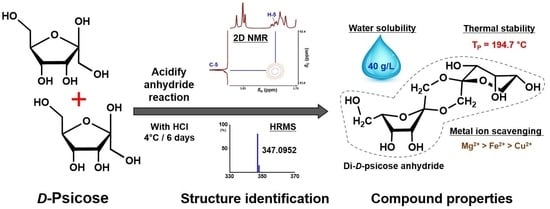
Synthesis of a New Glycoconjugate with Di-ᴅ-Psicose Anhydride Structure
Abstract
:1. Introduction
2. Results
2.1. Anhydrous Acidification
2.2. Structure Identification
2.3. Water Solubility and Endothermic Heat Flow
2.4. Metal Ion Reduction
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Anhydrous Acidification
4.3. HPLC Analysis
4.4. Preparation of Single Compound
4.5. Structure Identification
4.6. Compound Properties
4.7. Metal Ion Reduction
4.8. Data Expression and Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behrens, M.; Meyerhof, W.; Hellfritsch, C.; Hofmann, T. Sweet and umami taste: Natural products, their chemosensory targets, and beyond. Angew. Chem. Int. Edit. 2011, 50, 2220–2242. [Google Scholar] [CrossRef] [PubMed]
- McCain, H.R.; Kaliappan, S.; Drake, M.A. Invited review: Sugar reduction in dairy products. J. Dairy Sci. 2018, 101, 8619–8640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Muñoz, R.; Correa-Delgado, M.; Córdova-Almeida, R.; Lara-Nava, D.; Chávez-Muñoz, M.; Velásquez-Chávez, V.F.; Hernández-Torres, C.E.; Gontarek-Castro, E.; Ahmad, M.Z. Natural sweeteners: Sources, extraction and current uses in foods and food industries. Food Chem. 2022, 370, 130991. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, M.; Jiang, B.; Zhang, T. Bioproduction of ᴅ-allulose: Properties, applications, purification, and future perspectives. Compr. Rev. Food. Sci. Food Saf. 2021, 20, 6012–6026. [Google Scholar] [CrossRef] [PubMed]
- Eble, T.E.; Hoeksema, H.; Boyack, G.A.; Savage, G.M. Psicofuranine. I. Discovery, isolation, and properties. Antibiot. Chemother. 1959, 9, 419–420. [Google Scholar] [PubMed]
- Grembecka, M. Sugar alcohols—Their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef] [Green Version]
- García-Moreno, M.I.; Benito, J.M.; Mellet, C.O.; Fernández, J.M.G. Chemical and enzymatic approaches to carbohydrate-derived spiroketals: Di-ᴅ-fructose dianhydrides. Molecules 2008, 13, 1640–1670. [Google Scholar] [CrossRef]
- Cheng, M.; Wu, H.; Zhang, W.; Mu, W. Difructose anhydride III: A 50-year perspective on its production and physiological functions. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–12. [Google Scholar] [CrossRef]
- McDonald, E.J.; Jackson, R.F. Structure of difructose anhydride III (difructofuranose 1,2′, 2,3′-anhydride). J. Res. Natl. Inst. Stand. 1940, 24, 181–204. [Google Scholar] [CrossRef]
- Chan, T.-H.; Chen, P.-T.; Chang, H.-H.; Lai, M.-Y.; Hayashi, M.; Wang, J.-K.; Wang, Y.-L. Autocatalytic reaction in hydrolysis of difructose anhydride III. J. Phys. Chem. A 2011, 115, 10309–10314. [Google Scholar] [CrossRef]
- Christian, T.J.; Manley-Harris, M.; Field, R.J.; Parker, B.A. Kinetics of formation of di-ᴅ-fructose dianhydrides during thermal treatment of inulin. J. Agric. Food Chem. 2000, 48, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, S.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Recent advances on biological production of difructose dianhydride III. Appl. Microbiol. Biotechnol. 2018, 102, 3007–3015. [Google Scholar] [CrossRef]
- Manley-Harris, M.; Richards, G.N. Di-ᴅ-fructose dianhydrides and related oligomers from thermal treatments of inulin and sucrose. Carbohydr. Res. 1996, 287, 183–202. [Google Scholar] [CrossRef]
- Cheng, M.; Zhu, Y.; Mu, W. Difructose anhydrides-producing fructotransferase: Characteristics, catalytic mechanism, and applications. In Novel Enzymes for Functional Carbohydrates Production; Mu, W., Zhang, W., Chen, Q., Eds.; Springer: Singapore, 2021; pp. 147–174. [Google Scholar] [CrossRef]
- Fernández, J.M.G.; Gadelle, A.; Defaye, J. Difructose dianhydrides from sucrose and fructo-oligosaccharides and their use as building blocks for the preparation of amphiphiles, liquid crystals, and polymers. Carbohydr. Res. 1994, 265, 249–269. [Google Scholar] [CrossRef]
- Kim, N.-H.; Kim, H.-J.; Kang, D.-I.; Jeong, K.-W.; Lee, J.-K.; Kim, Y.; Oh, D.-K. Conversion shift of ᴅ-fructose to ᴅ-psicose for enzyme-catalyzed epimerization by addition of borate. Appl. Environ. Microbiol. 2008, 74, 3008–3013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manley-Harris, M.; Richards, G.N. Dihexulose dianhydrides. Adv. Carbohydr. Chem. Biochem. 1997, 52, 207–266. [Google Scholar] [CrossRef]
- Fukada, K.; Ishii, T.; Tanaka, K.; Yamaji, M.; Yamaoka, Y.; Kobashi, K.-I.; Izumori, K. Crystal structure, solubility, and mutarotation of the rare monosaccharide ᴅ-psicose. Chem. Soc. Jpn. 2010, 83, 1193–1197. [Google Scholar] [CrossRef]
- Alves, L.A.; Silva, J.B.A.E.; Giulietti, M. Solubility of ᴅ-glucose in water and ethanol/water mixtures. J. Chem. Eng. Data 2007, 52, 2166–2170. [Google Scholar] [CrossRef]
- Hanover, L.M.; White, J.S. Manufacturing, composition, and applications of fructose. Am. J. Clin. Nutr. 1993, 58, 724S–732S. [Google Scholar] [CrossRef]
- Kikuchi, H.; Nagura, T.; Inoue, M.; Kishida, T.; Sakurai, H.; Yokota, A.; Asano, K.; Tomita, F.; Sayama, K.; Senba, Y. Physical, chemical and physiological properties of difructose anhydride III produced from inulin by enzymatic reaction. J. Appl. Glycosci. 2004, 51, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, Y.; Iwagaki, S.; Hishida, M.; Nagatomo, S.; Fukada, K.; Saito, K. Heat capacity and standard thermodynamic functions of three ketohexoses in monosaccharides including rare sugars: ᴅ-fructose, ᴅ-psicose, and ᴅ-tagatose. J. Chem. Thermodyn. 2019, 131, 420–430. [Google Scholar] [CrossRef]
- Pocan, P.; Ilhan, E.; Oztop, M.H. Effect of ᴅ-psicose substitution on gelatin based soft candies: A TD-NMR study. Magn. Reson. Chem. 2019, 57, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Angyal, S.J.; Craig, D.C.; Defaye, J.; Gadelle, A. Complexes of carbohydrates with metal cations. XVI.1 Di-D-fructose and di-L-sorbose dianhydrides. Can. J. Chem. 1990, 68, 1140–1144. [Google Scholar] [CrossRef]
- Chemistry Data Booklet. 2014, pp. 1–40. Available online: https://www.ibchem.com/root_pdf/data_booklet_2016.pdf (accessed on 20 September 2022).
- Talbot, C. Reactivity series, activity series and electrochemical series. Sch. Sci. Rev. 2019, 100, 9–12. Available online: https://www.sji.edu.sg/qql/slot/u560/News%20and%20Events/News%20Highlights/2019/Talbot%207352%20SSR_reactivity_series_activity_series_electrochemical_series.pdf (accessed on 20 October 2022).
- Lee, S.I.; Kim, I.H. Difructose dianhydride improves intestinal calcium absorption, wound healing, and barrier function. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Woo, J.-B.; Ryu, S.-I.; Moon, S.-K.; Han, N.S.; Lee, S.-B. Glucosylation of flavonol and flavanones by Bacillus cyclodextrin glucosyltransferase to enhance their solubility and stability. Food Chem. 2017, 229, 75–83. [Google Scholar] [CrossRef] [PubMed]






| Compound | Temp (°C) | Anhydrous Acidify Reaction (mM) 1 | ||||
|---|---|---|---|---|---|---|
| 1 Day | 2 Day | 4 Day | 6 Day | 8 Day | ||
| Di-ᴅ-psicose anhydride | –20 | 440.9 ± 0.1 G | 480.9 ± 0.4 FG | 502.9 ± 0.2 F | 487.1 ± 0.4 FG | 569.2 ± 0.3 DE |
| 4 | 521.5 ± 0.1 EF | 579.8 ± 0.2 D | 668.5 ± 0.3 C | 733.1 ± 0.2 B | 893.5 ± 0.5 A | |
| 25 | 719.5 ± 0.3 B | 474.0 ± 0.4 FG | N.A | N.A | N.A | |
| 40 | N.A 2 | N.A | N.A | N.A | N.A | |
| ᴅ-Psicose | –20 | 1096.0 ± 0.2 AB | 1113.0 ± 0.4 A | 972.6 ± 0.3 C | 866.9 ± 0.4 CD | 929.4 ± 0.7 C |
| 4 | 986.3 ± 0.6 BC | 881.8 ± 0.3 C | 760.3 ± 0.5 D | 697.5 ± 0.2 E | 738.0 ± 0.6 E | |
| 25 | 403.0 ± 0.5 F | 340.8 ± 0.7 F | N.A | N.A | N.A | |
| 40 | N.A | N.A | N.A | N.A | N.A | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.S.; Kim, H.-G.; Lim, M.-C.; Park, J.-S.; Sa, S.; Yoo, M. Synthesis of a New Glycoconjugate with Di-ᴅ-Psicose Anhydride Structure. Int. J. Mol. Sci. 2022, 23, 12827. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232112827
Jung YS, Kim H-G, Lim M-C, Park J-S, Sa S, Yoo M. Synthesis of a New Glycoconjugate with Di-ᴅ-Psicose Anhydride Structure. International Journal of Molecular Sciences. 2022; 23(21):12827. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232112827
Chicago/Turabian StyleJung, Young Sung, Hyoung-Geun Kim, Min-Cheol Lim, Ji-Su Park, Soonok Sa, and Miyoung Yoo. 2022. "Synthesis of a New Glycoconjugate with Di-ᴅ-Psicose Anhydride Structure" International Journal of Molecular Sciences 23, no. 21: 12827. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232112827