Nature-Inspired Surface Structures Design for Antimicrobial Applications
Abstract
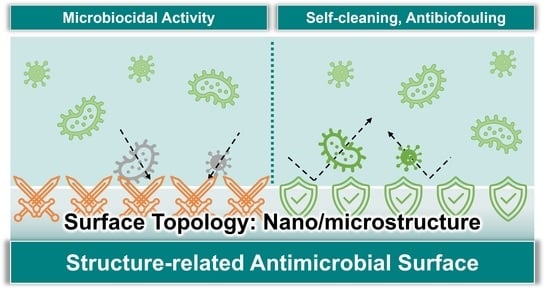
:1. Introduction
2. Microbial Cell-Surface Interaction
2.1. Bacterial Attachment and Colonization
2.2. Extracellular Polymeric Substance
2.3. Main factors Affecting Cell-Surface Interactions
2.4. Surface-Associated Motility
3. Microbicidal Surface
3.1. Mechano-Bactericidal Surface
3.2. Bactericidal Mechanisms of Mechano-Bactericidal Surface
3.3. Virucidal Surfaces
4. Anti-Biofouling Surface
4.1. Superhydrophobic Surface
4.2. Slippery Surface
5. Summary and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; de Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcon, K.P.; Melo, W.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal Biofilms and Polymicrobial Diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Maali, Y.; Journo, C.; Mahieux, R.; Dutartre, H. Microbial Biofilms: Human T-cell Leukemia Virus Type 1 First in Line for Viral Biofilm but Far Behind Bacterial Biofilms. Front. Microbiol. 2020, 11, 2041. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbourne, A.; Crawford, R.J.; Ivanova, E.P. Nano-structured antimicrobial surfaces: From nature to synthetic analogues. J. Colloid Interface Sci. 2017, 508, 603–616. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Sun, Z.; Ostrikov, K. Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustain. Mater. Technol. 2020, 25, e00203. [Google Scholar] [CrossRef]
- Huang, H.; Fan, C.; Li, M.; Nie, H.L.; Wang, F.B.; Wang, H.; Wang, R.; Xia, J.; Zheng, X.; Zuo, X.; et al. COVID-19: A Call for Physical Scientists and Engineers. ACS Nano 2020, 14, 3747–3754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Costerton, J. Introduction to biofilm. Int. J. Antimicrob. Agents 1999, 11, 217–221. [Google Scholar] [CrossRef]
- Kochkodan, V.; Tsarenko, S.; Potapchenko, N.; Kosinova, V.; Goncharuk, V. Adhesion of microorganisms to polymer membranes: A photobactericidal effect of surface treatment with TiO2. Desalination 2008, 220, 380–385. [Google Scholar] [CrossRef]
- Mahanta, U.; Khandelwal, M.; Deshpande, A.S. Antimicrobial surfaces: A review of synthetic approaches, applicability and outlook. J. Mater. Sci. 2021, 56, 17915–17941. [Google Scholar] [CrossRef] [PubMed]
- Kumada, Y.; Benson, D.R.; Hillemann, D.; Hosted, T.J.; Rochefort, D.A.; Thompson, C.J.; Wohlleben, W.; Tateno, Y. Evolution of the glutamine synthetase gene, one of the oldest existing and functioning genes. Proc. Natl. Acad. Sci. USA 1993, 90, 3009–3013. [Google Scholar] [CrossRef] [Green Version]
- Schidlowski, M. A 3,800-million-year isotopic record of life from carbon in sedimentary rocks. Nature 1988, 333, 313–318. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Boks, N.P.; Busscher, H.J.; van der Mei, H.C.; Norde, W. Bond-strengthening in staphylococcal adhesion to hydrophilic and hydrophobic surfaces using atomic force microscopy. Langmuir 2008, 24, 12990–12994. [Google Scholar] [CrossRef] [PubMed]
- Boks, N.P.; Kaper, H.J.; Norde, W.; Busscher, H.J.; van der Mei, H.C. Residence time dependent desorption of Staphylococcus epidermidis from hydrophobic and hydrophilic substrata. Colloids Surf. B Biointerfaces 2008, 67, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Norde, W.; Sharma, P.K.; van der Mei, H.C. Interfacial re-arrangement in initial microbial adhesion to surfaces. Curr. Opin. Colloid Interface Sci. 2010, 15, 510–517. [Google Scholar] [CrossRef]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef]
- Flemming, H.C. EPS-Then and Now. Microorganisms 2016, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Payne, D.E.; Boles, B.R. Emerging interactions between matrix components during biofilm development. Curr. Genet. 2016, 62, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Giaouris, E.; Chapot-Chartier, M.P.; Briandet, R. Surface physicochemical analysis of natural Lactococcus lactis strains reveals the existence of hydrophobic and low charged strains with altered adhesive properties. Int. J. Food Microbiol. 2009, 131, 2–9. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Graham, L.L. Surface layers of bacteria. Microbiol. Rev. 1991, 55, 684–705. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast: New structure and new challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkhipenko, M.V.; Nikitin, N.A.; Baranov, O.A.; Evtushenko, E.A.; Atabekov, J.G.; Karpova, O.V. Surface Charge Mapping on Virions and Virus-Like Particles of Helical Plant Viruses. Acta Nat. 2019, 11, 73–78. [Google Scholar] [CrossRef]
- Hernando-Perez, M.; Cartagena-Rivera, A.X.; Losdorfer Bozic, A.; Carrillo, P.J.; San Martin, C.; Mateu, M.G.; Raman, A.; Podgornik, R.; de Pablo, P.J. Quantitative nanoscale electrostatics of viruses. Nanoscale 2015, 7, 17289–17298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [Green Version]
- Diu, T.; Faruqui, N.; Sjostrom, T.; Lamarre, B.; Jenkinson, H.F.; Su, B.; Ryadnov, M.G. Cicada-inspired cell-instructive nanopatterned arrays. Sci. Rep. 2014, 4, 7122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, M.; Robinson, R.C.; Uyeda, T.Q.P.; Fukumori, Y.; Fukushima, S.I.; Haruta, S.; Homma, M.; Inaba, K.; Ito, M.; Kaito, C.; et al. Tree of motility—A proposed history of motility systems in the tree of life. Genes Cells 2020, 25, 6–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehes, E.; Vicsek, T. Collective motion of cells: From experiments to models. Integr. Biol. 2014, 6, 831–854. [Google Scholar] [CrossRef] [PubMed]
- Vicsek, T.; Zafeiris, A. Collective motion. Phys. Rep. 2012, 517, 71–140. [Google Scholar] [CrossRef] [Green Version]
- Harshey, R.M. Bacterial motility on a surface: Many ways to a common goal. Annu. Rev. Microbiol. 2003, 57, 249–273. [Google Scholar] [CrossRef]
- Kearns, D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef] [Green Version]
- Rather, P.N. Swarmer cell differentiation in Proteus mirabilis. Environ. Microbiol. 2005, 7, 1065–1073. [Google Scholar] [CrossRef]
- Harshey, R.M.; Matsuyama, T. Dimorphic transition in Escherichia coli and Salmonella typhimurium: Surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. USA 1994, 91, 8631–8635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, L.; Harshey, R.M. Differentiation of Serratia marcescens 274 into swimmer and swarmer cells. J. Bacteriol. 1990, 172, 4322–4328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, B.; Wong, G.C.L. How Bacteria Use Type IV Pili Machinery on Surfaces. Trends Microbiol. 2015, 23, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Burrows, L.L. Pseudomonas aeruginosa twitching motility: Type IV pili in action. Annu. Rev. Microbiol. 2012, 66, 493–520. [Google Scholar] [CrossRef] [Green Version]
- Kranz, W.T.; Golestanian, R. Trail-mediated self-interaction. J. Chem. Phys. 2019, 150, 214111. [Google Scholar] [CrossRef] [Green Version]
- McBride, M.J. Bacterial gliding motility: Multiple mechanisms for cell movement over surfaces. Annu. Rev. Microbiol. 2001, 55, 49–75. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Pogodin, S.; Baulin, V.A.; Watson, G.S.; Watson, J.A.; Crawford, R.J.; Ivanova, E.P. Selective bactericidal activity of nanopatterned superhydrophobic cicada Psaltoda claripennis wing surfaces. Appl. Microbiol. Biotechnol. 2012, 97, 9257–9262. [Google Scholar] [CrossRef] [PubMed]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [Green Version]
- Mainwaring, D.E.; Nguyen, S.H.; Webb, H.; Jakubov, T.; Tobin, M.; Lamb, R.N.; Wu, A.H.; Marchant, R.; Crawford, R.J.; Ivanova, E.P. The nature of inherent bactericidal activity: Insights from the nanotopology of three species of dragonfly. Nanoscale 2016, 8, 6527–6534. [Google Scholar] [CrossRef]
- Green, D.W.; Lee, K.K.; Watson, J.A.; Kim, H.Y.; Yoon, K.S.; Kim, E.J.; Lee, J.M.; Watson, G.S.; Jung, H.S. High Quality Bioreplication of Intricate Nanostructures from a Fragile Gecko Skin Surface with Bactericidal Properties. Sci. Rep. 2017, 7, 41023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, E.P.; Nguyen, S.H.; Webb, H.K.; Hasan, J.; Truong, V.K.; Lamb, R.N.; Duan, X.; Tobin, M.J.; Mahon, P.J.; Crawford, R.J. Molecular organization of the nanoscale surface structures of the dragonfly Hemianax papuensis wing epicuticle. PLoS ONE 2013, 8, e67893. [Google Scholar] [CrossRef] [Green Version]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Tobin, M.J.; Gervinskas, G.; Juodkazis, S.; Wang, J.Y.; Crawford, R.J.; et al. Spatial variations and temporal metastability of the self-cleaning and superhydrophobic properties of damselfly wings. Langmuir 2012, 28, 17404–17409. [Google Scholar] [CrossRef]
- Watson, G.S.; Green, D.W.; Schwarzkopf, L.; Li, X.; Cribb, B.W.; Myhra, S.; Watson, J.A. A gecko skin micro/nano structure—A low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Li, X.; Cheung, G.S.; Watson, G.S.; Watson, J.A.; Lin, S.; Schwarzkopf, L.; Green, D.W. The nanotipped hairs of gecko skin and biotemplated replicas impair and/or kill pathogenic bacteria with high efficiency. Nanoscale 2016, 8, 18860–18869. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Bhadra, C.M.; Yen Dang, T.H.; Buividas, R.; Wang, J.; Crawford, R.J.; Ivanova, E.P.; Juodkazis, S. A bactericidal microfluidic device constructed using nano-textured black silicon. RSC Adv. 2016, 6, 26300–26306. [Google Scholar] [CrossRef]
- Hasan, J.; Xu, Y.; Yarlagadda, T.; Schuetz, M.; Spann, K.; Yarlagadda, P.K. Antiviral and Antibacterial Nanostructured Surfaces with Excellent Mechanical Properties for Hospital Applications. ACS Biomater. Sci. Eng. 2020, 6, 3608–3618. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Berton, P.; Moraes, C.; Rogers, R.D.; Tufenkji, N. Nanodarts, nanoblades, and nanospikes: Mechano-bactericidal nanostructures and where to find them. Adv. Colloid Interface Sci. 2018, 252, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassallo, E.; Pedroni, M.; Silvetti, T.; Morandi, S.; Toffolatti, S.; Angella, G.; Brasca, M. Bactericidal performance of nanostructured surfaces by fluorocarbon plasma. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Hazell, G.; May, P.W.; Taylor, P.; Nobbs, A.H.; Welch, C.C.; Su, B. Studies of black silicon and black diamond as materials for antibacterial surfaces. Biomater. Sci. 2018, 6, 1424–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.; Choi, W.T.; Johnson, C.T.; Garcia, A.J.; Singh, P.M.; Breedveld, V.; Hess, D.W.; Champion, J.A. Inhibition of Bacterial Adhesion on Nanotextured Stainless Steel 316L by Electrochemical Etching. ACS Biomater. Sci. Eng. 2018, 4, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutey, A.H.A.; Gemini, L.; Romoli, L.; Lazzini, G.; Fuso, F.; Faucon, M.; Kling, R. Towards Laser-Textured Antibacterial Surfaces. Sci. Rep. 2018, 8, 10112. [Google Scholar] [CrossRef] [Green Version]
- Dickson, M.N.; Liang, E.I.; Rodriguez, L.A.; Vollereaux, N.; Yee, A.F. Nanopatterned polymer surfaces with bactericidal properties. Biointerphases 2015, 10, 021010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Zuber, F.; Maniura-Weber, K.; Brugger, J.; Ren, Q. Nanostructured surface topographies have an effect on bactericidal activity. J. Nanobiotechnol. 2018, 16, 20. [Google Scholar] [CrossRef]
- Wu, S.; Zuber, F.; Brugger, J.; Maniura-Weber, K.; Ren, Q. Antibacterial Au nanostructured surfaces. Nanoscale 2016, 8, 2620–2625. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, Y. Enhanced biomimic bactericidal surfaces by coating with positively-charged ZIF nano-dagger arrays. Nanomedicine 2017, 13, 2199–2207. [Google Scholar] [CrossRef]
- Yick, S.; Mai-Prochnow, A.; Levchenko, I.; Fang, J.; Bull, M.K.; Bradbury, M.; Murphy, A.B.; Ostrikov, K. The effects of plasma treatment on bacterial biofilm formation on vertically-aligned carbon nanotube arrays. RSC Adv. 2015, 5, 5142–5148. [Google Scholar] [CrossRef] [Green Version]
- Linklater, D.P.; Nguyen, H.K.D.; Bhadra, C.M.; Juodkazis, S.; Ivanova, E.P. Influence of nanoscale topology on bactericidal efficiency of black silicon surfaces. Nanotechnology 2017, 28, 245301. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Raj, S.; Yadav, L.; Chatterjee, K. Engineering a nanostructured “super surface” with superhydrophobic and superkilling properties. RSC Adv. 2015, 5, 44953–44959. [Google Scholar] [CrossRef] [Green Version]
- Sengstock, C.; Lopian, M.; Motemani, Y.; Borgmann, A.; Khare, C.; Buenconsejo, P.J.; Schildhauer, T.A.; Ludwig, A.; Koller, M. Structure-related antibacterial activity of a titanium nanostructured surface fabricated by glancing angle sputter deposition. Nanotechnology 2014, 25, 195101. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, C.M.; Truong, V.K.; Pham, V.T.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [Green Version]
- Hizal, F.; Zhuk, I.; Sukhishvili, S.; Busscher, H.J.; van der Mei, H.C.; Choi, C.H. Impact of 3D Hierarchical Nanostructures on the Antibacterial Efficacy of a Bacteria-Triggered Self-Defensive Antibiotic Coating. ACS Appl. Mater. Interfaces 2015, 7, 20304–20313. [Google Scholar] [CrossRef]
- Cao, Y.; Su, B.; Chinnaraj, S.; Jana, S.; Bowen, L.; Charlton, S.; Duan, P.; Jakubovics, N.S.; Chen, J. Nanostructured titanium surfaces exhibit recalcitrance towards Staphylococcus epidermidis biofilm formation. Sci. Rep. 2018, 8, 1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, J.; Jain, S.; Chatterjee, K. Nanoscale Topography on Black Titanium Imparts Multi-biofunctional Properties for Orthopedic Applications. Sci. Rep. 2017, 7, 41118. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Marshall, M.; Parivar, A.; Ly, V.K.; Pearlman, E.; Yee, A.F. Biomimetic Nanopillared Surfaces Inhibit Drug Resistant Filamentous Fungal Growth. ACS Appl. Bio Mater. 2019, 2, 3159–3163. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Liu, T.; Li, X.; Song, K.; Ge, D. Nanopillared Polycarbonate Surfaces Having Variable Feature Parameters as Bactericidal Coatings. ACS Appl. Nano Mater. 2020, 3, 4599–4609. [Google Scholar] [CrossRef]
- Linklater, D.P.; De Volder, M.; Baulin, V.A.; Werner, M.; Jessl, S.; Golozar, M.; Maggini, L.; Rubanov, S.; Hanssen, E.; Juodkazis, S.; et al. High Aspect Ratio Nanostructures Kill Bacteria via Storage and Release of Mechanical Energy. ACS Nano 2018, 12, 6657–6667. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Truong, V.K.; Quinn, M.D.; Notley, S.M.; Guo, Y.; Baulin, V.A.; Al Kobaisi, M.; Crawford, R.J.; Ivanova, E.P. Graphene Induces Formation of Pores That Kill Spherical and Rod-Shaped Bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Pyke, A.; Nair, N.; Yarlagadda, T.; Will, G.; Spann, K.; Yarlagadda, P. Antiviral Nanostructured Surfaces Reduce the Viability of SARS-CoV-2. ACS Biomater. Sci. Eng. 2020, 6, 4858–4861. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Phong Nguyen, T.H.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Zahir, T.; Pesek, J.; Franke, S.; Pee, J.V.; Rathore, A.; Smeets, B.; Ramon, H.; Xu, X.; Fauvart, M.; Michiels, J. Model-Driven Controlled Alteration of Nanopillar Cap Architecture Reveals its Effects on Bactericidal Activity. Microorganisms 2020, 8, 186. [Google Scholar] [CrossRef] [Green Version]
- Velic, A.; Hasan, J.; Li, Z.; Yarlagadda, P. Mechanics of Bacterial Interaction and Death on Nanopatterned Surfaces. Biophys. J. 2021, 120, 217–231. [Google Scholar] [CrossRef]
- Mo, S.; Mehrjou, B.; Tang, K.; Wang, H.; Huo, K.; Qasim, A.M.; Wang, G.; Chu, P.K. Dimensional-dependent antibacterial behavior on bioactive micro/nano polyetheretherketone (PEEK) arrays. Chem. Eng. J. 2020, 392, 123736. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Aizenberg, J. Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett. 2010, 10, 3717–3721. [Google Scholar] [CrossRef]
- Mirzaali, M.J.; van Dongen, I.C.P.; Tumer, N.; Weinans, H.; Yavari, S.A.; Zadpoor, A.A. In-silico quest for bactericidal but non-cytotoxic nanopatterns. Nanotechnology 2018, 29, 43LT02. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modaresifar, K.; Kunkels, L.B.; Ganjian, M.; Tumer, N.; Hagen, C.W.; Otten, L.G.; Hagedoorn, P.L.; Angeloni, L.; Ghatkesar, M.K.; Fratila-Apachitei, L.E.; et al. Deciphering the Roles of Interspace and Controlled Disorder in the Bactericidal Properties of Nanopatterns against Staphylococcus aureus. Nanomaterials 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velic, A.; Jaggessar, A.; Tesfamichael, T.; Li, Z.; Yarlagadda, P. Effects of Nanopillar Size and Spacing on Mechanical Perturbation and Bactericidal Killing Efficiency. Nanomaterials 2021, 11, 2472. [Google Scholar] [CrossRef] [PubMed]
- Shahali, H.; Hasan, J.; Mathews, A.; Wang, H.; Yan, C.; Tesfamichael, T.; Yarlagadda, P. Multi-biofunctional properties of three species of cicada wings and biomimetic fabrication of nanopatterned titanium pillars. J. Mater. Chem. B 2019, 7, 1300–1310. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Linklater, D.P.; Werner, M.; Baulin, V.A.; Xu, X.; Vrancken, N.; Rubanov, S.; Hanssen, E.; Wandiyanto, J.; Truong, V.K.; et al. The multi-faceted mechano-bactericidal mechanism of nanostructured surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 12598–12605. [Google Scholar] [CrossRef]
- Ganjian, M.; Angeloni, L.; Mirzaali, M.J.; Modaresifar, K.; Hagen, C.W.; Ghatkesar, M.K.; Hagedoorn, P.L.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Quantitative mechanics of 3D printed nanopillars interacting with bacterial cells. Nanoscale 2020, 12, 21988–22001. [Google Scholar] [CrossRef]
- Nakade, K.; Jindai, K.; Sagawa, T.; Kojima, H.; Shimizu, T.; Shingubara, S.; Ito, T. Single cell / real-time imaging of bactericidal effect on the nano-structural surface. Mater. Today Proc. 2019, 7, 497–500. [Google Scholar] [CrossRef]
- Suo, Z.; Avci, R.; Deliorman, M.; Yang, X.; Pascual, D.W. Bacteria survive multiple puncturings of their cell walls. Langmuir 2009, 25, 4588–4594. [Google Scholar] [CrossRef]
- Linklater, D.P.; Juodkazis, S.; Rubanov, S.; Ivanova, E.P. Comment on “Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli”. ACS Appl. Mater. Interfaces 2017, 9, 29387–29393. [Google Scholar] [CrossRef] [Green Version]
- Jindai, K.; Nakade, K.; Masuda, K.; Sagawa, T.; Kojima, H.; Shimizu, T.; Shingubara, S.; Ito, T. Adhesion and bactericidal properties of nanostructured surfaces dependent on bacterial motility. RSC Adv. 2020, 10, 5673–5680. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dollwet, H. Historic uses of copper compounds in medicine. Trace Elem. Med. 1985, 2, 80–87. [Google Scholar]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudha, V.P.; Singh, K.O.; Prasad, S.; Venkatasubramanian, P. Killing of enteric bacteria in drinking water by a copper device for use in the home: Laboratory evidence. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Chatterjee, K. Biomaterials-based formulations and surfaces to combat viral infectious diseases. APL Bioeng. 2021, 5, 011503. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef]
- Tavakoli, A.; Ataei-Pirkooh, A.; Mm Sadeghi, G.; Bokharaei-Salim, F.; Sahrapour, P.; Kiani, S.J.; Moghoofei, M.; Farahmand, M.; Javanmard, D.; Monavari, S.H. Polyethylene glycol-coated zinc oxide nanoparticle: An efficient nanoweapon to fight against herpes simplex virus type 1. Nanomedicine 2018, 13, 2675–2690. [Google Scholar] [CrossRef]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anti-Viral Surface Coating to Prevent the Spread of COVID-19. Focus Powder Coat. 2020, 2020, 5. [CrossRef]
- Hathout, R.M.; Kassem, D.H. Positively Charged Electroceutical Spun Chitosan Nanofibers Can Protect Health Care Providers From COVID-19 Infection: An Opinion. Front. Bioeng. Biotechnol. 2020, 8, 885. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasher, P.; Sharma, M. Nanotechnology-based self-sterilizing surfaces and their potential in combating COVID-19. Nanomedicine 2021, 16, 1183–1186. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Z.; Zhang, X.; Diao, D. Superhydrophobic, photo-sterilize, and reusable mask based on graphene nanosheet-embedded carbon (GNEC) film. Nano Res. 2021, 14, 1110–1115. [Google Scholar] [CrossRef]
- Hamouda, T.; Ibrahim, H.M.; Kafafy, H.H.; Mashaly, H.M.; Mohamed, N.H.; Aly, N.M. Preparation of cellulose-based wipes treated with antimicrobial and antiviral silver nanoparticles as novel effective high-performance coronavirus fighter. Int. J. Biol. Macromol. 2021, 181, 990–1002. [Google Scholar] [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Onses, M.S.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [Google Scholar] [CrossRef]
- Kiew, L.V.; Chang, C.Y.; Huang, S.Y.; Wang, P.W.; Heh, C.H.; Liu, C.T.; Cheng, C.H.; Lu, Y.X.; Chen, Y.C.; Huang, Y.X.; et al. Development of flexible electrochemical impedance spectroscopy-based biosensing platform for rapid screening of SARS-CoV-2 inhibitors. Biosens. Bioelectron. 2021, 183, 113213. [Google Scholar] [CrossRef]
- Huang, S.Y.; Kung, Y.A.; Huang, P.N.; Chang, S.Y.; Gong, Y.N.; Han, Y.J.; Chiang, H.J.; Liu, K.T.; Lee, K.M.; Chang, C.Y.; et al. Stability of SARS-CoV-2 Spike G614 Variant Surpasses That of the D614 Variant after Cold Storage. Msphere 2021, 6, e00104–e00121. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Huang, Y.T.; Chang, P.C.; Su, C.H.; Hsu, K.C.; Li, X.; Wu, C.H.; Chang, C.C. Surface active flexible palladium nano-thin-film electrode development for biosensing. Inorg. Chem. Commun. 2019, 107, 107461. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chen, W.; Su, C.H.; Chang, P.C.; Huang, Y.T.; Hsu, K.C.; Yuan, C.J.; Chang, C.C. Enhanced bioconjugation on sputtered palladium nano-thin-film electrode. Appl. Phys. Lett. 2019, 114, 093702. [Google Scholar] [CrossRef] [Green Version]
- Suman, R.; Javaid, M.; Haleem, A.; Vaishya, R.; Bahl, S.; Nandan, D. Sustainability of Coronavirus on Different Surfaces. J. Clin. Exp. Hepatol. 2020, 10, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H.A.; Sharafeldin, T.A.; Goyal, S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbound. Emerg. Dis. 2021, 68, 296–312. [Google Scholar] [CrossRef]
- Tiwari, A.; Patnayak, D.P.; Chander, Y.; Parsad, M.; Goyal, S.M. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 2006, 50, 284–287. [Google Scholar] [CrossRef]
- Lai, M.Y.; Cheng, P.K.; Lim, W.W. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005, 41, e67–e71. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.; Poon, L.L.M.; Chin, A.W.H.; Ducker, W.A. Effect of Surface Porosity on SARS-CoV-2 Fomite Infectivity. ACS Omega 2022, 7, 18238–18246. [Google Scholar] [CrossRef]
- Hosseini, M.; Behzadinasab, S.; Benmamoun, Z.; Ducker, W.A. The viability of SARS-CoV-2 on solid surfaces. Curr. Opin. Colloid Interface Sci. 2021, 55, 101481. [Google Scholar] [CrossRef]
- Chatterjee, S.; Murallidharan, J.S.; Agrawal, A.; Bhardwaj, R. A review on coronavirus survival on impermeable and porous surfaces. Sādhanā 2021, 47, 5. [Google Scholar] [CrossRef]
- Crick, C.R.; Ismail, S.; Pratten, J.; Parkin, I.P. An investigation into bacterial attachment to an elastomeric superhydrophobic surface prepared via aerosol assisted deposition. Thin Solid Film. 2011, 519, 3722–3727. [Google Scholar] [CrossRef]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Scheuerman, T.R.; Camper, A.K.; Hamilton, M.A. Effects of Substratum Topography on Bacterial Adhesion. J. Colloid Interface Sci. 1998, 208, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Yu, S.; Amirfazli, A.; Rahim Siddiqui, A.; Li, W. Recent Advances in Antibacterial Superhydrophobic Coatings. Adv. Eng. Mater. 2021, 24, 2101053. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Shang, H.M.; Wang, Y.; Limmer, S.J.; Chou, T.P.; Takahashi, K.; Cao, G.Z. Optically transparent superhydrophobic silica-based films. Thin Solid Film. 2005, 472, 37–43. [Google Scholar] [CrossRef]
- Gao, Y.N.; Wang, Y.; Yue, T.N.; Weng, Y.X.; Wang, M. Multifunctional cotton non-woven fabrics coated with silver nanoparticles and polymers for antibacterial, superhydrophobic and high performance microwave shielding. J. Colloid Interface Sci. 2021, 582, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, L.; Zhan, Y.; Lan, J.; Shang, J.; Zhou, M.; Lin, S. A robust and antibacterial superhydrophobic cotton fabric with sunlight-driven self-cleaning performance for oil/water separation. Cellulose 2021, 28, 1715–1729. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, X.; Yang, F.; Gan, J.; Jia, H. The preparation of cotton fabric with super-hydrophobicity and antibacterial properties by the modification of the stearic acid. J. Appl. Polym. Sci. 2021, 138, 50717. [Google Scholar] [CrossRef]
- Shaban, M.; Mohamed, F.; Abdallah, S. Production and Characterization of Superhydrophobic and Antibacterial Coated Fabrics Utilizing ZnO Nanocatalyst. Sci. Rep. 2018, 8, 3925. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Guo, Y.; Xu, L.; Chang, X.; Zhang, X.; Xu, G.; Shi, J. Plasma Enhanced Fluorine-Free Superhydrophobic Polyester (PET) Fabric with Ultra-Robust Antibacterial and Antibacterial Adhesion Properties. Coatings 2020, 11, 15. [Google Scholar] [CrossRef]
- Suryaprabha, T.; Sethuraman, M.G. Fabrication of copper-based superhydrophobic self-cleaning antibacterial coating over cotton fabric. Cellulose 2016, 24, 395–407. [Google Scholar] [CrossRef]
- Raeisi, M.; Kazerouni, Y.; Mohammadi, A.; Hashemi, M.; Hejazi, I.; Seyfi, J.; Khonakdar, H.A.; Davachi, S.M. Superhydrophobic cotton fabrics coated by chitosan and titanium dioxide nanoparticles with enhanced antibacterial and UV-protecting properties. Int. J. Biol. Macromol. 2021, 171, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Aslanidou, D.; Karapanagiotis, I. Superhydrophobic, Superoleophobic and Antimicrobial Coatings for the Protection of Silk Textiles. Coatings 2018, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Chen, P.; Liu, W. A Superhydrophobic and Antibacterial Surface Coated on Cotton Fabrics by Polydopamine. Fibers Polym. 2019, 20, 1380–1386. [Google Scholar] [CrossRef]
- Agbe, H.; Sarkar, D.K.; Chen, X.G.; Faucheux, N.; Soucy, G.; Bernier, J.L. Silver-Polymethylhydrosiloxane Nanocomposite Coating on Anodized Aluminum with Superhydrophobic and Antibacterial Properties. ACS Appl. Bio Mater. 2020, 3, 4062–4073. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Markova, N.; Rashkov, I. Superhydrophobic PVDF and PVDF-HFP nanofibrous mats with antibacterial and anti-biofouling properties. Appl. Surf. Sci. 2016, 363, 363–371. [Google Scholar] [CrossRef]
- Wang, T.; Lu, Z.; Wang, X.; Zhang, Z.; Zhang, Q.; Yan, B.; Wang, Y. A compound of ZnO/PDMS with photocatalytic, self-cleaning and antibacterial properties prepared via two-step method. Appl. Surf. Sci. 2021, 550, 149286. [Google Scholar] [CrossRef]
- Ren, T.; Yang, M.; Wang, K.; Zhang, Y.; He, J. CuO Nanoparticles-Containing Highly Transparent and Superhydrophobic Coatings with Extremely Low Bacterial Adhesion and Excellent Bactericidal Property. ACS Appl. Mater. Interfaces 2018, 10, 25717–25725. [Google Scholar] [CrossRef]
- Subhadarshini, S.; Singh, R.; Goswami, D.K.; Das, A.K.; Das, N.C. Electrodeposited Cu2O Nanopetal Architecture as a Superhydrophobic and Antibacterial Surface. Langmuir 2019, 35, 17166–17176. [Google Scholar] [CrossRef]
- Duan, X.; Liu, S.; Huang, E.; Shen, X.; Wang, Z.; Li, S.; Jin, C. Superhydrophobic and antibacterial wood enabled by polydopamine-assisted decoration of copper nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125145. [Google Scholar] [CrossRef]
- Agbe, H.; Sarkar, D.K.; Chen, X.G. Tunable Superhydrophobic Aluminum Surfaces with Anti-Biofouling and Antibacterial Properties. Coatings 2020, 10, 982. [Google Scholar] [CrossRef]
- Bartlet, K.; Movafaghi, S.; Dasi, L.P.; Kota, A.K.; Popat, K.C. Antibacterial activity on superhydrophobic titania nanotube arrays. Colloids Surf. B Biointerfaces 2018, 166, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Chien, Y.; Teng, P.C.; Huang, X.Y.; Lin, Y.Y.; Lin, T.Y.; Chou, S.J.; Chien, C.S.; Hsiao, Y.J.; Yang, Y.P.; et al. Superrepellent Doubly Reentrant Geometry Promotes Antibiofouling and Prevention of Coronavirus Contamination. Adv. Mater. Technol. 2022, 2200387. [Google Scholar] [CrossRef]
- Liu, T.L.; Kim, C.J. Repellent surfaces. Turning a surface superrepellent even to completely wetting liquids. Science 2014, 346, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.S.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Lafuma, A.; Quéré, D. Slippery pre-suffused surfaces. EPL (Europhys. Lett.) 2011, 96, 56001. [Google Scholar] [CrossRef]
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef] [Green Version]
- Bauer, U.; Federle, W. The insect-trapping rim of Nepenthes pitchers: Surface structure and function. Plant Signal. Behav. 2009, 4, 1019–1023. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Jiang, L. Bio-inspired design of multiscale structures for function integration. Nano Today 2011, 6, 155–175. [Google Scholar] [CrossRef]
- Wier, K.A.; McCarthy, T.J. Condensation on ultrahydrophobic surfaces and its effect on droplet mobility: Ultrahydrophobic surfaces are not always water repellant. Langmuir 2006, 22, 2433–2436. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Guo, Z. Facile Fabrication of Slippery Lubricant-Infused CuO-Coated Surfaces with Different Morphologies for Efficient Water Collection and Excellent Slippery Stability. Langmuir 2020, 36, 8983–8992. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent Flame-Retardant and Self-Healing Superhydrophobic Coatings on Cotton Fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W. Superhydrophobic, superamphiphobic and SLIPS materials as anti-corrosion and anti-biofouling barriers. New J. Chem. 2021, 45, 15170–15179. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Kleintschek, T.; Rieder, A.; Cheng, Y.; Baumbach, T.; Obst, U.; Schwartz, T.; Levkin, P.A. Hydrophobic liquid-infused porous polymer surfaces for antibacterial applications. ACS Appl. Mater. Interfaces 2013, 5, 6704–6711. [Google Scholar] [CrossRef]
- Leslie, D.C.; Waterhouse, A.; Berthet, J.B.; Valentin, T.M.; Watters, A.L.; Jain, A.; Kim, P.; Hatton, B.D.; Nedder, A.; Donovan, K.; et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014, 32, 1134–1140. [Google Scholar] [CrossRef]
- Howell, C.; Vu, T.L.; Lin, J.J.; Kolle, S.; Juthani, N.; Watson, E.; Weaver, J.C.; Alvarenga, J.; Aizenberg, J. Self-replenishing vascularized fouling-release surfaces. ACS Appl. Mater. Interfaces 2014, 6, 13299–13307. [Google Scholar] [CrossRef] [PubMed]
- Manna, U.; Raman, N.; Welsh, M.A.; Zayas-Gonzalez, Y.M.; Blackwell, H.E.; Palecek, S.P.; Lynn, D.M. Slippery Liquid-Infused Porous Surfaces that Prevent Microbial Surface Fouling and Kill Non-Adherent Pathogens in Surrounding Media: A Controlled Release Approach. Adv. Funct. Mater. 2016, 26, 3599–3611. [Google Scholar] [CrossRef] [Green Version]
- Kratochvil, M.J.; Welsh, M.A.; Manna, U.; Ortiz, B.J.; Blackwell, H.E.; Lynn, D.M. Slippery Liquid-Infused Porous Surfaces that Prevent Bacterial Surface Fouling and Inhibit Virulence Phenotypes in Surrounding Planktonic Cells. ACS Infect. Dis. 2016, 2, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoo, J.; Kim, J.; Jang, Y.; Shin, K.; Ha, E.; Ryu, S.; Kim, B.G.; Wooh, S.; Char, K. Development of Multimodal Antibacterial Surfaces Using Porous Amine-Reactive Films Incorporating Lubricant and Silver Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 6550–6560. [Google Scholar] [CrossRef] [PubMed]
- Wylie, M.P.; Bell, S.E.J.; Nockemann, P.; Bell, R.; McCoy, C.P. Phosphonium Ionic Liquid-Infused Poly(vinyl chloride) Surfaces Possessing Potent Antifouling Properties. ACS Omega 2020, 5, 7771–7781. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Jiang, R.; Gao, J.; Xu, J.-N.; Tian, L.; Zhang, X.; Zhou, S.; Zhao, J.; Ren, L. Metal-organic framework (MOF)-based slippery liquid-infused porous surface (SLIPS) for purely physical antibacterial applications. Appl. Mater. Today 2022, 27, 101430. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Ma, S.; Zhang, H. Slippery liquid-infused porous surface (SLIPS) with super-repellent and contact-killing antimicrobial performances. Colloids Surf. B Biointerfaces 2022, 220, 112878. [Google Scholar] [CrossRef]





| Material | Substrate | Fabrication | Structure Type | Structure Dimensions (nm) | Microbes (Bactericidal Activity) | Notes and/or Potential Applications | Refs | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diameter | Pitch | Height | Gram-Negative | Gram-Positive | Others | ||||||
| Black Silicon (Si/SiO2) | Silicon | Reactive ion beam etching (RIE) | Nanopillar | 20–80 | NI * | 500 | P. aeruginosa (~50% after 3 h) | B. subtilis (~15% after 3 h), S. aureus (~50% after 3 h) | NT * | First artificial mechano-bactericidal surfaces device | [61] |
| 62 | 62 | 280 | P. aeruginosa (89%) | S. aureus (85%) | NT | High bactericidal activity | [74] | ||||
| Nanograss | 10–20 | NI | 4000 | E. coli (83% after 3 h) | S. aureus (86% after 3 h) | NT |
| [75] | |||
| Plasma etching | Nanopillar | 150–200 | 100–250 | NI | E. coli (~95% after 3 h, ~99% after 24 h) | S. aureus (~63% after 3 h, ~99% after 24 h) | B. cereus (~30% after 3 h, ~99% after 24 h) | Effective against spore-forming bacteria | [65] | ||
| Titanium (Ti) | Titanium | Glancing angle sputter deposition (GLAD) | Nanocolumn | NI | 158 | 478 | E. coli (60%) | S. aureus (N/A *) | NT | Not affect tissue-like cells (hMSCs) and leukocytes (PBMCs) | [76] |
| Titania (TiO2) | Titanium | Hydrothermal process | Nanowire | 100 | NI | 3000 | P. aeruginosa (>60%), E. coli (>60%), K. pneumonia (<5%) | S. aureus (<5%,) B. subtilis (>60%), E. faecalis (<5%) | NT |
| [38] |
| Nanopattern array | 40 | NI | NI | P. aeruginosa (50%) | S. aureus (20%) | NT |
| [77] | |||
| Nanospike | 10–30 | 2000 | 2000 | NT | S. aureus (15%) | NT |
| [78] | |||
| Nanospears | 50 | 3000–5000 | 4000 | NT | S. epidermidis (47%) | NT |
| [79] | |||
| Black Titanium (Ti/TiO2 /Cl) | Titanium | Chlorine-based reactive ion etching (RIE) | Nanopillar | 80 | random | 1000 | E. coli (95% after 4 h), P. aeruginosa (92% after 4 h) | S. aureus (~22% after 4 h, ~76% after 24 h), M. smegmatis (~92% after 4 h) | NT |
| [80] |
| Gold (Au/W) | Silicon | Sputtering W and Al film, Al film anodization, Au electrodeposition | Nanopillar | 50 | NI | 100 | NT | S. aureus (~99%) | NT |
| [71] |
| Sputtering W and Al film, Al film anodization, W plasma etching, Au electrodeposition | Nanoring | 200 | NI | 100 | |||||||
| Polymer | Glass | Nanoporous template molding, ormostamp solution, UV curing | Nanopillar | 80 | 130 | 200 | NT | S. aureus (23%) | NT | Optimum NP density: ∼40 pillars μm−2 | [70] |
| 80 | 170 | 400 | S. aureus (100%) | ||||||||
| 80 | 170 | 200 | S. aureus (98%) | ||||||||
| 80 | 300 | 300 | S. aureus (26%) | ||||||||
| PMMA | PMMA | Nanoimprint lithography (NIL) | Nanopillar | 70 | 170 | 210 | NT | NT | A. fumigatus F. oxysporum | Inhibits the growth of filamentous fungi | [81] |
| 120 | 320 | 300 | |||||||||
| 100 | 500 | 700 | |||||||||
| PC | PC | Nanoporous anodic aluminum oxide (AAO) template-assisted hot embossing, wet etching | Nanopillar | <60 | 170 | 200 | E. coli (98.4%) | NT | NT | Polymer nanostructure surfaces | [82] |
| Carbon nanotubes | Silicon | Chemical vapor deposition (CVD) | Vertically aligned nanotube forest | 10 | <10 | 20,000 | E. coli, (N/A) P. aeruginosa (N/A) | B. subtilis, (45%) S. epidermidis (~90%) | NT | Plasma treatment may affect bactericidal activity | [73] |
| 10 | <10 | 1000 | P. aeruginosa (99%) | S. aureus (84%) | NT |
| [83] | ||||
| 10 | <10 | 30,000 | S. aureus (17%) | ||||||||
| Graphene | Glass | liquid-phase exfoliation procedure, vacuum filtration process | Nanoblade | 5 | NI | Horizontal length 79.7 | P. aeruginosa (71.4%) | S. aureus (77.1%) | NT |
| [84] |
| Zeolitic Imidazole framework (ZIF) | Multi-substrate compatible | Coating | Nanodagger | 2000 | <2000 | 1000 | E. coli (~99%) | S. aureus (~99%) | C. albicans (~80%) |
| [72] |
| Aluminum alloy (AA) | Aluminum alloy 6063 | Sodium hydroxide based wet etching | Nanopillar | 23 | 161 | NI | P. aeruginosa (92% after 3 h), | S. aureus (87% after 3 h) | Respiratory Syncytial Virus (RSV), Rhinovirus (RV) (3–4 log reduction) | With both antibacterial and antiviral activity | [63] |
| NT | NT | SARS-CoV-2 (2.5 log reduction) | [85] | ||||||||
| Substrate | Structure and Material | Fabrication | Microbes | Note and/or Potential Applications | Refs | |
|---|---|---|---|---|---|---|
| Gram-Negative | Gram-Positive | |||||
| Cotton fabric | PDMS/AgNPs/PDA PI/AgNPs/PDA | PDA modification, and immersing | E. coli | S. aureus |
| [138] |
| PDMS/Ag/AgCl | PDA modification, electrostatic adsorption, and immersing | E. coli | S. aureus |
| [139] | |
| UV-curable waterborne coatings, the silver nanoparticles, and the stearic acid | Electric spraying, UV curing, immersing, and stearic acid modification | E. coli | S. aureus |
| [140] | |
| ZnO NPs | Sol-gel method, and spin coating | K. pneumonia, P. aeruginosa, E. coli, and S. typhimurium | S. aureus, B. subtilis, E. faecalis, and B. cereus |
| [141] | |
| Cu/stearic acid | Chemical reduction of copper acetate and stearic acid immersing | E. coli | S. aureus |
| [143] | |
| chitosan/TiO2 nanocomposites | Immersing | E. coli | S. aureus | Promising UV-protecting properties | [144] | |
| HDTMS, EPDDAC, and SiO2 NPs | Immersing | E. coli | S. aureus | Good washability | [146] | |
| PET fabric | PDMS/ZnO NPs | Immersing, and Ar plasma treatment | E. coli | S. aureus |
| [142] |
| Silk | alkoxy silanes, organic fluoropolymer, silane quaternary ammonium salt, and silica nanoparticles | Spray | NI * (Microorganisms) |
| [145] | |
| Anodized Al oxide (AAO) | Ag/PMHS nanocomposites | Dip-coating deposition | P. aeruginosa, and E. coli | S. aureus |
| [147] |
| nanofibrous PVDF and PVDF-HFP mats | PVDF, PVDF-HFP, and nanosized zinc oxide with a silanized surface | One-pot electrospinning technique | E. coli | S. aureus | ZnO NPs: a potent bactericidal agent | [148] |
| ITO Glass | PDMS/ZnO | Electrodeposition-grafting modification method | E. coli | NT * |
| [149] |
| Glass | hydrophobic silica sol, and CuO NPs | Oxygen plasma treatment, and spray-coating | E. coli | NT |
| [150] |
| Copper foil | Cu2O nano pedals | Electrochemical deposition (ECD) method | E. coli | B. subtilis |
| [151] |
| Wood | CuNPs | PDA modification, coating, and fluorosilane treatment | E. coli | S. aureus |
| [152] |
| Aluminum alloy | OTES, and QUATs | Chemical etching, and immersing | P. aeruginosa, and E. coli | S. aureus | Suitable applications: anti-biofouling healthcare consumables such as nose masks, bedsheets and medical scraps | [153] |
| Titanium sheets | (heptadecafluoro-1,1,2,2-tetrahydrodecyl) trichlorosilane, and titania nanotube | Anodization process, and chemical vapor deposition (CVD) | P. aeruginosa, | S. aureus |
| [154] |
| Substrate | Material | Lubricant | Fabrication | Microbes | Note and/or Potential Applications | Refs |
|---|---|---|---|---|---|---|
| Silicon | Si/SiO2/heptadecafluoro-1,1,2,2-tetrahydrodecyl trichlorosilane | Perfluoropolyether, perfluorotripentylamine, or perfluorodecalin | Bosch process, vapor coating | S. aureus, P. aeruginosa, E. coli | Stable in submerged, extreme pH, salinity, and UV environments | [166] |
| Commercially available porous Teflon film | NA | NA | ||||
| Porous BMA-EDMA | BMA-EDMA | PFPE liquid | Coating | P. aeruginos | This device may have different surface antibiofouling ability of the same bacterial strain from field or laboratory. | [167] |
| Perfluorocarbon | Tethered perfluorocarbon | Perfluorodecalin | Plasma modification | P. aeruginos |
| [168] |
| PDMS | PDMS | Silicon oil | Micro molding, and immersing | S. aureus, E. coli, green microalgae, Botryococcus braunii, Chlamydomonas reinhardtii, Dunaliella salina, and Nannochloropsis oculata | With lubricant self-replenishment function of this device | [169] |
| Glass | PEI/PVDMA multilayers, triclosan loading | Silicon oil | Repeatedly soaking | C. albicans | Antimicrobial agent (triclosan) releasing to kill non-adherent pathogens | [170] |
| Glass | PEI/ PVDMA multilayers, QSIs loading | Silicon oil | Submerged iteratively | P. aeruginos | Small-molecule quorum sensing inhibitors (QSIs) releasing to attenuate virulence phenotypes in non-adherent cell | [171] |
| PAR film | PPFPA porous surface AgNPs loading | Silicon oil | Selective removal method, soaking modification | E. coil | AgNPs releasing to attenuate virulence phenotypes in non-adherent cell | [172] |
| Roughened PVC | PVC | Phosphonium ionic liquids (PILs) | NI | S. aureus, and P. aeruginos | The PIL: a potent bactericidal agent | [173] |
| Silica wafers, silicone, and polyurethane films or catheters | Oriented zeolitic imidazolate framework-L (ZIF-L) layer | Fluorinated lubricant oil | seeding and secondary growth technique | S. aureus, and P. aeruginos | Dual functions: mechano-bactericidal activity and lubricant antibiofouling | [174] |
| Glass slide, PC plate, PET plate, PE film, and silicone catheter tube | PS porous surface | Silicon oil | Dip coating, plasma treatment | S. aureus | Simple, low-cost, fast, and multi-substrate available fabrication | [175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-S.; Hussein, H.R.; Chang, S.-W.; Chang, C.-Y.; Lin, Y.-Y.; Chien, Y.; Yang, Y.-P.; Kiew, L.-V.; Chen, C.-Y.; Chiou, S.-H.; et al. Nature-Inspired Surface Structures Design for Antimicrobial Applications. Int. J. Mol. Sci. 2023, 24, 1348. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24021348
Lee M-S, Hussein HR, Chang S-W, Chang C-Y, Lin Y-Y, Chien Y, Yang Y-P, Kiew L-V, Chen C-Y, Chiou S-H, et al. Nature-Inspired Surface Structures Design for Antimicrobial Applications. International Journal of Molecular Sciences. 2023; 24(2):1348. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24021348
Chicago/Turabian StyleLee, Meng-Shiue, Hussein Reda Hussein, Sheng-Wen Chang, Chia-Yu Chang, Yi-Ying Lin, Yueh Chien, Yi-Ping Yang, Lik-Voon Kiew, Ching-Yun Chen, Shih-Hwa Chiou, and et al. 2023. "Nature-Inspired Surface Structures Design for Antimicrobial Applications" International Journal of Molecular Sciences 24, no. 2: 1348. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24021348