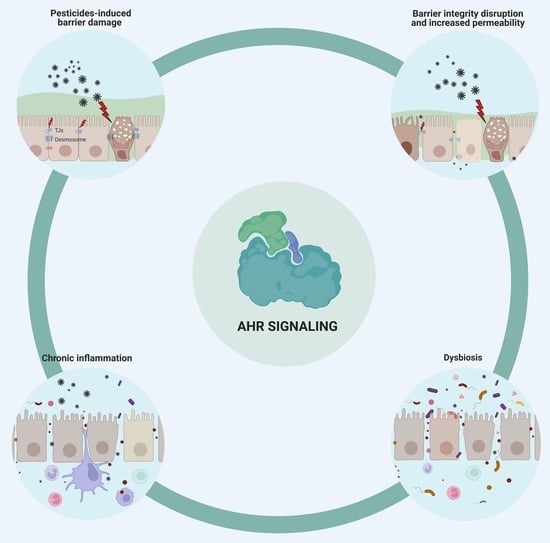
Pesticides and Their Impairing Effects on Epithelial Barrier Integrity, Dysbiosis, Disruption of the AhR Signaling Pathway and Development of Immune-Mediated Inflammatory Diseases
Abstract
:1. Pesticides Disrupt the Integrity of the Epithelial Barriers
2. Pesticide-Induced Dysbiosis May Be Associated with AhR Signaling
3. Immune-Mediated Inflammatory Disease Development Is Enhanced by Pesticides
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| 5-HT | 5-hydroxytryptamine |
| AChE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| AHR | aryl hydrocarbon receptor |
| AJ | adherent junctions |
| ALP | alkaline phosphatase |
| ASD | autism spectrum disorder |
| BBB | blood–brain barrier |
| BEAS | human bronchial epithelial cells |
| BHCH | β-hexachlorocyclohexane |
| BHLH | basic helix-loop-helix |
| CD | Crohn′s disease |
| CHN | clothianidin |
| COX | cyclooxygenase |
| CP | chloropicrin |
| DAMP | damage-associated molecular patterns |
| DDE | dichlorodiphenyldichloroethylene |
| DSB | double strand breaks |
| DSS | dextran sulphate sodium |
| DTM | deltamethrin |
| EU | European Union |
| EWG | Environmental Working Group |
| FFA | free fatty acids |
| FICZ | 6-formylindolo(3,2-b)carbazole |
| FNT | fenitrothion |
| FPN | fipronil |
| GABA | γ-aminobutyric acid |
| GBH | glyphosate-based herbicides |
| GI | gastrointestinal |
| GLY | glyphosate |
| GM | genetically modified |
| GNRH | gonadotropin-releasing hormone |
| HCE | human corneal epithelial |
| HOSE | human ovary surface epithelial cells |
| IARC | International Agency for Research on Cancer |
| IBW | inflammatory bowel disease |
| IEL | intestinal intraepithelial lymphocytes |
| IL | interleukin |
| ILC | innate lymphoid cells |
| IMI | imidacloprid |
| IMID | immune-mediated inflammatory diseases |
| IPAN | intrinsic primary afferent neurons |
| ISC | intestinal stem cells |
| LBP | lipopolysaccharide-binding protein |
| LCT | λ-cyhalothrin |
| LH | luteinizing hormone |
| NFKB | nuclear factor kappa B |
| NLR | NOD-like receptor |
| NMDA | N-methyl-D-aspartate |
| OCP | organochlorine pesticides |
| OP | organophosphorus |
| PAMP | pathogen-associated molecular patterns |
| PAS | PER-ARNT-SIM |
| PD | Parkinson’s disease |
| PRR | Pattern Recognition Receptors |
| RA | Rheumatoid Arthritis |
| RLR | RIG-I-Like Receptor |
| ROS | Reactive Oxygen Species |
| SCFA | Short-Chain Fatty Acids |
| SD | Sprague-Dawley |
| SSB | Single-Strand Breaks |
| T2D | Type 3 Diabetes Mellitus |
| TBZ | Teflubenzuron |
| TGF-b | Transforming Growth Factor Beta |
| TJ | Tight Junctions |
| TLR | Toll-Like Receptor |
| TNF | Tumor Necrosis factor |
| UC | Ulcerative Colitis |
| US | United States |
| VIP | Vasoactive Intestinal Peptide |
| ZO | Zonula Occludens |
References
- Agache, I.; Miller, R.; Gern, J.E.; Hellings, P.W.; Jutel, M.; Muraro, A.; Phipatanakul, W.; Quirce, S.; Peden, D. Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: A Practall document. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 449–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.-L.; Miller, G.W. The exposome and health: Where chemistry meets biology. Science 2020, 367, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Nawrot, T.S.; Baccarelli, A.A. Hallmarks of Environmental Insults. Cell 2021, 184, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Celebi Sözener, Z.; Cevhertas, L.; Nadeau, K.; Akdis, M.; Akdis, C.A. Environmental factors in epithelial barrier dysfunction. J. Allergy Clin. Immunol. 2020, 145, 1517–1528. [Google Scholar] [CrossRef]
- Mitamura, Y.; Ogulur, I.; Pat, Y.; Rinaldi, A.O.; Ardicli, O.; Cevhertas, L.; Brüggen, M.C.; Traidl-Hoffmann, C.; Akdis, M.; Akdis, C.A. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermat. 2021, 85, 615–626. [Google Scholar] [CrossRef]
- FAO Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/RP/visualize (accessed on 1 June 2022).
- FAOSTATa Definitions and Standards Used in FAOSTAT. Available online: http://www.fao.org/faostat/en/#definitions (accessed on 1 June 2022).
- Brasil Ato, No. 91. Available online: http://www.in.gov.br/web/dou/-/ato-n-91-de-26-de-dezembro-de-2019-235559622 (accessed on 22 February 2022).
- Brasil Portaria, No. 43, de 21 de Fevereiro de 2020. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/agrotoxicos/informacoes-tecnicas (accessed on 14 June 2022).
- IBAMA Instituto Brasileiro Do Meio Ambiente e Dos Recursos Naturais (IBRN). Relatórios de Comercialização de Agrotóxicos. Available online: http://ibama.gov.br/agrotoxicos/relatorios-de-comercializacao-de-agrotoxicos (accessed on 28 May 2022).
- Sergievich, A.A.; Khoroshikh, P.P.; Artemenko, A.F.; Zakharenko, A.M.; Chaika, V.V.; Kodintsev, V.V.; Stroeva, O.A.; Lenda, E.G.; Tsatsakis, A.; Burykina, T.I.; et al. Behavioral impacts of a mixture of six pesticides on rats. Sci. Total Environ. 2020, 727, 138491. [Google Scholar] [CrossRef]
- Islam, F.; Wang, J.; Farooq, M.A.; Khan, M.S.S.; Xu, L.; Zhu, J.; Zhao, M.; Muños, S.; Li, Q.X.; Zhou, W. Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ. Int. 2018, 111, 332–351. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Akash, S.; Sivaprakash, B.; Rajamohan, N.; Pandiyan, C.M.; Vo, D.-V.N. Pesticide pollutants in the environment—A critical review on remediation techniques, mechanism and toxicological impact. Chemosphere 2022, 301, 134754. [Google Scholar] [CrossRef]
- Perry, E.D.; Ciliberto, F.; Hennessy, D.A.; Moschini, G.C. Genetically engineered crops and pesticide use in U.S. maize and soybeans. Sci. Adv. 2016, 2, e1600850. [Google Scholar] [CrossRef] [Green Version]
- FAOSTATb International Code of Conduct on the Distribution and Use of Pesticides. Available online: https://apps.who.int/iris/bitstream/handle/10665/70293/WHO_HTM_NTD_WHOPES_2010.7_eng.pdf (accessed on 1 June 2022).
- Samarghandi, M.R.; Mohammadi, M.; Karami, A.; Tabandeh, L.; Dargahi, A.; Amirian, F. Residue Analysis of Pesticides, Herbicides, and Fungicides in Various Water Sources Using Gas Chromatography-Mass Detection. Pol. J. Environ. Stud. 2017, 26, 2189–2195. [Google Scholar] [CrossRef]
- Sjerps, R.M.A.; Kooij, P.J.F.; van Loon, A.; van Wezel, A.P. Occurrence of pesticides in Dutch drinking water sources. Chemosphere 2019, 235, 510–518. [Google Scholar] [CrossRef]
- El-Nahhal, I.; El-Nahhal, Y. Pesticide residues in drinking water, their potential risk to human health and removal options. J. Environ. Manag. 2021, 299, 113611. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Cao, F.; Souders, C.L.; Li, P.; Pang, S.; Qiu, L.; Martyniuk, C.J. Developmental toxicity of the triazole fungicide cyproconazole in embryo-larval stages of zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2019, 26, 4913–4923. [Google Scholar] [CrossRef]
- Es Ruiz de Arcaute, C.; Ossana, N.A.; Pérez-Iglesias, J.M.; Soloneski, S.; Larramendy, M.L. Auxinic herbicides induce oxidative stress on Cnesterodon decemmaculatus (Pisces: Poeciliidae). Environ. Sci. Pollut. Res. 2019, 26, 20485–20498. [Google Scholar] [CrossRef]
- De Brito Rodrigues, L.; Gonçalves Costa, G.; Lundgren Thá, E.; da Silva, L.R.; de Oliveira, R.; Morais Leme, D.; Cestari, M.M.; Koppe Grisolia, C.; Campos Valadares, M.; de Oliveira, G.A.R. Impact of the glyphosate-based commercial herbicide, its components and its metabolite AMPA on non-target aquatic organisms. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2019, 842, 94–101. [Google Scholar] [CrossRef]
- Valadas, J.; Mocelin, R.; Sachett, A.; Marcon, M.; Zanette, R.A.; Dallegrave, E.; Herrmann, A.P.; Piato, A. Propiconazole induces abnormal behavior and oxidative stress in zebrafish. Environ. Sci. Pollut. Res. 2019, 26, 27808–27815. [Google Scholar] [CrossRef]
- Moutinho, M.F.; De Almeida, E.A.; Espíndola, E.L.G.; Daam, M.A.; Schiesari, L. Herbicides employed in sugarcane plantations have lethal and sublethal effects to larval Boana pardalis (Amphibia, Hylidae). Ecotoxicology 2020, 29, 1043–1051. [Google Scholar] [CrossRef]
- Bhagat, J.; Singh, N.; Nishimura, N.; Shimada, Y. A comprehensive review on environmental toxicity of azole compounds to fish. Chemosphere 2021, 262, 128335. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chen, L.; Liu, X.; Wang, L.; Wu, S.; Zhao, X. Histology and multi-omic profiling reveal the mixture toxicity of tebuconazole and difenoconazole in adult zebrafish. Sci. Total Environ. 2021, 795, 148777. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.S.F.; Venâncio, C.A.S.; Félix, L.M. Behavioural impairment and oxidative stress by acute exposure of zebrafish to a commercial formulation of tebuconazole. Environ. Toxicol. Pharmacol. 2022, 91, 103823. [Google Scholar] [CrossRef] [PubMed]
- Disner, G.R.; Falcão, M.A.P.; Andrade-Barros, A.I.; Leite dos Santos, N.V.; Soares, A.B.S.; Marcolino-Souza, M.; Gomes, K.S.; Lima, C.; Lopes-Ferreira, M. The Toxic Effects of Glyphosate, Chlorpyrifos, Abamectin, and 2,4-D on Animal Models: A Systematic Review of Brazilian Studies. Integr. Environ. Assess. Manag. 2021, 17, 507–520. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Maleski, A.L.A.; Balan-Lima, L.; Bernardo, J.T.G.; Hipolito, L.M.; Seni-Silva, A.C.; Batista-Filho, J.; Falcao, M.A.P.; Lima, C. Impact of Pesticides on Human Health in the Last Six Years in Brazil. Int. J. Environ. Res. Public Health 2022, 19, 3198. [Google Scholar] [CrossRef]
- Calaf, G.M.; Ponce-Cusi, R.; Aguayo, F.; Muñoz, J.P.; Bleak, T.C. Endocrine disruptors from the environment affecting breast cancer (Review). Oncol. Lett. 2020, 20, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Guida, Y.; de Carvalho, G.O.; Capella, R.; Pozo, K.; Lino, A.S.; Azeredo, A.; Carvalho, D.F.P.; Braga, A.L.F.; Torres, J.P.M.; Meire, R.O. Atmospheric Occurrence of Organochlorine Pesticides and Inhalation Cancer Risk in Urban Areas at Southeast Brazil. Environ. Pollut. 2021, 271, 116359. [Google Scholar] [CrossRef]
- Costa, M.B.; Farias, I.R.; da Silva Monte, C.; Filho, L.I.P.F.; de Paula Borges, D.; de Oliveira, R.T.G.; Ribeiro-Junior, H.L.; Magalhães, S.M.M.; Pinheiro, R.F. Chromosomal abnormalities and dysregulated DNA repair gene expression in farmers exposed to pesticides. Environ. Toxicol. Pharmacol. 2021, 82, 103564. [Google Scholar] [CrossRef]
- Dos Santos, N.F.; Contrera, L.; Teston, E.F.; Kawakame, P.M.G.; Reis, L.E.A.; Amarilha, K.J.d.O. Evidências Dos Efeitos Neurotóxicos Por Exposição Ao Agrotóxico: Uma Revisão Integrativa / Evidence of Neurotoxic Effects by Exposure to Pesticide. Braz. J. Dev. 2020, 6, 102160–102170. [Google Scholar] [CrossRef]
- Bonvoisin, T.; Utyasheva, L.; Knipe, D.; Gunnell, D.; Eddleston, M. Suicide by pesticide poisoning in India: A review of pesticide regulations and their impact on suicide trends. BMC Public Health 2020, 20, 251. [Google Scholar] [CrossRef]
- Dardiotis, E.; Aloizou, A.-M.; Sakalakis, E.; Siokas, V.; Koureas, M.; Xiromerisiou, G.; Petinaki, E.; Wilks, M.; Tsatsakis, A.; Hadjichristodoulou, C.; et al. Organochlorine pesticide levels in Greek patients with Parkinson’s disease. Toxicol. Rep. 2020, 7, 596–601. [Google Scholar] [CrossRef]
- Schneider Medeiros, M.; Reddy, S.P.; Socal, M.P.; Schumacher-Schuh, A.F.; Mello Rieder, C.R. Occupational pesticide exposure and the risk of death in patients with Parkinson’s disease: An observational study in southern Brazil. Environ. Health A Glob. Access Sci. Source 2020, 19, 68. [Google Scholar] [CrossRef]
- Daniali, M.; Baeeri, M.; Farhadi, R.; Gholami, M.; Hassani, S.; Navaei-Nigjeh, M.; Rahimifard, M.; Abdollahi, M. Molecular Evidence on the Inhibitory Potential of Metformin against Chlorpyrifos-Induced Neurotoxicity. Toxics 2022, 10, 197. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Stapleton, H.M. Endocrine-Mediated Mechanisms of Metabolic Disruption and New Approaches to Examine the Public Health Threat. Front. Endocrinol. 2019, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environ. Res. 2021, 194, 110694. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Vandenberg, L.N.; Demeneix, B.A.; Porta, M.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020, 8, 719–730. [Google Scholar] [CrossRef]
- Zhang, C.; Schilirò, T.; Gea, M.; Bianchi, S.; Spinello, A.; Magistrato, A.; Gilardi, G.; Di Nardo, G. Molecular Basis for Endocrine Disruption by Pesticides Targeting Aromatase and Estrogen Receptor. Int. J. Environ. Res. Public Health 2020, 17, 5664. [Google Scholar] [CrossRef]
- Draskau, M.K.; Svingen, T. Azole Fungicides and Their Endocrine Disrupting Properties: Perspectives on Sex Hormone-Dependent Reproductive Development. Front. Toxicol. 2022, 4, 883254. [Google Scholar] [CrossRef]
- He, B.; Ni, Y.; Jin, Y.; Fu, Z. Pesticides-induced energy metabolic disorders. Sci. Total Environ. 2020, 729, 139033. [Google Scholar] [CrossRef]
- Javeres, M.N.L.; Habib, R.; Laure, N.J.; Shah, S.T.A.; Valis, M.; Kuca, K.; Nurulain, S.M. Chronic Exposure to Organophosphates Pesticides and Risk of Metabolic Disorder in Cohort from Pakistan and Cameroon. Int. J. Environ. Res. Public Health 2021, 18, 2310. [Google Scholar] [CrossRef]
- Ku, T.; Zhou, M.; Hou, Y.; Xie, Y.; Li, G.; Sang, N. Tebuconazole induces liver injury coupled with ROS-mediated hepatic metabolism disorder. Ecotoxicol. Environ. Saf. 2021, 220, 112309. [Google Scholar] [CrossRef]
- Yamamoto, I.; Casida, J.E. Syntheses of 14C-labeled pyrethrin I, allethrin, phthalthrin, and dimethrin on a submillimole scale. Agric. Biol. Chem. 1968, 32, 1382–1391. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef]
- Stillerman, K.P.; Mattison, D.R.; Giudice, L.C.; Woodruff, T.J. Environmental Exposures and Adverse Pregnancy Outcomes: A Review of the Science. Reprod. Sci. 2008, 15, 631–650. [Google Scholar] [CrossRef]
- Raanan, R.; Harley, K.G.; Balmes, J.R.; Bradman, A.; Lipsett, M.; Eskenazi, B. Early-life Exposure to Organophosphate Pesticides and Pediatric Respiratory Symptoms in the CHAMACOS Cohort. Environ. Health Perspect. 2015, 123, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Helou, K.; Matta, J.; Harmouche-Karaki, M.; Sayegh, N.; Younes, H.; Mahfouz, Y.; Mahfouz, M.; Karake, S.; Finan, R.; Abi-Tayeh, G.; et al. Maternal and cord serum levels of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) among Lebanese pregnant women and predictors of exposure. Chemosphere 2021, 266, 129211. [Google Scholar] [CrossRef]
- Criswell, R.; Crawford, K.A.; Bucinca, H.; Romano, M.E. Endocrine-disrupting chemicals and breastfeeding duration: A review. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 388–395. [Google Scholar] [CrossRef]
- Roberts, J.R.; Karr, C.J. Pesticide Exposure in Children. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef] [Green Version]
- EWG—Environmental Working Group Report. Available online: https://static.ewg.org/reports/2020/EWG_AnnualReport-2020.pdf?_ga=2.250700486.1567527694.1658418128-903861400.1658418128 (accessed on 1 June 2022).
- Widdicombe, J.H. Early studies on the surface epithelium of mammalian airways. Am. J. Physiol.-Lung Cell Mol. Physiol. 2019, 317, L486–L495. [Google Scholar] [CrossRef]
- Yoshida, T.; Beck, L.A.; De Benedetto, A. Skin barrier defects in atopic dermatitis: From old idea to new opportunity. Allergol. Int. 2022, 71, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Jiang, X.; Li, G.; Zhu, Y.; Zhang, H. Junctional complexes in epithelial cells: Sentinels for extracellular insults and intracellular homeostasis. FEBS J. 2022, 1–20, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, V.; Catone, T.; Turco, L.; Di Consiglio, E.; Testai, E.; De Angelis, I. Effects of the pesticide chlorpyrifos on an in vitro model of intestinal barrier. Toxicol. Vitr. 2007, 21, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Ilboudo, S.; Fouche, E.; Rizzati, V.; Toé, A.M.; Gamet-Payrastre, L.; Guissou, P.I. In vitro impact of five pesticides alone or in combination on human intestinal cell line Caco-2. Toxicol. Rep. 2014, 1, 474–489. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Song, W.; Shan, A. Vitamin E alleviates phoxim-induced toxic effects on intestinal oxidative stress, barrier function, and morphological changes in rats. Environ. Sci. Pollut. Res. 2018, 25, 26682–26692. [Google Scholar] [CrossRef]
- Shah, H.K.; Sharma, T.; Banerjee, B.D. Organochlorine pesticides induce inflammation, ROS production, and DNA damage in human epithelial ovary cells: An in vitro study. Chemosphere 2020, 246, 125691. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Wu, G.; Yang, J.; Wen, Y.; Meng, S.; Lin, X.; Pang, X.; An, L. Toxic effects of the Emamectin Benzoate exposure on cultured human bronchial epithelial (16HBE) cells. Environ. Pollut. 2020, 257, 113618. [Google Scholar] [CrossRef]
- Goswami, D.G.; Kant, R.; Ammar, D.A.; Agarwal, C.; Gomez, J.; Agarwal, R.; Saba, L.M.; Fritz, K.S.; Tewari-Singh, N. Toxic consequences and oxidative protein carbonylation from chloropicrin exposure in human corneal epithelial cells. Toxicol. Lett. 2020, 322, 1–11. [Google Scholar] [CrossRef]
- Atlı Şekeroğlu, Z.A.; Şekeroğlu, V.; Aydın, B.; Kontaş Yedier, S.; İlkun, E. Clothianidin induces DNA damage and oxidative stress in bronchial epithelial cells. Environ. Mol. Mutagen. 2020, 61, 647–655. [Google Scholar] [CrossRef]
- Zhao, G.-P.; Wang, X.-Y.; Li, J.-W.; Wang, R.; Ren, F.-Z.; Pang, G.-F.; Li, Y.-X. Imidacloprid increases intestinal permeability by disrupting tight junctions. Ecotoxicol. Environ. Saf. 2021, 222, 112476. [Google Scholar] [CrossRef]
- Del Castilo, I.; Neumann, A.S.; Lemos, F.S.; De Bastiani, M.A.; Oliveira, F.L.; Zimmer, E.R.; Rêgo, A.M.; Hardoim, C.C.P.; Antunes, L.C.M.; Lara, F.A.; et al. Lifelong Exposure to a Low-Dose of the Glyphosate-Based Herbicide RoundUp® Causes Intestinal Damage, Gut Dysbiosis, and Behavioral Changes in Mice. Int. J. Mol. Sci. 2022, 23, 5583. [Google Scholar] [CrossRef]
- Brewster, D.W.; Warren, J.A.; Hopkins, W.E. Metabolism of glyphosate in Sprague-Dawley rats: Tissue distribution, identification, and quantitation of glyphosate-derived materials following a single oral dose. Fundam. Appl. Toxicol. 1991, 17, 43–51. [Google Scholar] [CrossRef]
- Chiu, L.; Bazin, T.; Truchetet, M.-E.; Schaeverbeke, T.; Delhaes, L.; Pradeu, T. Protective Microbiota: From Localized to Long-Reaching Co-Immunity. Front. Immunol. 2017, 8, 1678. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [Green Version]
- Linares, R.; Francés, R.; Gutiérrez, A.; Juanola, O. Bacterial Translocation as Inflammatory Driver in Crohn’s Disease. Front. Cell Dev. Biol. 2021, 9, 703310. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Moradkhani, F.; Asghari, N.; Fallah, M.; Asghari, M.H.; Moghadamnia, A.A.; Abdollahi, M. NLRP inflammasome as a key role player in the pathogenesis of environmental toxicants. Life Sci. 2019, 231, 116585. [Google Scholar] [CrossRef]
- Zhu, S.-Y.; Guo, J.-Y.; Li, J.-Y.; Dai, X.-Y.; Li, X.-N. Lycopene ameliorates atrazine-induced pyroptosis in spleen by suppressing the Ox-mtDNA/Nlrp3 inflammasome pathway. Food Funct. 2022, 13, 3551–3560. [Google Scholar] [CrossRef]
- Rosenblum, D.; Naik, S. Epithelial–immune crosstalk in health and disease. Curr. Opin. Genet. Dev. 2022, 74, 101910. [Google Scholar] [CrossRef]
- Naik, S.; Larsen, S.B.; Gomez, N.C.; Alaverdyan, K.; Sendoel, A.; Yuan, S.; Polak, L.; Kulukian, A.; Chai, S.; Fuchs, E. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 2017, 550, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.; Fuchs, E. Inflammatory memory and tissue adaptation in sickness and in health. Nature 2022, 607, 249–255. [Google Scholar] [CrossRef]
- Del Poggetto, E.; Ho, I.-L.; Balestrieri, C.; Yen, E.-Y.; Zhang, S.; Citron, F.; Shah, R.; Corti, D.; Diaferia, G.R.; Li, C.-Y.; et al. Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis. Science 2021, 373, eabj0486. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, M.-C.; Lin, L.-L.; Christian, D.A.; Min, B.; Hunter, C.A.; Lu, L.-F. Gut epithelial IL-27 confers intestinal immunity through the induction of intraepithelial lymphocytes. J. Exp. Med. 2021, 218, e20210021. [Google Scholar] [CrossRef]
- Duan, Z.; Liu, M.; Yuan, L.; Du, X.; Wu, M.; Yang, Y.; Wang, L.; Zhou, K.; Yang, M.; Zou, Y.; et al. Innate lymphoid cells are double-edged swords under the mucosal barrier. J. Cell Mol. Med. 2021, 25, 8579–8587. [Google Scholar] [CrossRef]
- Moreau, J.M.; Dhariwala, M.O.; Gouirand, V.; Boda, D.P.; Boothby, I.C.; Lowe, M.M.; Cohen, J.N.; Macon, C.E.; Leech, J.M.; Kalekar, L.A.; et al. Regulatory T cells promote innate inflammation after skin barrier breach via TGF-β activation. Sci. Immunol. 2021, 6, eabg2329. [Google Scholar] [CrossRef]
- Trier, A.M.; Mack, M.R.; Kim, B.S. The Neuro-Immune Axis in Skin Sensation, Inflammation, and Immunity. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Heiman, M.G. When is a neuron like an epithelial cell. Dev. Biol. 2022, 489, 161–164. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Zhu, H.; Zhang, J.; Wan, D. The Pivotal Role of Microbiota in Modulating the Neuronal–Glial–Epithelial Unit. Infect. Drug Resist. 2021, 14, 5613–5628. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Blicharz, L.; Rudnicka, L.; Czuwara, J.; Waśkiel-Burnat, A.; Goldust, M.; Olszewska, M.; Samochocki, Z. The Influence of Microbiome Dysbiosis and Bacterial Biofilms on Epidermal Barrier Function in Atopic Dermatitis—An Update. Int. J. Mol. Sci. 2021, 22, 8403. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Li, J.; Phadnis-Moghe, A.S.; Crawford, R.B.; Kaminski, N.E. Aryl Hydrocarbon Receptor Activation by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Impairs Human B Lymphopoiesis. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhang, Q.; Henriquez, J.E.; Crawford, R.B.; Kaminski, N.E. Lymphocyte-Specific Protein Tyrosine Kinase (LCK) is Involved in the Aryl Hydrocarbon Receptor-Mediated Impairment of Immunoglobulin Secretion in Human Primary B Cells. Toxicol. Sci. 2018, 165, 322–334. [Google Scholar] [CrossRef] [Green Version]
- Diny, N.L.; Schonfeldova, B.; Shapiro, M.; Winder, M.L.; Varsani-Brown, S.; Stockinger, B. The aryl hydrocarbon receptor contributes to tissue adaptation of intestinal eosinophils in mice. J. Exp. Med. 2022, 219, e20210970. [Google Scholar] [CrossRef]
- Blevins, L.K.; Zhou, J.; Crawford, R.B.; Kaminski, N.E. Identification of a Sensitive Human Immunological Target of Aryl Hydrocarbon Receptor Activation: CD5+ Innate-Like B Cells. Front. Immunol. 2021, 12, 635748. [Google Scholar] [CrossRef]
- Stockinger, B.; Shah, K.; Wincent, E. AHR in the intestinal microenvironment: Safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 559–570. [Google Scholar] [CrossRef]
- Barroso, A.; Mahler, J.V.; Fonseca-Castro, P.H.; Quintana, F.J. The aryl hydrocarbon receptor and the gut–brain axis. Cell Mol. Immunol. 2021, 18, 259–268. [Google Scholar] [CrossRef]
- Gupta, A.; Sasse, S.K.; Gruca, M.A.; Sanford, L.; Dowell, R.D.; Gerber, A.N. Deconvolution of multiplexed transcriptional responses to wood smoke particles defines rapid aryl hydrocarbon receptor signaling dynamics. J. Biol. Chem. 2021, 279, 101147. [Google Scholar] [CrossRef]
- Metidji, A.; Omenetti, S.; Crotta, S.; Li, Y.; Nye, E.; Ross, E.; Li, V.; Maradana, M.R.; Schiering, C.; Stockinger, B. The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity 2018, 49, 353–362.e5. [Google Scholar] [CrossRef] [Green Version]
- Postal, B.G.; Ghezzal, S.; Aguanno, D.; André, S.; Garbin, K.; Genser, L.; Brot-Laroche, E.; Poitou, C.; Soula, H.; Leturque, A.; et al. AhR activation defends gut barrier integrity against damage occurring in obesity. Mol. Metab. 2020, 39, 101007. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Q.; Ma, Y.; Li, L.; Yu, K.; Zhang, Z.; Chen, G.; Li, X.; Xiao, W.; Xu, P.; et al. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int. J. Biol. Sci. 2018, 14, 69–77. [Google Scholar] [CrossRef]
- Kyoreva, M.; Li, Y.; Hoosenally, M.; Hardman-Smart, J.; Morrison, K.; Tosi, I.; Tolaini, M.; Barinaga, G.; Stockinger, B.; Mrowietz, U.; et al. CYP1A1 Enzymatic Activity Influences Skin Inflammation Via Regulation of the AHR Pathway. J. Investig. Dermatol. 2020, 141, 1553–1563.e3. [Google Scholar] [CrossRef]
- Fernández-Gallego, N.; Sánchez-Madrid, F.; Cibrian, D. Role of AHR Ligands in Skin Homeostasis and Cutaneous Inflammation. Cells 2021, 10, 3176. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, L.; Qi, Y.; Zhang, J.; Zhu, H.; Tang, J. Intestinal Flora-Derived Kynurenic Acid Protects Against Intestinal Damage Caused by Candida albicans Infection via Activation of Aryl Hydrocarbon Receptor. Front. Microbiol. 2022, 13, 934786. [Google Scholar] [CrossRef]
- Taddese, R.; Roelofs, R.; Draper, D.; Wu, X.; Wu, S.; Swinkels, D.W.; Tjalsma, H.; Boleij, A. Streptococcus gallolyticus Increases Expression and Activity of Aryl Hydrocarbon Receptor-Dependent CYP1 Biotransformation Capacity in Colorectal Epithelial Cells. Front. Cell Infect. Microbiol. 2021, 11, 740704. [Google Scholar] [CrossRef]
- Mendler, A.; Geier, F.; Haange, S.-B.; Pierzchalski, A.; Krause, J.L.; Nijenhuis, I.; Froment, J.; Jehmlich, N.; Berger, U.; Ackermann, G.; et al. Mucosal-associated invariant T-Cell (MAIT) activation is altered by chlorpyrifos- and glyphosate-treated commensal gut bacteria. J. Immunotoxicol. 2020, 17, 10–20. [Google Scholar] [CrossRef]
- Giambò, F.; Teodoro, M.; Costa, C.; Fenga, C. Toxicology and Microbiota: How Do Pesticides Influence Gut Microbiota? A Review. Int. J. Environ. Res. Public Health 2021, 18, 5510. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef]
- Yip, W.; Hughes, M.R.; Li, Y.; Cait, A.; Hirst, M.; Mohn, W.W.; McNagny, K.M. Butyrate Shapes Immune Cell Fate and Function in Allergic Asthma. Front. Immunol. 2021, 12, 628453. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Baldi, I.; Robert, C.; Piantoni, F.; Tual, S.; Bouvier, G.; Lebailly, P.; Raherison, C. Agricultural exposure and asthma risk in the AGRICAN French cohort. Int. J. Hyg. Environ. Health 2014, 217, 435–442. [Google Scholar] [CrossRef]
- Henneberger, P.K.; Liang, X.; London, S.J.; Umbach, D.M.; Sandler, D.P.; Hoppin, J.A. Exacerbation of symptoms in agricultural pesticide applicators with asthma. 2014, 87, 423–432. Int. Arch. Occup. Environ. Health 2014, 87, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Ndlovu, V.; Dalvie, M.A.; Jeebhay, M.F. Asthma associated with pesticide exposure among women in rural Western Cape of South Africa. Am. J. Ind. Med. 2014, 57, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.; Syamlal, G.; Henneberger, P.K.; Alarcon, W.A.; Mazurek, J.M. Pesticide use, allergic rhinitis, and asthma among US farm operators. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Chittrakul, J.; Sapbamrer, R.; Sirikul, W. Pesticide Exposure and Risk of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Toxics 2022, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhan, J.; Liu, D.; Luo, M.; Han, J.; Liu, X.; Liu, C.; Cheng, Z.; Zhou, Z.; Wang, P. Organophosphorus Pesticide Chlorpyrifos Intake Promotes Obesity and Insulin Resistance through Impacting Gut and Gut Microbiota. Microbiome 2019, 3, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 2019, 123, 325–336. [Google Scholar] [CrossRef]
- Wang, B.; Tsakiridis, E.E.; Zhang, S.; Llanos, A.; Desjardins, E.M.; Yabut, J.M.; Green, A.E.; Day, E.A.; Smith, B.K.; Lally, J.S.V.; et al. The pesticide chlorpyrifos promotes obesity by inhibiting diet-induced thermogenesis in brown adipose tissue. Nat. Commun. 2021, 12, 5163. [Google Scholar] [CrossRef]
- Djekkoun, N.; Lalau, J.-D.; Bach, V.; Depeint, F.; Khorsi-Cauet, H. Chronic oral exposure to pesticides and their consequences on metabolic regulation: Role of the microbiota. Eur. J. Nutr. 2021, 60, 4131–4149. [Google Scholar] [CrossRef]
- Gutgesell, R.M.; Tsakiridis, E.E.; Jamshed, S.; Steinberg, G.R.; Holloway, A.C. Impact of pesticide exposure on adipose tissue development and function. Biochem. J. 2020, 477, 2639–2653. [Google Scholar] [CrossRef]
- Di Gregorio, I.; Busiello, R.A.; Aceves, M.A.B.; Lepretti, M.; Paolella, G.; Lionetti, L. Environmental Pollutants Effect on Brown Adipose Tissue. Front. Physiol. 2019, 10, 1891. [Google Scholar] [CrossRef]
- Jellali, R.; Jacques, S.; Essaouiba, A.; Gilard, F.; Letourneur, F.; Gakière, B.; Legallais, C.; Leclerc, E. Investigation of steatosis profiles induced by pesticides using liver organ-on-chip model and omics analysis. Food Chem. Toxicol. 2021, 152, 112155. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Mansouri, E.H.; Reggabi, M. Association between type 2 diabetes and exposure to chlorinated persistent organic pollutants in Algeria: A case-control study. Chemosphere 2021, 264, 128596. [Google Scholar] [CrossRef]
- Tyagi, S.; Siddarth, M.; Mishra, B.K.; Banerjee, B.D.; Urfi, A.J.; Madhu, S.V. High levels of organochlorine pesticides in drinking water as a risk factor for type 2 diabetes: A study in north India. Environ. Pollut. 2021, 271, 116287. [Google Scholar] [CrossRef]
- Han, X.; Zhang, F.; Meng, L.; Xu, Y.; Li, Y.; Li, A.; Turyk, M.E.; Yang, R.; Wang, P.; Zhang, J.; et al. Exposure to organochlorine pesticides and the risk of type 2 diabetes in the population of East China. Ecotoxicol. Environ. Saf. 2020, 190, 110125. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.-K.; Kim, J.-Y.; Lee, K.; Choi, J.R.; Chang, S.-J.; Chung, C.H.; Park, K.-S.; Oh, S.-S.; Koh, S.-B. Exposure to pesticides and the prevalence of diabetes in a rural population in Korea. NeuroToxicology 2019, 70, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Chan, H.M. Persistent organic pollutants and diabetes among Inuit in the Canadian Arctic. Environ. Int. 2017, 101, 183–189. [Google Scholar] [CrossRef]
- Guo, X.; Wang, H.; Song, Q.; Li, N.; Liang, Q.; Su, W.; Liang, M.; Ding, X.; Sun, C.; Lowe, S.; et al. Association between exposure to organophosphorus pesticides and the risk of diabetes among US Adults: Cross-sectional findings from the National Health and Nutrition Examination Survey. Chemosphere 2022, 301, 134471. [Google Scholar] [CrossRef]
- Park, J.; Park, S.K.; Choi, Y.-H. Environmental pyrethroid exposure and diabetes in U.S. adults. Environ. Res. 2019, 172, 399–407. [Google Scholar] [CrossRef]
- Ho, S.-M.; Lewis, J.D.; Mayer, E.A.; Plevy, S.E.; Chuang, E.; Rappaport, S.M.; Croitoru, K.; Korzenik, J.R.; Krischer, J.; Hyams, J.S.; et al. Challenges in IBD Research: Environmental Triggers. Inflamm. Bowel Dis. 2019, 25, S13–S23. [Google Scholar] [CrossRef] [Green Version]
- Vuyyuru, S.K.; Kedia, S.; Sahu, P.; Ahuja, V. Immune-mediated inflammatory diseases of the gastrointestinal tract: Beyond Crohn’s disease and ulcerative colitis. JGH Open 2022, 6, 100–111. [Google Scholar] [CrossRef]
- Hu, Z.; Brooks, S.A.; Dormoy, V.; Hsu, C.-W.; Hsu, H.-Y.; Lin, L.-T.; Massfelder, T.; Kimryn Rathmell, W.; Xia, M.; Al-Mulla, F.; et al. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: Focus on the cancer hallmark of tumor angiogenesis. Carcinogenesis 2015, 36, S184–S202. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, C.; Falcão, M.A.P.; Rosa, J.G.S.; Disner, G.R.; Lopes-Ferreira, M. Pesticides and Their Impairing Effects on Epithelial Barrier Integrity, Dysbiosis, Disruption of the AhR Signaling Pathway and Development of Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 12402. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232012402
Lima C, Falcão MAP, Rosa JGS, Disner GR, Lopes-Ferreira M. Pesticides and Their Impairing Effects on Epithelial Barrier Integrity, Dysbiosis, Disruption of the AhR Signaling Pathway and Development of Immune-Mediated Inflammatory Diseases. International Journal of Molecular Sciences. 2022; 23(20):12402. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232012402
Chicago/Turabian StyleLima, Carla, Maria Alice Pimentel Falcão, João Gabriel Santos Rosa, Geonildo Rodrigo Disner, and Monica Lopes-Ferreira. 2022. "Pesticides and Their Impairing Effects on Epithelial Barrier Integrity, Dysbiosis, Disruption of the AhR Signaling Pathway and Development of Immune-Mediated Inflammatory Diseases" International Journal of Molecular Sciences 23, no. 20: 12402. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232012402