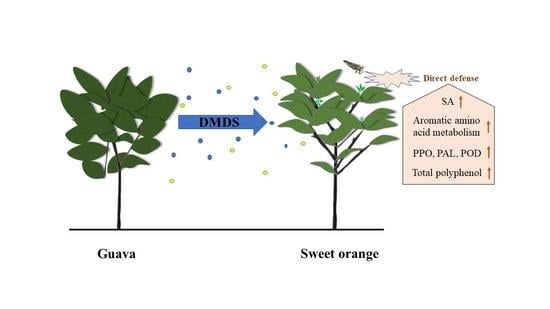
Volatile Dimethyl Disulfide from Guava Plants Regulate Developmental Performance of Asian Citrus Psyllid through Activation of Defense Responses in Neighboring Orange Plants
Abstract
:1. Introduction
2. Results
2.1. Dimethyl Disulfide Is the Dominant Headspace Sulfur Volatile of Guava Plants
2.2. The Developmental Performance of Psyllids Was Suppressed on Dimethyl Disulfide-Exposed Orange Plants
2.3. The Content of Salicylic Acid and the Expression of Defense-Associated Genes Were Induced in Dimethyl Disulfide-Exposed Orange Plants
2.4. The Activity of Defense-Associated Enzymes and the Content of Total Polyphenol Were Enhanced in Dimethyl Disulfide-Exposed Orange Plants
2.5. Volatile and Semipolar Metabolomics of Orange Plants Were Changed after Exposure to Dimethyl Disulfide
3. Discussion
4. Materials and Methods
4.1. Plants and Insect Rearing
4.2. Real-Time Determination of Sulfur Volatiles from Guava Plants
4.3. Exposure of Orange Plant to Sulfur Volatiles
4.4. Insect Preference and Performance Experiments
4.5. Phytohormone Quantification
4.6. Gene Expression Analysis
4.7. Determination of Enzyme Activity and Total Polyphenol Content
4.8. Analysis of Volatile Metabolites
4.9. Analysis of Semipolar Metabolites
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Cai, X.; Li, X.; Xin, Z. (E)-Nerolidol is a volatile signal that induces defenses against insects and pathogens in tea plants. Hortic. Res. 2020, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, V.; Markovic, D.; Dahlin, I. Decoding neighbour volatiles in preparation for future competition and implications for tritrophic interactions. Perspect. Plant Ecol. Evol. Syst. 2016, 23, 11–17. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.-M. Social networking in crop plants: Wired and wireless cross-plant communications. Plant Cell Environ. 2021, 44, 1095–1110. [Google Scholar] [CrossRef]
- Takabayashi, J.; Shiojiri, K. Multifunctionality of herbivory-induced plant volatiles in chemical communication in tritrophic interactions. Curr. Opin. Insect Sci. 2019, 32, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Riahi, C.; González-Rodríguez, J.; Alonso-Valiente, M.; Urbaneja, A.; Pérez-Hedo, M. Eliciting plant defenses through herbivore-induced plant volatiles’ exposure in sweet peppers. Front. Ecol. Evol. 2022, 9, 776827. [Google Scholar] [CrossRef]
- Karban, R.; Yang, L.H.; Edwards, K.F. Volatile communication between plants that affects herbivory: A meta-analysis. Ecol. Lett. 2014, 17, 44–52. [Google Scholar] [CrossRef]
- Dani, K.G.S.; Loreto, F. Plant volatiles as regulators of hormone homeostasis. New Phytol. 2022, 234, 804–812. [Google Scholar] [CrossRef]
- Sukegawa, S.; Shiojiri, K.; Higami, T.; Suzuki, S.; Arimura, G.-I. Pest management using mint volatiles to elicit resistance in soy: Mechanism and application potential. Plant J. 2018, 96, 910–920. [Google Scholar] [CrossRef]
- Dahlin, I.; Ninkovic, V. Aphid performance and population development on their host plants is affected by weed–crop interactions. J. Appl. Ecol. 2013, 50, 1281–1288. [Google Scholar] [CrossRef]
- Ninkovic, V.; Dahlin, I.; Vucetic, A.; Petrovic-Obradovic, O.; Glinwood, R.; Webster, B. Volatile exchange between undamaged plants-a new mechanism affecting insect orientation in intercropping. PLoS ONE 2013, 8, e69431. [Google Scholar]
- Dahlin, I.; Vucetic, A.; Ninkovic, V. Changed host plant volatile emissions induced by chemical interaction between unattacked plants reduce aphid plant acceptance with intermorph variation. J. Pest Sci. 2015, 88, 249–257. [Google Scholar] [CrossRef]
- Tolosa, T.A.; Tamiru, A.; Midega, C.A.O.; Berg, J.V.D.; Khan, Z.R. Molasses grass induces direct and indirect defense responses in neighbouring maize plants. J. Chem. Ecol. 2019, 45, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Rizvi, S.A.H.; Xiong, T.; Liu, J.; Gu, Y.; Wang, S.; Zeng, X. Volatile signals from guava plants prime defense signaling and increase jasmonate-dependent herbivore resistance in neighboring citrus plants. Front. Plant Sci. 2022, 13, 833562. [Google Scholar] [CrossRef] [PubMed]
- Erb, M. Volatiles as inducers and suppressors of plant defense and immunity-origins, specificity, perception and signaling. Curr. Opin. Plant Biol. 2018, 44, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant volatiles as cues and signals in plant communication. Plant Cell Environ. 2021, 44, 1030–1043. [Google Scholar] [CrossRef]
- Kigathi, R.N.; Weisser, W.W.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 2019, 19, 58. [Google Scholar] [CrossRef]
- Beattie, G.A.C.; Holford, P.; Mabberley, D.J.; Haigh, A.M.; Bayer, R.; Broadbent, P. In Aspects and Insights of Australia-Asia CollaboratiVe Research on Huanglongbing. In Proceedings of the International Workshop for Prevention of Citrus Greening Diseases in Severely Infested Areas, Ishigaki, Japan, 7–9 December 2006; Multilateral Research Network for Food and Agricultural Safety: Tokyo, Japan, 2006; pp. 47–64. [Google Scholar]
- Gottwald, T.R.; Hall, D.G.; Kriss, A.B.; Salinas, E.J.; Parker, P.E.; Beattie, G.A.C.; Nguyen, M.C. Orchard and nursery dynamics of the effect of interplanting citrus with guava for huanglongbing, vector, and disease management. Crop Prot. 2014, 64, 93–103. [Google Scholar] [CrossRef]
- Hall, D.G.; Gottwald, T.R.; Nguyen, N.C.; Ichinose, K.; Stover, E. Greenhouse investigations on the effect of guava on infestations of Asian citrus psyllid in grapefruit. Proc. Fla. State Hort. Soc. 2008, 121, 104–109. [Google Scholar]
- Hall, D.G.; Richardson, M.L.; Ammar, E.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Spreen, T.H.; Baldwin, J.P.; Futch, S.H. An economic assessment of the impact of huanglongbing on citrus tree plantings in Florida. HortScience 2014, 49, 1052–1055. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, F.; Li, G.; Chi, X.; Xu, B. Metabolite support of long-term storage of sperm in the spermatheca of honeybee (Apis mellifera) queens. Front. Physiol. 2020, 11, 574856. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Huang, T.; Shi, K.; Wu, Z. Chemical compositions, antioxidant and antimicrobial activities of essential oils of Psidium guajava L. Leaves from different geographic regions in China. Chem. Biodivers. 2017, 14, e1700114. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.D.S.; Ferreira, M.F.D.S.; Menini, L.; Souza, J.R.C.L.; Bernardes, C.O.; Ferreira, A. Chemotype diversity of Psidium guajava L. Phytochemistry 2018, 153, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.A.; Hall, D.G.; Gottwald, T.R.; Andrade, M.S.; Maldonado, W.; Alessandro, R.T.; Lapointe, S.L.; Andrade, E.C.; Machado, M.A. Repellency of selected Psidium guajava cultivars to the Asian citrus psyllid, Diaphorina citri. Crop Prot. 2016, 84, 14–20. [Google Scholar] [CrossRef]
- Alquézar, B.; Volpe, H.; Magnani, R.F.; Miranda, M.D.; Santos, M.A.; Wulff, N.A.; Bento, J.; Parra, J.; Bouwmeester, H.; Peña, L. β-Caryophyllene emitted from a transgenic Arabidopsis or chemical dispenser repels Diaphorina citri, vector of Candidatus Liberibacters. Sci. Rep. 2017, 7, 5639. [Google Scholar] [CrossRef] [PubMed]
- Rouseff, R.L.; Onagbola, E.O.; Smoot, J.M.; Stelinski, L.L. Sulfur volatiles in guava (Psidium guajava L.) leaves: Possible defense mechanism. J. Agric. Food Chem. 2008, 56, 8905–8910. [Google Scholar] [CrossRef]
- Cannon, R.J.; Ho, C.-T. Volatile sulfur compounds in tropical fruits. J. Food Drug Anal. 2018, 26, 445–468. [Google Scholar] [CrossRef]
- Zaka, S.M.; Zeng, X.N.; Holfor, P. Repellent effect of guava leaf volatiles on settlement of adults of citrus psylla, Diaphorina citri Kuwayama, on citrus. Insect Sci. 2010, 17, 39–45. [Google Scholar] [CrossRef]
- Onagbola, E.O.; Rouseff, R.L.; Smoot, J.M.; Stelinski, L.L. Guava leaf volatiles and dimethyl disulphide inhibit response of Diaphorina citri Kuwayama to host plant volatiles. J. Appl. Entomol. 2011, 135, 404–414. [Google Scholar] [CrossRef]
- Barman, J.C.; Campbell, S.A.; Zeng, X. Exposure to guava affects citrus olfactory cues and attractiveness to Diaphorina citri (Hemiptera: Psyllidae). Environ. Entomol. 2016, 45, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Haneklaus, S.; Kok, L.; Schnug, E.; Singh, B. Effect of H2S and dimethyl sulfide (DMS) on growth and enzymatic activities of Rhizoctonia solani and its implications for sulfur-induced resistance (SIR) of agricultural crops. Phyton-Ann. REI Bot. 2006, 46, 55–70. [Google Scholar]
- Choudhary, A.K.; Singh, S.; Khatri, N.; Gupta, R. Hydrogen sulphide: An emerging regulator of plant defence signalling. Plant Biol. 2022, 24, 532–539. [Google Scholar] [CrossRef]
- Tyagi, S.; Lee, K.-J.; Shukla, P.; Chae, J.-C. Dimethyl disulfide exerts antifungal activity against Sclerotinia minor by damaging its membrane and induces systemic resistance in host plants. Sci. Rep. 2020, 10, 6547. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Duan, Y.; Liu, J.P.; Fu, J.; Huang, Y. Efficacy of dimethyl trisulfide on the suppression of ring rot disease caused by Botryosphaeria dothidea and induction of defense-related genes on apple fruits. Front. Microbiol. 2022, 13, 796167. [Google Scholar] [CrossRef]
- Li, T.; Blande, J.D. Associational susceptibility in broccoli: Mediated by plant volatiles, impeded by ozone. Glob. Chang. Biol. 2015, 21, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Zebelo, S.A.; Matsui, K.; Ozawa, R.; Maffei, M.E. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. Plant Sci. 2012, 196, 93–100. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Ye, M.; Liu, M.; Erb, M.; Glauser, G.; Zhang, J.; Li, X.; Sun, X. Indole primes defence signalling and increases herbivore resistance in tea plants. Plant Cell Environ. 2021, 44, 1165–1177. [Google Scholar] [CrossRef]
- Zeng, L.; Liao, Y.; Li, J.; Zhou, Y.; Tang, J. α-Farnesene and ocimene induce metabolite changes by volatile signaling in neighboring tea (Camellia sinensis) plants. Plant Sci. 2017, 264, 29–36. [Google Scholar] [CrossRef]
- Riedlmeier, M.; Ghirardo, A.; Wenig, M.; Knappe, C.; Koch, K.; Georgii, E.; Dey, S.; Parker, J.E.; Schnitzler, J.P.; Vlot, C. Monoterpenes support systemic acquired resistance within and between plants. Plant Cell 2017, 29, 1440–1459. [Google Scholar] [CrossRef]
- Wei, X.; Vrieling, K.; Kim, H.K.; Mulder, P.P.J.; Klinkhamer, P.G.L. Application of methyl jasmonate and salicylic acid lead to contrasting effects on the plant’s metabolome and herbivory. Plant Sci. 2021, 303, 110784. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Bouwmeester, H.J.; Kappers, I.F. Combined transcriptome and metabolome analysis identifies defence responses in spider mite-infested pepper (Capsicum annuum). J. Exp. Bot. 2020, 71, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Duan, X.N.; Jin, S.; Li, X.W. Regurgitant derived from the tea geometrid Ectropis obliqua suppresses wound-induced polyphenol oxidases activity in tea plants. J. Chem. Ecol. 2013, 39, 744–751. [Google Scholar] [CrossRef]
- Ye, M.; Song, Y.-Y.; Baerson, S.R.; Long, J.; Wang, J.; Pan, Z.; Lin, W.-X.; Zeng, R.-S. Ratoon rice generated from primed parent plants exhibit enhanced herbivore resistance. Plant Cell Environ. 2017, 40, 779–787. [Google Scholar] [CrossRef]
- Duan, C.; Yu, J.; Bai, J.; Zhu, Z.; Wang, X. Induced defense responses in rice plants against small brown planthopper infestation. Crop J. 2014, 2, 55–62. [Google Scholar] [CrossRef]
- Martini, X.; Pelz-Stelinski, K.S.; Stelinski, L.L. Plant pathogen-induced volatiles attract parasitoids to increase parasitism of an insect vector. Front. Ecol. Evol. 2014, 2, 8. [Google Scholar] [CrossRef]
- Liu, X.Q.; Jiang, H.B.; Fan, J.Y.; Liu, T.Y.; Meng, L.W.; Liu, Y.; Yu, H.Z.; Dou, W.; Wang, J.J. An odorant-binding protein of Asian citrus psyllid, Diaphorina citri, participates in the response of host plant volatiles. Pest Manag. Sci. 2021, 77, 3068–3079. [Google Scholar] [CrossRef]
- ZEIER, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef]
- Kisa, D.; İmamoğlu, R.; Genç, N.; Şahin, S.; Qayyum, M.A.; Elmastaş, M. The interactive effect of aromatic amino acid composition on the accumulation of phenolic compounds and the expression of biosynthesis-related genes in Ocimum basilicum. Physiol. Mol. Biol. Plants 2021, 27, 2057–2069. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and tyrosine as exogenous precursors of wheat (Triticum aestivum L.) secondary metabolism through PAL-associated pathways. Plants 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Girón-Calva, P.; Molina-Torres, J.; Heil, M. Volatile dose and exposure time impact perception in neighboring plants. J. Chem. Ecol. 2012, 38, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Šimpraga, M.; Takabayashi, J.; Holopainen, J.K. Language of plants: Where is the word? J. Integr. Plant Biol. 2016, 58, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.; Wenig, M.; Ghirardo, A.; Krol, A.; Rosenkranz, M. Isoprene and β-aryophyllene confer plant resistance via different plant internal signaling pathways. Plant Cell Environ. 2021, 44, 1151–1164. [Google Scholar] [CrossRef]
- Cipollini, D.; Purrington, C.B.; Bergelson, J. Costs of induced responses in plants. Basic Appl. Ecol. 2003, 4, 79–89. [Google Scholar] [CrossRef]
- van Hulten, M.; Pelser, M.; van Loon, L.C.; Pieterse, C.M.J.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef]
- Karban, R. Tradeoff between resistance induced by volatile communication and over-topping vertical growth. Plant Signal. Behav. 2017, 12, e1309491. [Google Scholar] [CrossRef]
- Freundlich, G.E.; Shields, M.; Frost, C.J. Dispensing a synthetic green leaf volatile to two plant species in a common garden differentially alters physiological responses and herbivory. Agronomy 2021, 11, 958. [Google Scholar] [CrossRef]
- Engelberth, J.; Engelberth, M. The costs of green leaf volatile-induced defense priming: Temporal diversity in growth responses to mechanical wounding and insect herbivory. Plants 2019, 8, 23. [Google Scholar] [CrossRef]
- Douma, J.C.; Vermeulen, P.J.; Poelman, E.H.; Dicke, M.; Anten, N.P.R. When does it pay off to prime for defense? A modeling analysis. New Phytol. 2017, 216, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, I.A.; Pieterse, C.M.J.; van Wees, S.C.M. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55. [Google Scholar] [CrossRef]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Märk, L.; Seehauser, H.; Schottkowsky, R.; Sulzer, P.; Märk, T.D. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int. J. Mass Spectrom. 2009, 286, 122–128. [Google Scholar] [CrossRef]
- Danner, H.; Brown, P.; Cator, E.A.; Harren, F.J.M.; van Dam, N.M.; Cristescu, S.M. Aboveground and belowground herbivores synergistically induce volatile organic sulfur compound emissions from shoots but not from roots. J. Chem. Ecol. 2015, 41, 631–640. [Google Scholar] [CrossRef]
- Samudrala, D.; Brown, P.A.; Mandon, J.; Cristescu, S.M.; Harren, F.J.M. Optimization and sensitive detection of sulfur compounds emitted from plants using proton transfer reaction mass spectrometry. Int. J. Mass Spectrom. 2015, 386, 6–14. [Google Scholar] [CrossRef]
- George, J.; Shi, Q.; Stelinski, L.L.; Stover, E.; Lapointe, S.L. Host selection, oviposition and feeding by a phytopathogen vector, Diaphorina citri (Hemiptera: Liviidae), modulated by plant exposure to formic acid. Front. Ecol. Evol. 2019, 7, 78. [Google Scholar] [CrossRef]
- Ibanez, F.; Suh, J.H.; Wang, Y.; Stelinski, L.L. Long-term, sustained feeding by Asian citrus psyllid disrupts salicylic acid homeostasis in sweet orange. BMC Plant Biol. 2019, 19, 493. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Mafra, V.; Kubo, K.S.; Alves-Ferreira, M.; Ribeiro-Alves, M.; Stuart, R.M.; Boava, L.P.; Rodrigues, C.M.; Machado, M.A. Reference genes for accurate transcript tormalization in citrus genotypes under different experimental conditions. PLoS ONE 2012, 7, e31263. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vos, R.; Moco, S.; Lommen, A.; Keurentjes, J.J.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Reig, M.; Jaumot, J.; García-Reiriz, A.; Tauler, R. Evaluation of changes induced in rice metabolome by Cd and Cu exposure using LC-MS with XCMS and MCR-ALS data analysis strategies. Anal. Bioanal. Chem. 2015, 407, 8835–8847. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinf. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, S.; Qiu, H.; Xu, J.; Gu, Y.; Yu, J.; Wang, W.; Liu, J.; Zeng, X. Volatile Dimethyl Disulfide from Guava Plants Regulate Developmental Performance of Asian Citrus Psyllid through Activation of Defense Responses in Neighboring Orange Plants. Int. J. Mol. Sci. 2022, 23, 10271. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231810271
Ling S, Qiu H, Xu J, Gu Y, Yu J, Wang W, Liu J, Zeng X. Volatile Dimethyl Disulfide from Guava Plants Regulate Developmental Performance of Asian Citrus Psyllid through Activation of Defense Responses in Neighboring Orange Plants. International Journal of Molecular Sciences. 2022; 23(18):10271. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231810271
Chicago/Turabian StyleLing, Siquan, Hualong Qiu, Jinzhu Xu, Yanping Gu, Jinxin Yu, Wei Wang, Jiali Liu, and Xinnian Zeng. 2022. "Volatile Dimethyl Disulfide from Guava Plants Regulate Developmental Performance of Asian Citrus Psyllid through Activation of Defense Responses in Neighboring Orange Plants" International Journal of Molecular Sciences 23, no. 18: 10271. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231810271