh.ai/samples/8848/908d030b1d6a4807bc2ac15fee8d51f9/SIST-ISO-3496-1995.pdf (accessed on 2 January 2021).
- Vázquez-Ortiz, F.A.; González-Méndez, N.F. Determination of collagen as a quality index in Bologna from Northwestern Mexico. J. Food Compos. Anal. 1996, 9, 269–276. [Google Scholar] [CrossRef]
- Antony, J. Design of Experiments for Engineers and Scientists, 2nd ed.; Elsevier: London, UK, 2014; pp. 33–85. [Google Scholar] [CrossRef]
- Official Procedure of the Gelatin Manufacturers Institute of America, Inc. Standard Testing Methods for Edible Gelatin. Available online: http://www.gelatin-gmia.com/images/GMIA_Official_Methods_of_Gelatin_Revised_2013.pdf/ (accessed on 20 July 2021).
- Nasrin, T.A.A.; Noomhorm, A.; Anal, A.K. Physico-chemical characterization of culled plantain pulp starch, peel starch and flour. Int. J. Food Prop. 2015, 18, 165–177. [Google Scholar] [CrossRef]
- Sathe, S.K.; Deshpande, S.S.; Salunkhe, D.K. Functional properties of lupin seed (Supinus mutabilis) proteins and protein concentrates. J. Food Sci. 1982, 47, 491–497. [Google Scholar] [CrossRef]
- Neto, V.Q.; Narain, N.; Silva, J.B.; Bora, P.S. Functional properties of raw and heat processed cashew nut (Anarcardium occidentale L.) kernel protein isolates. Nahrung 2001, 45, 258–262. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Yazdani-Dehnavi, M.; Mirzapour-Kouhdasht, A. The effects of enzymatically aided acid-swelling process on gelatin extracted from fish by-products. Food Sci. Nutr. 2020, 8, 5017–5025. [Google Scholar] [CrossRef] [PubMed]




| Exp. No. | Factor A (wt%) | Factor B (wt%) | YH (%) | YG1 (%) | YG2 (%) | YG3 (%) | YG4 (%) | YP (%) | YRF (%) | UR (%) | MBE (%) | ∑YG (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 0 | 3.86 | 3.97 | 3.90 | 6.68 | 4.13 | 0.11 | 0.34 | 74.96 | 2.05 | 18.68 |
| 2 | 0.5 | 0.1 | 5.08 | 6.54 | 5.76 | 4.50 | 2.22 | 0.24 | 0.51 | 74.36 | 0.79 | 19.02 |
| 3 | 0.5 | 0.2 | 7.30 | 11.39 | 10.00 | 3.47 | 2.06 | 0.65 | 1.30 | 62.95 | 0.88 | 26.92 |
| 4 | 1.0 | 0 | 8.75 | 4.20 | 4.77 | 5.87 | 6.04 | 0.31 | 0.24 | 69.32 | 0.50 | 20.88 |
| 5 | 1.0 | 0.1 | 12.29 | 17.30 | 6.30 | 3.81 | 3.56 | 0.59 | 0.92 | 53.16 | 2.07 | 30.97 |
| 6 | 1.0 | 0.2 | 15.27 | 24.92 | 13.13 | 3.33 | 2.81 | 0.98 | 1.88 | 35.21 | 2.47 | 44.19 |
| 7 | 2.0 | 0 | 11.09 | 9.81 | 5.56 | 4.83 | 7.22 | 0.33 | 0.30 | 59.26 | 1.60 | 27.42 |
| 8 | 2.0 | 0.1 | 12.83 | 20.53 | 7.24 | 3.75 | 6.30 | 0.60 | 1.15 | 47.10 | 0.50 | 37.82 |
| 9 | 2.0 | 0.2 | 17.84 | 34.02 | 15.24 | 2.06 | 4.35 | 1.00 | 2.02 | 21.36 | 2.11 | 55.67 |
| 10 * | 0 | 0 | 0.65 | 3.62 | 3.55 | 1.94 | 1.86 | 0.56 | 1.23 | 86.43 | 0.16 | 10.97 |
| Degree of Freedom | Sum of Squares | Mean Squares | F-Value | p-Value | |
|---|---|---|---|---|---|
| Response: The yield of the 1st gelatin fraction, YG1 (%) = 1.72 + 5.12 A + 27.85 B; R2 = 80.24% | |||||
| Regression | 2 | 138.30 | 69.152 | 12.18 | 0.008 |
| Factor A (Concentration of HCl) | 1 | 91.77 | 91.767 | 16.16 | 0.007 ● |
| Factor B (The amount of enzyme) | 1 | 46.54 | 46.537 | 8.20 | 0.029 ● |
| Error | 6 | 34.07 | 5.678 | ||
| Total | 8 | 172.37 | |||
| Response: The yield of the 2nd gelatin fraction, YG2 (%) = −4.41 + 8.94 A + 87.2 B; R2 = 87.14% | |||||
| Regression | 2 | 736.6 | 368.30 | 20.33 | 0.002 |
| Factor A (Concentration of HCl) | 1 | 279.9 | 279.85 | 15.45 | 0.008 ● |
| Factor B (The amount of enzyme) | 1 | 456.8 | 456.75 | 25.22 | 0.002 ● |
| Error | 6 | 108.7 | 18.11 | ||
| Total | 8 | 845.3 | |||
| Response: The yield of the 3rd gelatin fraction, YG3 (%) = 6.698 − 0.877 A − 14.20 B; R2 = 94.66% | |||||
| Regression | 2 | 14.7883 | 7.3942 | 53.19 | 0.000 |
| Factor A (Concentration of HCl) | 1 | 2.6899 | 2.6899 | 19.35 | 0.005 ● |
| Factor B (The amount of enzyme) | 1 | 12.0984 | 12.0984 | 87.03 | 0.000 ● |
| Error | 6 | 0.8341 | 0.1390 | ||
| Total | 8 | 15.6224 | |||
| Response: The yield of the 4th gelatin fraction, YG4 (%) = 3.255 + 2.062 A–13.62 B; R2 = 93.89% | |||||
| Regression | 2 | 26.005 | 13.0024 | 46.10 | 0.000 |
| Factor A (Concentration of HCl) | 1 | 14.880 | 14.8801 | 52.76 | 0.000 ● |
| Factor B (The amount of enzyme) | 1 | 11.125 | 11.1248 | 39.45 | 0.001 ● |
| Error | 6 | 1.692 | 0.2820 | ||
| Total | 8 | 27.697 | |||
| Process Factors | Gelatin Gel-Forming Properties | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. No. | Factor A (wt%) | Factor B (wt%) | GGS (Bloom) | MP (°C) | GP (°C) | WHC (g/g) | FBC (g/g) | GGS (Bloom) | MP (°C) | GP (°C) | WHC (g/g) | FBC (g/g) |
| 1st gelatin fractions | 2nd gelatin fractions | |||||||||||
| 1 | 0.5 | 0 | 128 | 29.4 | 15.3 | 5.0 | 10.2 | 10 | 18.6 | 9.6 | 1.0 | 2.6 |
| 2 | 0.5 | 0.1 | 30 | 22.5 | 11.2 | 1.1 | 2.8 | 0 | NA | NA | 0.3 | 0.9 |
| 3 | 0.5 | 0.2 | 0 | NA | NA | 0.6 | 1.4 | 0 | NA | NA | 0.3 | 0.9 |
| 4 | 1.0 | 0 | 45 | 23.0 | 11.5 | 1.5 | 3.7 | 59 | 23.4 | 11.7 | 2.0 | 8.3 |
| 5 | 1.0 | 0.1 | 0 | NA | NA | 0.3 | 1.0 | 23 | 21.0 | 11.0 | 1.0 | 6.5 |
| 6 | 1.0 | 0.2 | 0 | NA | NA | 0.2 | 0.8 | 0 | NA | NA | 0.3 | 0.7 |
| 7 | 2.0 | 0 | 0 | NA | NA | 0.2 | 0.7 | 65 | 24.8 | 12.4 | 1.6 | 5.6 |
| 8 | 2.0 | 0.1 | 0 | NA | NA | 0.1 | 0.6 | 63 | 23.8 | 12.1 | 0.8 | 2.8 |
| 9 | 2.0 | 0.2 | 0 | NA | NA | 0.1 | 0.3 | 0 | NA | NA | 0.2 | 0.7 |
| 10 * | 0 | 0 | 0 | NA | NA | 0.5 | 0.6 | 0 | NA | NA | 0.5 | 0.5 |
| 3rd gelatin fractions | 4th gelatin fractions | |||||||||||
| 1 | 0.5 | 0 | 114 | 28.0 | 14.1 | 1.5 | 8.3 | 101 | 26.7 | 14.7 | 0.5 | 4.6 |
| 2 | 0.5 | 0.1 | 11 | 13.7 | 4.2 | 0.6 | 5.6 | 0 | NA | NA | 0.6 | 1.1 |
| 3 | 0.5 | 0.2 | 0 | NA | NA | 0.2 | 1.3 | 0 | NA | NA | 0.4 | 0.9 |
| 4 | 1.0 | 0 | 122 | 28.2 | 15.5 | 2.5 | 7.4 | 169 | 29.9 | 15.6 | 1.0 | 4.6 |
| 5 | 1.0 | 0.1 | 55 | 27.0 | 11.9 | 0.6 | 3.7 | 0 | NA | NA | 0.5 | 1.1 |
| 6 | 1.0 | 0.2 | 18 | 18.0 | 7.8 | 0.8 | 0.9 | 0 | NA | NA | 0.4 | 1.0 |
| 7 | 2.0 | 0 | 124 | 28.6 | 15.7 | 4.5 | 11.1 | 145 | 29.5 | 15.3 | 0.9 | 7.4 |
| 8 | 2.0 | 0.1 | 97 | 27.9 | 12.6 | 1.2 | 7.4 | 0 | NA | NA | 0.3 | 0.7 |
| 9 | 2.0 | 0.2 | 90 | 27.6 | 12.0 | 1.0 | 4.6 | 0 | NA | NA | 0.3 | 0.8 |
| 10 * | 0 | 0 | 0 | NA | NA | 0.6 | 0.6 | 0 | NA | NA | 0.5 | 0.7 |
| Degree of Freedom | Sum of Squares | Mean Squares | F-Value | p-Value | |
|---|---|---|---|---|---|
| Response: 1st gelatin fraction gel strength, GGS1 (Bloom) = 89.0 − 32.2 A − 288 B; R2 = 58.56% | |||||
| Regression | 2 | 8626 | 4313 | 4.24 | 0.071 |
| Factor A (Concentration of HCl) | 1 | 3638 | 3638 | 3.58 | 0.108 |
| Factor B (The amount of enzyme) | 1 | 4988 | 4988 | 4.90 | 0.069 |
| Error | 6 | 6105 | 1017 | ||
| Total | 8 | 14,730 | |||
| Response: 2nd gelatin fraction gel strength, GGS2 (Bloom) = 18.0 + 24.67 A − 223.3 B; R2 = 73.95% | |||||
| Regression | 2 | 5122 | 2561.1 | 8.52 | 0.018 |
| Factor A (Concentration of HCl) | 1 | 2130 | 2129.6 | 7.08 | 0.037 ● |
| Factor B (The amount of enzyme) | 1 | 2993 | 2992.7 | 9.95 | 0.020 ● |
| Error | 6 | 1804 | 300.7 | ||
| Total | 8 | 6926 | |||
| Response: 3rd gelatin fraction gel strength, GGS3 (Bloom) = 64.3 + 41.0 A − 420.0 B; R2 = 82.29% | |||||
| Regression | 2 | 16,454 | 8226.9 | 13.94 | 0.006 |
| Factor A (Concentration of HCl) | 1 | 5870 | 5869.8 | 9.95 | 0.020 ● |
| Factor B (The amount of enzyme) | 1 | 10,584 | 10,584.0 | 17.93 | 0.005 ● |
| Error | 6 | 3541 | 590.2 | ||
| Total | 8 | 19,995 | |||
| Response: 4th gelatin fraction gel strength, GGS4 (Bloom) = 106.8 + 7.2 A − 692 B; R2 = 71.06% | |||||
| Regression | 2 | 28,887.5 | 14,443.8 | 7.37 | 0.024 |
| Factor A (Concentration of HCl) | 1 | 183.4 | 183.4 | 0.09 | 0.770 |
| Factor B (The amount of enzyme) | 1 | 28,704.2 | 28,704.2 | 14.64 | 0.009 ● |
| Error | 6 | 11,763.4 | 1960.6 | ||
| Total | 8 | 40,650.9 | |||
| Process Factors | Gelatin Surface Properties | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. No. | Factor A (wt%) | Factor B (wt%) | FC (%) | FS (%) | EC (%) | ES (%) | υ (mPa·s) | FC (%) | FS (%) | EC (%) | ES (%) | υ (mPa·s) |
| 1st gelatin fractions | 2nd gelatin fractions | |||||||||||
| 1 | 0.5 | 0 | 20 | 4 | 50.0 | 100 | 2.86 | 6 | 2 | 48.3 | 96.6 | 1.20 |
| 2 | 0.5 | 0.1 | 4 | 0 | 48.3 | 96.6 | 1.65 | 4 | 0 | 48.3 | 96.6 | 1.18 |
| 3 | 0.5 | 0.2 | 4 | 0 | 48.3 | 96.6 | 1.20 | 4 | 0 | 48.3 | 96.6 | 1.14 |
| 4 | 1.0 | 0 | 8 | 0 | 51.7 | 96.8 | 1.7 | 16 | 4 | 48.3 | 96.6 | 1.44 |
| 5 | 1.0 | 0.1 | 6 | 2 | 48.3 | 96.6 | 1.20 | 12 | 4 | 50.0 | 96.6 | 1.32 |
| 6 | 1.0 | 0.2 | 4 | 0 | 48.3 | 96.6 | 1.18 | 4 | 1 | 48.3 | 87.2 | 1.17 |
| 7 | 2.0 | 0 | 3 | 1 | 48.3 | 96.6 | 1.12 | 20 | 0 | 46.7 | 96.3 | 2.08 |
| 8 | 2.0 | 0.1 | 3 | 0 | 48.3 | 96.6 | 1.12 | 12 | 4 | 51.7 | 96.8 | 1.84 |
| 9 | 2.0 | 0.2 | 3 | 0 | 45.0 | 96.6 | 1.06 | 4 | 1 | 48.3 | 96.6 | 1.06 |
| 10 * | 0 | 0 | 4 | 0 | 48.3 | 87.2 | 1.20 | 4 | 1 | 48.3 | 87.2 | 1.20 |
| 3rd gelatin fractions | 4th gelatin fractions | |||||||||||
| 1 | 0.5 | 0 | 25 | 16 | 50.0 | 83.3 | 3.34 | 24 | 16 | 50.0 | 93.3 | 2.75 |
| 2 | 0.5 | 0.1 | 8 | 4 | 45.0 | 96.3 | 1.15 | 8 | 4 | 48.3 | 96.3 | 1.16 |
| 3 | 0.5 | 0.2 | 3 | 1 | 48.3 | 96.6 | 1.07 | 3 | 1 | 48.3 | 96.6 | 1.15 |
| 4 | 1.0 | 0 | 26 | 20 | 50.0 | 66.7 | 2.97 | 52 | 40 | 55.0 | 90.9 | 4.88 |
| 5 | 1.0 | 0.1 | 20 | 8 | 50.0 | 66.7 | 1.86 | 8 | 4 | 46.7 | 100 | 1.96 |
| 6 | 1.0 | 0.2 | 8 | 4 | 46.7 | 100 | 1.37 | 6 | 2 | 48.3 | 96.6 | 1.15 |
| 7 | 2.0 | 0 | 28 | 8 | 50.0 | 96.7 | 4.50 | 48 | 32 | 53.3 | 93.8 | 4.59 |
| 8 | 2.0 | 0.1 | 23 | 20 | 46.7 | 100 | 2.72 | 4 | 1 | 48.3 | 96.6 | 1.26 |
| 9 | 2.0 | 0.2 | 20 | 8 | 48.3 | 17.2 | 2.00 | 3 | 1 | 48.3 | 96.6 | 1.17 |
| 10 * | 0 | 0 | 6 | 2 | 50.0 | 96.7 | 1.13 | 6 | 2 | 50.0 | 96.7 | 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gál, R.; Mokrejš, P.; Pavlačková, J.; Janáčová, D. Cyprinus carpio Skeleton Byproduct as a Source of Collagen for Gelatin Preparation. Int. J. Mol. Sci. 2022, 23, 3164. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23063164
Gál R, Mokrejš P, Pavlačková J, Janáčová D. Cyprinus carpio Skeleton Byproduct as a Source of Collagen for Gelatin Preparation. International Journal of Molecular Sciences. 2022; 23(6):3164. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23063164
Chicago/Turabian StyleGál, Robert, Pavel Mokrejš, Jana Pavlačková, and Dagmar Janáčová. 2022. "Cyprinus carpio Skeleton Byproduct as a Source of Collagen for Gelatin Preparation" International Journal of Molecular Sciences 23, no. 6: 3164. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23063164