Effect of Transglutaminase Post-Treatment on the Stability and Swelling Behavior of Casein Micro-Particles
Abstract
:1. Introduction
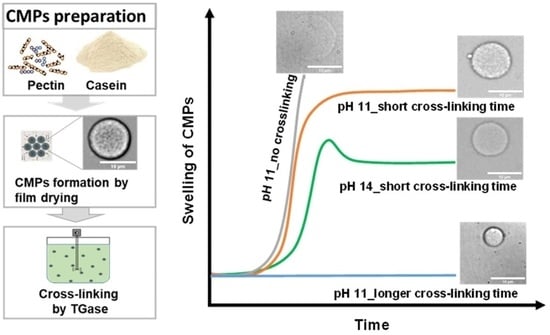
2. Results and Discussion
3. Material and Methods
3.1. Materials
3.2. Preparation of Working Solutions
3.3. Preparation of Casein Microparticles
3.4. Enzymatic Cross-Linking
3.5. CMPs Morphology Using Line Cut Data
3.6. Stability Experiments
3.7. pH-Dependent Swelling Experiments
3.8. Dynamic Model and Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcuello, C.; Chambel, L.; Rodrigues, M.S.; Ferreira, L.P.; Cruz, M.M. Magnetotactic bacteria: Magnetism beyond magnetosomes. IEEE Trans. Nanobiosci. 2018, 17, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, E. Magnetic nanoparticles in targeted drug delivery: A review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Tavares, G.M.; Croguennec, T.; Carvalho, A.F.; Bouhallab, S. Milk proteins as encapsulation devices and delivery vehicles: Applications and trends. Trends Food Sci. Technol. 2014, 37, 5–20. [Google Scholar] [CrossRef]
- Santiago, L.G.; Castro, G.R. Novel technologies for the encapsulation of bioactive food compounds. Curr. Opin. Food Sci. 2016, 7, 78–85. [Google Scholar] [CrossRef]
- Nascimento, L.G.L.; Casanova, F.; Silva, N.F.N.; de Carvalho Teixeira, A.V.N.; de Carvalho, A.F. Casein-based hydrogels: A mini-review. Food Chem. 2020, 314, 126063. [Google Scholar] [CrossRef]
- Sadiq, U.; Gill, H.; Chandrapala, J. Casein micelles as an emerging delivery system for bioactive food components. Foods 2021, 10, 1965. [Google Scholar] [CrossRef]
- Holt, C. A quantitative calcium phosphate nanocluster model of the casein micelle: The average size, size distribution and surface properties. Eur. Biophys. J. 2021, 50, 847–866. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Brodkorb, A. The casein micelle: Historical aspects, current concepts and significance. Int. Dairy J. 2008, 18, 677–684. [Google Scholar] [CrossRef]
- Bouchoux, A.; Gesan-Guiziou, G.; Pérez, J.; Cabane, B. How to squeeze a sponge: Casein micelles under osmotic stress, a SAXS study. Biophys. J. 2010, 99, 3754–3762. [Google Scholar] [CrossRef] [Green Version]
- Huppertz, T.; Gazi, I.; Luyten, H.; Nieuwenhuijse, H.; Alting, A.; Schokker, E. Hydration of casein micelles and caseinates: Implications for casein micelle structure. Int. Dairy J. 2017, 74, 1–11. [Google Scholar] [CrossRef]
- Dalgleish, D.G. On the structural models of bovine casein micelles—review and possible improvements. Soft Matter 2011, 7, 2265–2272. [Google Scholar] [CrossRef]
- Sherstneva, A.A.; Demina, T.S.; Monteiro, A.P.; Akopova, T.A.; Grandfils, C.; Ilangala, A.B. Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application. Polymers 2022, 14, 1314. [Google Scholar] [CrossRef] [PubMed]
- Głąb, T.K.; Boratyński, J. Potential of Casein as a Carrier for Biologically Active Agents. Top. Curr. Chem. 2017, 375, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matalanis, A.; Decker, E.A.; McClements, D.J. Inhibition of lipid oxidation by encapsulation of emulsion droplets within hydrogel microspheres. Food Chem. 2012, 13, 766–772. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sterr, J.; Kulozik, U.; Gebhardt, R. Application of confocal Raman microscopy to investigate casein micro-particles in blend casein/pectin films. Int. J. Biol. Macromol. 2015, 74, 44–48. [Google Scholar] [CrossRef]
- Maroziene, A.; De Kruif, C.G. Interaction of pectin and casein micelles. Food Hydrocoll. 2000, 14, 391–394. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sterr, J.; Schulte, J.; Kulozik, U.; Gebhardt, R. Casein Microparticles from Blend Films Forming Casein/α-Tocopherol Emulsion Droplets in Solution. Food Biophys. 2016, 11, 332–338. [Google Scholar] [CrossRef]
- Schulte, J.; Pütz, T.; Gebhardt, R. Influence of pectin and drying conditions on the structure, stability and swelling behaviour of casein microparticles. Int. Dairy J. 2022, 133, 105422. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R. Interaction between casein and sodium dodecyl sulfate. J. Colloid Interface Sci. 2007, 315, 685–692. [Google Scholar] [CrossRef]
- Lefebvre-Cases, E.; Gastaldi, E.; Tarodo De La Fuente, B. Influence of chemical agents on interactions in dairy products: Effect of SDS on casein micelles. Colloids Surf. B Biointerfaces 1998, 11, 281–285. [Google Scholar] [CrossRef]
- Schulte, J.; Stöckermann, M.; Thill, S.; Gebhardt, R. Calcium effect on the swelling behaviour and stability of casein microparticles. Int. Dairy J. 2020, 105, 104692. [Google Scholar] [CrossRef]
- Schulte, J.; Stöckermann, M.; Gebhardt, R. Influence of pH on the stability and structure of single casein microparticles. Food Hydrocoll. 2020, 105, 105741. [Google Scholar] [CrossRef]
- De Kruif, C.K.; Anema, S.G.; Zhu, C.; Havea, P.; Coker, C. Water holding capacity and swelling of casein hydrogels. Food Hydrocoll. 2015, 44, 372–379. [Google Scholar] [CrossRef]
- Saraydın, D.; Karadag, E.; Işıkver, Y.; Şahiner, N.; Güven, O. The influence of preparation methods on the swelling and network properties of acrylamide hydrogels with crosslinkers. J. Macromol. Sci. Part A 2004, 41, 419–431. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z. Acid and rennet-induced coagulation behavior of casein micelles with modified structure. Food Chem. 2019, 291, 231–238. [Google Scholar] [CrossRef]
- Partschefeld, C.; Schwarzenbolz, U.; Richter, S.; Henle, T. Crosslinking of casein by microbial transglutaminase and its resulting influence on the stability of micelle structure. Biotechnol. J. Healthc. Nutr. Technol. 2007, 2, 456–461. [Google Scholar] [CrossRef]
- Smiddy, M.A.; Martin, J.E.; Kelly, A.L.; De Kruif, C.G.; Huppertz, T. Stability of casein micelles cross-linked by transglutaminase. Int. J. Dairy Sci. 2006, 89, 1906–1914. [Google Scholar] [CrossRef] [Green Version]
- Huppertz, T.; de Kruif, C.G. Structure and stability of nanogel particles prepared by internal cross-linking of casein micelles. Int. Dairy J. 2008, 18, 556–565. [Google Scholar] [CrossRef]
- Nogueira, M.H.; Tavares, G.M.; Silva, N.F.N.; Casanova, F.; Stringheta, P.C.; Gaucheron, F.; Teixeira, A.V.N.C.; Perrone, I.T.; Carvalho, A.F. Physico-chemical stability of casein micelles cross-linked by transglutaminase as a function of acidic pH. Food struct. 2019, 19, 100103. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of probiotic cells by means of rennet-gelation of milk proteins. Food Hydrocoll. 2009, 23, 1670–1677. [Google Scholar] [CrossRef]
- Huppertz, T.; Smiddy, M.A.; de Kruif, C.G. Biocompatible micro-gel particles from cross-linked casein micelles. Biomacromolecules 2007, 8, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Amsterdam, The Netherlands, 2015; pp. 1–195. [Google Scholar]
- Fänger, C.; Wack, H.; Ulbricht, M. Macroporous Poly (N-isopropylacrylamide) Hydrogels with Adjustable Size “Cut-off” for the Efficient and Reversible Immobilization of Biomacromolecules. Macromol. Biosci. 2006, 6, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of probiotic cells for food applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R.; Pechkova, E.; Riekel, C.; Nicolini, C. In situ μGISAXS: II. thaumatin crystal growth kinetic. Biophys. J. 2010, 99, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Eedara, B.B.; Tucker, I.G.; Das, S.C. A STELLA simulation model for in vitro dissolution testing of respirable size particles. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Vasbinder, A.J.; Rollema, H.S.; Bot, A.; De Kruif, C.G. Gelation mechanism of milk as influenced by temperature and pH; studied by the use of transglutaminase cross-linked casein micelles. J. Dairy Sci. 2003, 86, 1556–1563. [Google Scholar] [CrossRef] [Green Version]
- Zeeb, B.; Grossmann, L.; Weiss, J. Accessibility of transglutaminase to induce protein crosslinking in gelled food matrices-impact of membrane structure. Food Biophys. 2016, 11, 176–183. [Google Scholar] [CrossRef]
- Grossmann, L.; Wefers, D.; Bunzel, M.; Weiss, J.; Zeeb, B. Accessibility of transglutaminase to induce protein crosslinking in gelled food matrices-Influence of network structure. LWT 2017, 75, 271–278. [Google Scholar] [CrossRef]
- Schott, H. Swelling kinetics of polymers. J. Macromol. Sci. Part B 1992, 31, 1–9. [Google Scholar] [CrossRef]
- Thill, S.; Schmidt, T.; Jana, S.; Wöll, D.; Gebhardt, R. Fine Structure and Swelling Properties of Fibers from Regenerated Rennet-treated Casein Micelles. Macromol. Mater. Eng. 2022, 2200272. [Google Scholar] [CrossRef]
- Vaia, B.; Smiddy, M.A.; Kelly, A.L.; Huppertz, T. Solvent-mediated disruption of bovine casein micelles at alkaline pH. J. Agric. Food Chem. 2006, 54, 8288–8293. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.F.N.; Saint-Jalmes, A.; de Carvalho, A.F.; Gaucheron, F. Development of casein microgels from cross-linking of casein micelles by genipin. Langmuir 2014, 30, 10167–10175. [Google Scholar] [CrossRef]
- Song, F.; Zhang, L.M.; Yang, C.; Yan, L. Genipin-crosslinked casein hydrogels for controlled drug delivery. Int. J. Pharm. 2009, 373, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chesnutt, B.M.; Utturkar, G.; Haggard, W.O.; Yang, Y.; Ong, J.L.; Bumgardner, J.D. The effect of cross-linking of chitosan microspheres with genipin on protein release. Carbohydrate Polymers 2007, 68, 561–567. [Google Scholar] [CrossRef]
- Schulte, J.; Pütz, T.; Gebhardt, R. Statistical analysis of the swelling process of casein microparticles based on single particle measurements. Food Hydrocoll. Health 2021, 1, 100014. [Google Scholar] [CrossRef]
- Dumpler, J.; Kieferle, I.; Wohlschläger, H.; Kulozik, U. Milk ultrafiltrate analysis by ion chromatography and calcium activity for SMUF preparation for different scientific purposes and prediction of its supersaturation. Int. Dairy J. 2017, 68, 60–69. [Google Scholar] [CrossRef]
- Marchin, S.; Putaux, J.L.; Pignon, F.; Léonil, J. Effects of the environmental factors on the casein micelle structure studied by cryo transmission electron microscopy and small-angle x-ray scattering/ultrasmall-angle x-ray scattering. J. Chem. Phys. 2007, 126, 045101. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Tuinier, R. Polysaccharide protein interactions. Food Hydrocoll. 2001, 15, 555–563. [Google Scholar] [CrossRef]
- Hinz, K.; Huppertz, T.; Kelly, A.L. Susceptibility of the individual caseins in reconstituted skim milk to cross-linking by transglutaminase: Influence of temperature, pH and mineral equilibria. J. Dairy Res. 2012, 79, 414–421. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Anzini, P.; Redoglio, D.; Rocco, M.; Masciocchi, N.; Ferri, F. Light Scattering and Turbidimetry Techniques for the Characterization of Nanoparticles and Nanostructured Networks. Nanomaterials 2022, 12, 2214. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Domínguez, S.; Caballero-Mancebo, S.; Marcuello, C.; Martínez-Júlvez, M.; Medina, M.; Lostao, A. Nanomechanical Study of Enzyme: Coenzyme Complexes: Bipartite Sites in Plastidic Ferredoxin-NADP+ Reductase for the Interaction with NADP+. Antioxidants 2022, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Velours, C.; Zhou, J.; Zecchin, P.; He, N.; Salameh, M.; Golinelli-Cohen, M.P.; Golinelli-Pimpaneau, B. Determination of the Absolute Molar Mass of [Fe-S]-Containing Proteins Using Size Exclusion Chromatography-Multi-Angle Light Scattering (SEC-MALS). Biomolecules 2022, 12, 270. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebhardt, R.; Khanna, S.; Schulte, J.; Asaduzzaman, M. Effect of Transglutaminase Post-Treatment on the Stability and Swelling Behavior of Casein Micro-Particles. Int. J. Mol. Sci. 2022, 23, 11837. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231911837
Gebhardt R, Khanna S, Schulte J, Asaduzzaman M. Effect of Transglutaminase Post-Treatment on the Stability and Swelling Behavior of Casein Micro-Particles. International Journal of Molecular Sciences. 2022; 23(19):11837. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231911837
Chicago/Turabian StyleGebhardt, Ronald, Sahel Khanna, Jann Schulte, and Md Asaduzzaman. 2022. "Effect of Transglutaminase Post-Treatment on the Stability and Swelling Behavior of Casein Micro-Particles" International Journal of Molecular Sciences 23, no. 19: 11837. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231911837