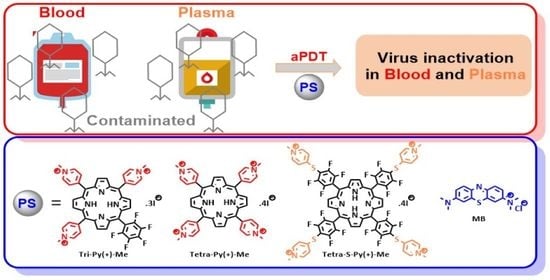
Anti-Viral Photodynamic Inactivation of T4-like Bacteriophage as a Mammalian Virus Model in Blood
Abstract
:1. Introduction
2. Results
2.1. Evaluation of aPDT Effect on Erythrocyte Osmotic Fragility
2.2. Photodynamic Inactivation of T4-Like Phage in PBS, Plasma, and Whole Blood
3. Discussion
4. Materials and Methods
4.1. Blood Samples
4.2. Bacterial Strain and Growth Conditions
4.3. Phage Stock Preparation and Quantification
4.4. Photosensitizers Stock Solutions
4.5. Irradiation Conditions
4.6. Evaluation of aPDT Effect on Erythrocyte Osmotic Fragility
4.7. Photodynamic Inactivation of T4-like Phage in PBS, Plasma, and Whole Blood
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wainwright, M. Pathogen Inactivation in Blood Products. Curr. Med. Chem. 2002, 9, 127–143. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Blood Transfusion Safety. The Clinical Use of Blood: In Medicine, Obstetrics, Paediatrics, Surgery & Anaesthesia, Trauma & Burns; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Ben-Hur, E.; Goodrich, R.P. Chapter 9. Pathogen Reduction in Blood for Transfusion Using Photodynamic Treatments. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications; RSC Publishing: Cambridge, UK, 2011; pp. 233–263. [Google Scholar]
- Liumbruno, G.M.; Bennardello, F.; Lattanzio, A.; Piccoli, P.L.; Rossetti, G. Recommendations for the Transfusion of Plasma and Platelets. Blood Transfus. 2009, 7, 132–150. [Google Scholar] [PubMed]
- Dean, C.L.; Wade, J.; Roback, J.D. Transfusion-Transmitted Infections: An Update on Product Screening, Diagnostic Techniques, and the Path Ahead. J. Clin. Microbiol. 2018, 56, e00352-18. [Google Scholar] [CrossRef]
- Politis, C.; Kavallierou, L.; Hantziara, S.; Parara, M.; Zervou, E.; Katsarou, O.; Hatzitaki, M.; Fountouli, P.; Gioka, A.; Tzioura, K.; et al. Haemovigilance Data on the Use of Methylene Blue Virally Inactivated Fresh Frozen Plasma with the Theraflex MB-Plasma System in Comparison to Quarantine Plasma: 11 Years’ Experience. Transfus. Med. 2014, 24, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, V.; van der Meer, P.F.; de Korte, D.; Seghatchian, J.; Gutiérrez, L. Development of Blood Transfusion Product Pathogen Reduction Treatments: A Review of Methods, Current Applications and Demands. Transfus. Apher. Sci. 2015, 52, 19–34. [Google Scholar] [CrossRef]
- di Minno, G.; Perno, C.F.; Tiede, A.; Navarro, D.; Canaro, M.; Güertler, L.; Ironside, J.W. Current Concepts in the Prevention of Pathogen Transmission via Blood/Plasma-Derived Products for Bleeding Disorders. Blood Rev. 2016, 30, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Aabdien, M.; Selim, N.; Himatt, S.; Hmissi, S.; Merenkov, Z.; AlKubaisi, N.; Abdel-Rahman, M.E.; Abdelmola, A.; Khelfa, S.; Farag, E.; et al. Prevalence and Trends of Transfusion Transmissible Infections among Blood Donors in the State of Qatar, 2013–2017. BMC Infect. Dis. 2020, 20, 617. [Google Scholar] [CrossRef]
- Bihl, F.; Castelli, D.; Marincola, F.; Dodd, R.Y.; Brander, C. Transfusion-Transmitted Infections. J. Transl. Med. 2007, 5, 25. [Google Scholar] [CrossRef]
- Schneider, B.; Becker, M.; Brackmann, H.-H.; Eis-Hübinger, A. Contamination of Coagulation Factor Concentrates with Human Parvovirus B19 Genotype 1 and 2. Thromb. Haemost. 2004, 92, 838–845. [Google Scholar] [CrossRef]
- Messina, G.; Ceriale, E.; Lenzi, D.; Burgassi, S.; Azzolini, E.; Manzi, P. Environmental Contaminants in Hospital Settings and Progress in Disinfecting Techniques. Biomed. Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R. Upconversion in Photodynamic Therapy: Plumbing the Depths. Dalton Trans. 2018, 47, 8571–8580. [Google Scholar] [CrossRef] [PubMed]
- Kashef, N.; Huang, Y.-Y.; Hamblin, M.R. Advances in Antimicrobial Photodynamic Inactivation at the Nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.; Tomé, J.P. Photodynamic Inactivation of Bacteria: Finding the Effective Targets. Future Med. Chem. 2015, 7, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Costa, L.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Evaluation of Resistance Development and Viability Recovery by a Non-Enveloped Virus after Repeated Cycles of APDT. Antivir. Res. 2011, 91, 278–282. [Google Scholar] [CrossRef]
- Alves, E.; Costa, L.; Carvalho, C.M.; Tomé, J.P.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Almeida, A. Charge Effect on the Photoinactivation of Gram-Negative and Gram-Positive Bacteria by Cationic Meso-Substituted Porphyrins. BMC Microbiol. 2009, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Skupin-Mrugalska, P.; Mielcarek, J.; Goslinski, T.; Balzarini, J. Photosensitizers Mediated Photodynamic Inactivation Against Virus Particles. Mini-Rev. Med. Chem. 2015, 15, 503–521. [Google Scholar] [CrossRef]
- Marciel, L.; Mesquita, M.Q.; Ferreira, R.; Moreira, B.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Efficient Formulation Based on Cationic Porphyrins to Photoinactivate Staphylococcus Aureus Escherichia coli. Future Med. Chem. 2018, 10, 1821–1833. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Almeida, A. Photodynamic Inactivation of Mammalian Viruses and Bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef] [Green Version]
- Lozano, M.; Cid, J.; Müller, T.H. Plasma Treated with Methylene Blue and Light: Clinical Efficacy and Safety Profile. Transfus. Med. Rev. 2013, 27, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Seghatchian, J.; Struff, W.G.; Reichenberg, S. Main Properties of the THERAFLEX MB-Plasma System for Pathogen Reduction. Transfus. Med. Hemother. 2011, 38, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Mohr, H.; Knüver-Hopf, J.; Gravemann, U.; Redecker-Klein, A.; Müller, T.H. West Nile Virus in Plasma Is Highly Sensitive to Methylene Blue-Light Treatment. Transfusion 2004, 44, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Gironés, N.; Bueno, J.L.; Carrión, J.; Fresno, M.; Castro, E. The Efficacy of Photochemical Treatment with Methylene Blue and Light for the Reduction of Trypanosoma Cruzi in Infected Plasma. Vox Sang. 2006, 91, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Mohr, H.; Bachmann, B.; Klein-Struckmeier, A.; Lambrecht, B. Virus Inactivation of Blood Products by Phenothiazine Dyes and Light. Photochem. Photobiol. 1997, 65, 441–445. [Google Scholar] [CrossRef]
- Arroyo, J.L.; Martínez, E.; Amunárriz, C.; Muñoz, C.; Romón, I.; Álvarez, I.; García, J.M. Methylene Blue-Treated Plasma, versus Quarantine Fresh Frozen Plasma, for Acute Thrombotic Thrombocytopenic Purpura Treatment: Comparison between Centres and Critical Review on Longitudinal Data. Transfus. Apher. Sci. 2020, 59, 102771. [Google Scholar] [CrossRef]
- del Río-Garma, J.; Alvarez-Larrán, A.; Martínez, C.; Muncunill, J.; Castellà, D.; de la Rubia, J.; Zamora, C.; Corral, M.; Viejo, A.; Peña, F.; et al. Methylene Blue-Photoinactivated Plasma versus Quarantine Fresh Frozen Plasma in Thrombotic Thrombocytopenic Purpura: A Multicentric, Prospective Cohort Study. Br. J. Haematol. 2008, 143, 39–45. [Google Scholar] [CrossRef]
- Jin, C.; Yu, B.; Zhang, J.; Wu, H.; Zhou, X.; Yao, H.; Liu, F.; Lu, X.; Cheng, L.; Jiang, M.; et al. Retraction Note to: Methylene Blue Photochemical Treatment as a Reliable SARS-CoV-2 Plasma Virus Inactivation Method for Blood Safety and Convalescent Plasma Therapy for COVID-19. BMC Infect. Dis. 2021, 21, 672. [Google Scholar] [CrossRef]
- Marciel, L.; Teles, L.; Moreira, B.; Pacheco, M.; Lourenço, L.M.O.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Faustino, M.A.F.; Almeida, A. An Effective and Potentially Safe Blood Disinfection Protocol Using Tetrapyrrolic Photosensitizers. Future Med. Chem. 2017, 9, 365–379. [Google Scholar] [CrossRef]
- Sousa, V.; Gomes, A.T.P.C.; Freitas, A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A. Photodynamic Inactivation of Candida albicans in Blood Plasma and Whole Blood. Antibiotics 2019, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Kaestner, L.; Juzeniene, A.; Moan, J. Erythrocytes—The ‘House Elves’ of Photodynamic Therapy. Photochem. Photobiol. Sci. 2004, 3, 981–989. [Google Scholar] [CrossRef]
- Beaton, S.; McPherson, R.A.; Tilley, L. Alterations in erythrocyte band 3 organization induced by the photosensitizer, hematoporphyrin derivative. Photochem. Photobiol. 1995, 62, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Bolodon, V.N.; Krut’ko, I.V.; Rozin, V.V.; Chernitskiĭ, E.A. Effect of Erythrocyte Membrane Structure on the Dose Dependence of Photohemolysis. Biofizika 1996, 41, 413–416. [Google Scholar] [PubMed]
- Hellstern, P.; Solheim, B.G. The Use of Solvent/Detergent Treatment in Pathogen Reduction of Plasma. Transfus. Med. Hemother. 2011, 38, 65–70. [Google Scholar] [CrossRef]
- Wainwright, M. Methylene Blue Derivatives—Suitable Photoantimicrobials for Blood Product Disinfection? Int. J. Antimicrob. Agents 2000, 16, 381–394. [Google Scholar] [CrossRef]
- Burnouf, T.; Radosevich, M. Reducing the Risk of Infection from Plasma Products: Specific Preventative Strategies. Blood Rev. 2000, 14, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Sadraeian, M.; da Cruz, E.F.; Boyle, R.W.; Bahou, C.; Chudasama, V.; Janini, L.M.R.; Diaz, R.S.; Guimarães, F.E.G. Photoinduced Photosensitizer–Antibody Conjugates Kill HIV Env-Expressing Cells, Also Inactivating HIV. ACS Omega 2021, 6, 16524–16534. [Google Scholar] [CrossRef]
- Sadraeian, M.; Bahou, C.; da Cruz, E.F.; Janini, L.M.R.; Sobhie Diaz, R.; Boyle, R.W.; Chudasama, V.; Eduardo Gontijo Guimarães, F. Photoimmunotherapy Using Cationic and Anionic Photosensitizer-Antibody Conjugates against HIV Env-Expressing Cells. Int. J. Mol. Sci. 2020, 21, 9151. [Google Scholar] [CrossRef]
- Costa, L.; Alves, E.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Sewage Bacteriophage Photoinactivation by Cationic Porphyrins: A Study of Charge Effect. Photochem. Photobiol. Sci. 2008, 7, 415. [Google Scholar] [CrossRef]
- Kutter, E.; Bryan, D.; Ray, G.; Brewster, E.; Blasdel, B.; Guttman, B. From Host to Phage Metabolism: Hot Tales of Phage T4’s Takeover of E. coli. Viruses 2018, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.P.S.; Faustino, M.A.F.; Almeida, A.; Lourenço, L.M.O. The Antimicrobial Photoinactivation Effect on Escherichia coli through the Action of Inverted Cationic Porphyrin–Cyclodextrin Conjugates. Microorganisms 2022, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.; Gamelas, S.R.D.; Vieira, C.; Faustino, M.A.F.; Tomé, J.P.C.; Almeida, A.; Gomes, A.T.P.C.; Lourenço, L.M.O. Pyrazole-Pyridinium Porphyrins and Chlorins as Powerful Photosensitizers for Photoinactivation of Planktonic and Biofilm Forms of E. coli. Dye. Pigment. 2021, 193, 109557. [Google Scholar] [CrossRef]
- Calmeiro, J.M.D.; Dias, C.J.; Ramos, C.I.V.; Almeida, A.; Tomé, J.P.C.; Faustino, M.A.F.; Lourenço, L.M.O. Comparative Photodynamic Inactivation of Bioluminescent E. coli by Pyridinium and Inverted Pyridinium Chlorins. Dye. Pigment. 2020, 173, 107410. [Google Scholar] [CrossRef]
- Ribeiro, C.P.S.; Lourenço, L.M.O. Overview of Cationic Phthalocyanines for Effective Photoinactivation of Pathogenic Microorganisms. J. Photochem. Photobiol. C Photochem. Rev. 2021, 48, 100422. [Google Scholar] [CrossRef]
- Simões, C.; Gomes, M.C.; Neves, M.G.P.M.S.; Cunha, Â.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Faustino, M.A.F. Photodynamic Inactivation of Escherichia coli with Cationic Meso-Tetraarylporphyrins—The Charge Number and Charge Distribution Effects. Catal. Today 2016, 266, 197–204. [Google Scholar] [CrossRef]
- Gomes, M.C.; Woranovicz-Barreira, S.M.; Faustino, M.A.F.; Fernandes, R.; Neves, M.G.P.M.S.; Tomé, A.C.; Gomes, N.C.M.; Almeida, A.; Cavaleiro, J.A.S.; Cunha, Â.; et al. Photodynamic Inactivation of Penicillium Chrysogenum Conidia by Cationic Porphyrins. Photochem. Photobiol. Sci. 2011, 10, 1735. [Google Scholar] [CrossRef]
- Ribeiro, C.P.S.; Gamelas, S.R.D.; Faustino, M.A.F.; Gomes, A.T.P.C.; Tomé, J.P.C.; Almeida, A.; Lourenço, L.M.O. Unsymmetrical Cationic Porphyrin-Cyclodextrin Bioconjugates for Photoinactivation of Escherichia coli. Photodiagn. Photodyn. Ther. 2020, 31, 101788. [Google Scholar] [CrossRef]
- Calmeiro, J.M.D.; Gamelas, S.R.D.; Gomes, A.T.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A.; Tomé, J.P.C.; Lourenço, L.M.O. Versatile Thiopyridyl/Pyridinone Porphyrins Combined with Potassium Iodide and Thiopyridinium/Methoxypyridinium Porphyrins on E. coli Photoinactivation. Dye. Pigment. 2020, 181, 108476. [Google Scholar] [CrossRef]
- Fedorov, V.; Kholina, E.; Khruschev, S.; Kovalenko, I.; Rubin, A.; Strakhovskaya, M. What Binds Cationic Photosensitizers Better: Brownian Dynamics Reveals Key Interaction Sites on Spike Proteins of SARS-CoV, MERS-CoV, and SARS-CoV-2. Viruses 2021, 13, 1615. [Google Scholar] [CrossRef]
- Sharshov, K.; Solomatina, M.; Kurskaya, O.; Kovalenko, I.; Kholina, E.; Fedorov, V.; Meerovich, G.; Rubin, A.; Strakhovskaya, M. The Photosensitizer Octakis (Cholinyl) Zinc Phthalocyanine with Ability to Bind to a Model Spike Protein Leads to a Loss of SARS-CoV-2 Infectivity In Vitro When Exposed to Far-Red LED. Viruses 2021, 13, 643. [Google Scholar] [CrossRef]
- Kim, G.; Karbaschi, M.; Cooke, M.; Gaitas, A. Light-Based Methods for Whole Blood Bacterial Inactivation Enabled by a Recirculating Flow System. Photochem. Photobiol. 2018, 94, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.Q.; Blanco, K.C.; Soares, J.M.; Inada, N.M.; Kurachi, C.; Golim, M.d.A.; Deffune, E.; Bagnato, V.S. Photodynamic Inactivation for in Vitro Decontamination of Staphylococcus Aureus in Whole Blood. Photodiagn. Photodyn. Ther. 2019, 28, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kufner, G.; Schlegel, H.; Jäger, R. A Spectrophotometric Micromethod for Determining Erythrocyte Protoporphyrin-IX in Whole Blood or Erythrocytes. Clin. Chem. Lab. Med. (CCLM) 2005, 43, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Pereira, C.; Gomes, A.; Almeida, A. Efficiency of Single Phage Suspensions and Phage Cocktail in the Inactivation of Escherichia coli and Salmonella Typhimurium: An In Vitro Preliminary Study. Microorganisms 2019, 7, 94. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, P.; Gomes, A.T.P.C.; Lourenço, L.M.O.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A. Anti-Viral Photodynamic Inactivation of T4-like Bacteriophage as a Mammalian Virus Model in Blood. Int. J. Mol. Sci. 2022, 23, 11548. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231911548
Santos P, Gomes ATPC, Lourenço LMO, Faustino MAF, Neves MGPMS, Almeida A. Anti-Viral Photodynamic Inactivation of T4-like Bacteriophage as a Mammalian Virus Model in Blood. International Journal of Molecular Sciences. 2022; 23(19):11548. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231911548
Chicago/Turabian StyleSantos, Patrícia, Ana T. P. C. Gomes, Leandro M. O. Lourenço, Maria A. F. Faustino, Maria G. P. M. S. Neves, and Adelaide Almeida. 2022. "Anti-Viral Photodynamic Inactivation of T4-like Bacteriophage as a Mammalian Virus Model in Blood" International Journal of Molecular Sciences 23, no. 19: 11548. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231911548