Use of PET Imaging to Assess the Efficacy of Thiethylperazine to Stimulate Cerebral MRP1 Transport Activity in Wild-Type and APP/PS1-21 Mice
Abstract
:1. Introduction
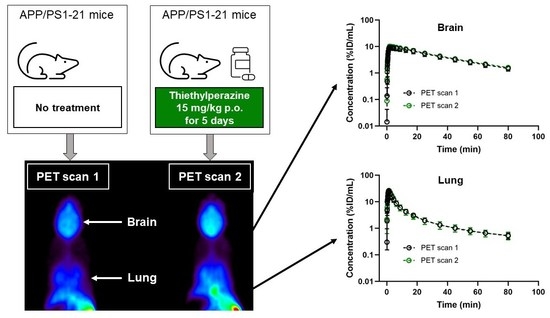
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Radiotracer Synthesis
4.3. Animals
4.4. Experimental Design
4.5. PET Imaging
4.6. PET Data Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, S.P. Targeting multidrug resistance protein 1 (MRP1, ABCC1): Past, present, and future. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.P. Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. J. Biol. Chem. 2014, 289, 30880–30888. [Google Scholar] [CrossRef]
- Krohn, M.; Lange, C.; Hofrichter, J.; Scheffler, K.; Stenzel, J.; Steffen, J.; Schumacher, T.; Bruning, T.; Plath, A.S.; Alfen, F.; et al. Cerebral amyloid-beta proteostasis is regulated by the membrane transport protein ABCC1 in mice. J. Clin. Investig. 2011, 121, 3924–3931. [Google Scholar] [CrossRef]
- Wesolowska, O.; Molnar, J.; Ocsovszki, I.; Michalak, K. Differential effect of phenothiazines on MRP1 and P-glycoprotein activity. In Vivo 2009, 23, 943–947. [Google Scholar]
- Zoufal, V.; Mairinger, S.; Krohn, M.; Wanek, T.; Filip, T.; Sauberer, M.; Stanek, J.; Traxl, A.; Schuetz, J.D.; Kuntner, C.; et al. Influence of multidrug resistance-associated proteins on the excretion of the ABCC1 imaging probe 6-bromo-7-[11C]methylpurine in mice. Mol. Imaging Biol. 2019, 21, 306–316. [Google Scholar] [CrossRef]
- Zoufal, V.; Mairinger, S.; Krohn, M.; Wanek, T.; Filip, T.; Sauberer, M.; Stanek, J.; Kuntner, C.; Pahnke, J.; Langer, O. Measurement of cerebral ABCC1 transport activity in wild-type and APP/PS1-21 mice with positron emission tomography. J. Cereb. Blood Flow Metab. 2020, 40, 954–965. [Google Scholar] [CrossRef]
- Okamura, T.; Okada, M.; Kikuchi, T.; Wakizaka, H.; Zhang, M.R. Mechanisms of glutathione-conjugate efflux from the brain into blood: Involvement of multiple transporters in the course. J. Cereb. Blood Flow Metab. 2020, 40, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Kikuchi, T.; Okada, M.; Wakizaka, H.; Zhang, M.R. Imaging of activity of multidrug resistance-associated protein 1 in the lungs. Am. J. Respir. Cell Mol. Biol. 2013, 49, 335–340. [Google Scholar] [CrossRef]
- Okamura, T.; Kikuchi, T.; Okada, M.; Toramatsu, C.; Fukushi, K.; Takei, M.; Irie, T. Noninvasive and quantitative assessment of the function of multidrug resistance-associated protein 1 in the living brain. J. Cereb. Blood Flow Metab. 2009, 29, 504–511. [Google Scholar] [CrossRef]
- Mairinger, S.; Sake, J.A.; Hernández Lozano, I.; Filip, T.; Sauberer, M.; Stanek, J.; Wanek, T.; Ehrhardt, C.; Langer, O. Assessing the activity of multidrug resistance-associated protein 1 at the lung epithelial barrier. J. Nucl. Med. 2020, 61, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Krohn, M.; Zoufal, V.; Mairinger, S.; Wanek, T.; Paarmann, K.; Bruning, T.; Eiriz, I.; Brackhan, M.; Langer, O.; Pahnke, J. Generation and characterization of an Abcc1 humanized mouse model (hABCC1flx/flx) with knockout capability. Mol. Pharmacol. 2019, 96, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-beta-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Bauer, B.; Hartz, A.M. ABC transporters and the Alzheimer’s disease enigma. Front. Psychiatry 2012, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S. Regulation of ABC transporters blood-brain barrier: The good, the bad, and the ugly. Adv. Cancer Res. 2015, 125, 43–70. [Google Scholar] [CrossRef] [PubMed]
- Hartz, A.M.; Miller, D.S.; Bauer, B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer’s disease. Mol. Pharmacol. 2010, 77, 715–723. [Google Scholar] [CrossRef]
- Zoufal, V.; Mairinger, S.; Brackhan, M.; Krohn, M.; Filip, T.; Sauberer, M.; Stanek, J.; Wanek, T.; Tournier, N.; Bauer, M.; et al. Imaging P-glycoprotein induction at the blood-brain barrier of a beta-amyloidosis mouse model with 11C-metoclopramide PET. J. Nucl. Med. 2020, 61, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.C.; Liu, R.; Lu, P.; Shapiro, A.B.; Renoir, J.M.; Sharom, F.J.; Reiner, P.B. Beta-Amyloid efflux mediated by p-glycoprotein. J. Neurochem. 2001, 76, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Kuhnke, D.; Jedlitschky, G.; Grube, M.; Krohn, M.; Jucker, M.; Mosyagin, I.; Cascorbi, I.; Walker, L.C.; Kroemer, H.K.; Warzok, R.W.; et al. MDR1-P-glycoprotein (ABCB1) mediates transport of Alzheimer’s amyloid-beta peptides–implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007, 17, 347–353. [Google Scholar] [CrossRef]
- Cirrito, J.R.; Deane, R.; Fagan, A.M.; Spinner, M.L.; Parsadanian, M.; Finn, M.B.; Jiang, H.; Prior, J.L.; Sagare, A.; Bales, K.R.; et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Investig. 2005, 115, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Kilic, E.; Spudich, A.; Kilic, U.; Rentsch, K.M.; Vig, R.; Matter, C.M.; Wunderli-Allenspach, H.; Fritschy, J.M.; Bassetti, C.L.; Hermann, D.M. ABCC1: A gateway for pharmacological compounds to the ischaemic brain. Brain 2008, 131, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.; Miller, D.S.; Bendayan, R. Multidrug resistance-associated proteins: Expression and function in the central nervous system. Pharmacol. Rev. 2006, 58, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Miller, D.S.; Pasinelli, P.; Trotti, D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015, 1628, 298–316. [Google Scholar] [CrossRef]
- Okamura, T.; Kikuchi, T.; Fukushi, K.; Irie, T. Reactivity of 6-halopurine analogs with glutathione as a radiotracer for assessing function of multidrug resistance-associated protein 1. J. Med. Chem. 2009, 52, 7284–7288. [Google Scholar] [CrossRef]
- Okamura, T.; Kikuchi, T.; Fukushi, K.; Arano, Y.; Irie, T. A novel noninvasive method for assessing glutathione-conjugate efflux systems in the brain. Bioorg. Med. Chem. 2007, 15, 3127–3133. [Google Scholar] [CrossRef]
- Okamura, T.; Kikuchi, T.; Zhang, M.-R. Attempts to image MRP1 function in the blood-brain barrier using the metabolite extrusion method. In PET and SPECT of Neurobiological Systems; Dierckx, R.A.J.O., Otte, A., de Vries, E.F.J., van Waarde, A., Lammertsma, A.A., Eds.; Springer: Cham, Switzerland, 2021; pp. 547–566. [Google Scholar]
- Maeno, K.; Nakajima, A.; Conseil, G.; Rothnie, A.; Deeley, R.G.; Cole, S.P. Molecular basis for reduced estrone sulfate transport and altered modulator sensitivity of transmembrane helix (TM) 6 and TM17 mutants of multidrug resistance protein 1 (ABCC1). Drug Metab. Dispos. 2009, 37, 1411–1420. [Google Scholar] [CrossRef]
- Seno, H.; Hattori, H.; Ishii, A.; Kumazawa, T.; Watanabe-Suzuki, K.; Suzuki, O. High performance liquid chromatography/electrospray tandem mass spectrometry for phenothiazines with heavy side chains in whole blood. Rapid Commun. Mass Spectrom. 1999, 13, 2394–2398. [Google Scholar] [CrossRef]
- Nickel, S.; Clerkin, C.G.; Selo, M.A.; Ehrhardt, C. Transport mechanisms at the pulmonary mucosa: Implications for drug delivery. Expert Opin. Drug Deliv. 2016, 13, 667–690. [Google Scholar] [CrossRef]
- Radde, R.; Bolmont, T.; Kaeser, S.A.; Coomaraswamy, J.; Lindau, D.; Stoltze, L.; Calhoun, M.E.; Jaggi, F.; Wolburg, H.; Gengler, S.; et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006, 7, 940–946. [Google Scholar] [CrossRef]
- Loening, A.M.; Gambhir, S.S. AMIDE: A free software tool for multimodality medical image analysis. Mol. Imaging 2003, 2, 131–137. [Google Scholar] [CrossRef]



| Group | Scan | Weight (g) | Injected Activity (MBq) | Sex 1 | n |
|---|---|---|---|---|---|
| Wild-type | Baseline | 23.4 ± 1.4 | 36.3 ± 5.3 | F | 7 |
| Thiethylperazine | 22.9 ± 0.6 | 31.4 ± 2.8 | 5 2 | ||
| APP/PS1-21 | Baseline | 23.5 ± 1.5 | 36.9 ± 3.0 | F | 5 |
| Thiethylperazine | 23.9 ± 0.9 | 37.3 ± 7.2 | |||
| Abcc1(−/−) | Baseline | 29.4 ± 4.4 | 34.2 ± 3.0 | M/F | 4/2 |
| Thiethylperazine | 28.3 ± 5.1 | 41.5 ± 6.8 |
| Group | Scan | Brain (h−1) | Lung (h−1) | Kidney (h−1) |
|---|---|---|---|---|
| Wild-type | Baseline | 1.39 ± 0.05 | 1.26 ± 0.29 | 1.54 ± 0.31 |
| Thiethylperazine | 1.36 ± 0.05 | 1.13 ± 0.24 | 1.72 ± 0.28 | |
| APP/PS1-21 | Baseline | 1.44 ± 0.11 | 1.26 ± 0.18 | 1.56 ± 0.43 |
| Thiethylperazine | 1.43 ± 0.19 | 1.23 ± 0.22 | 1.54 ± 0.28 | |
| Abcc1(−/−) | Baseline | 0.14 ± 0.01 | 0.30 ± 0.09 | 0.40 ± 0.45 |
| Thiethylperazine | 0.18 ± 0.04 | 0.39 ± 0.23 | 0.61 ± 0.30 |
| Group | Scan | Blood (%ID/mL) | Plasma (%ID/mL) | Plasma-Blood |
|---|---|---|---|---|
| Wild-type | Baseline | 0.35 ± 0.13 | 0.50 ± 0.09 | 1.52 ± 0.39 |
| Thiethylperazine | 0.32 ± 0.01 | 0.47 ± 0.02 | 1.48 ± 0.04 | |
| APP/PS1-21 | Baseline | 0.25 ± 0.03 | 0.39 ± 0.06 | 1.53 ± 0.13 |
| Thiethylperazine | 0.27 ± 0.10 | 0.41 ± 0.13 | 1.53 ± 0.12 | |
| Abcc1(−/−) | Baseline | 0.96 ± 0.21 | 0.59 ± 0.09 | 0.63 ± 0.05 |
| Thiethylperazine | 1.01 ± 0.28 | 0.58 ± 0.17 | 0.57 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wölfl-Duchek, M.; Mairinger, S.; Hernández-Lozano, I.; Filip, T.; Zoufal, V.; Löbsch, M.; Stanek, J.; Kuntner, C.; Wanek, T.; Bauer, M.; et al. Use of PET Imaging to Assess the Efficacy of Thiethylperazine to Stimulate Cerebral MRP1 Transport Activity in Wild-Type and APP/PS1-21 Mice. Int. J. Mol. Sci. 2022, 23, 6514. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23126514
Wölfl-Duchek M, Mairinger S, Hernández-Lozano I, Filip T, Zoufal V, Löbsch M, Stanek J, Kuntner C, Wanek T, Bauer M, et al. Use of PET Imaging to Assess the Efficacy of Thiethylperazine to Stimulate Cerebral MRP1 Transport Activity in Wild-Type and APP/PS1-21 Mice. International Journal of Molecular Sciences. 2022; 23(12):6514. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23126514
Chicago/Turabian StyleWölfl-Duchek, Michael, Severin Mairinger, Irene Hernández-Lozano, Thomas Filip, Viktoria Zoufal, Mathilde Löbsch, Johann Stanek, Claudia Kuntner, Thomas Wanek, Martin Bauer, and et al. 2022. "Use of PET Imaging to Assess the Efficacy of Thiethylperazine to Stimulate Cerebral MRP1 Transport Activity in Wild-Type and APP/PS1-21 Mice" International Journal of Molecular Sciences 23, no. 12: 6514. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23126514