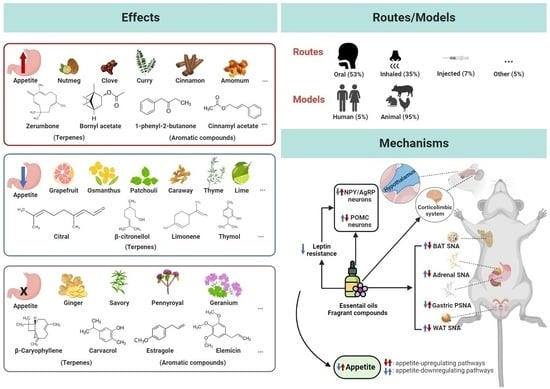
Effects of Essential Oils and Fragrant Compounds on Appetite: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategies
- For PubMed: ((appetite[MeSH Terms]) OR (food intake[MeSH Terms]) OR (food consumption[MeSH Terms])) AND ((aroma[MeSH Terms]) OR (aromas[MeSH Terms]) OR (scent[MeSH Terms]) OR (scents[MeSH Terms]) OR (fragrance[MeSH Terms]) OR (fragrances[MeSH Terms]) OR (smell[MeSH Terms]) OR (aromatherapy[MeSH Terms]) OR (aromatherapies[MeSH Terms]) OR (volatile oils[MeSH Terms]) OR (essential oils[MeSH Terms]) OR (essential oil[MeSH Terms])).
- For Web of Science (All Fields): (appetite OR food intake OR food consumption) AND (aroma OR aromas OR scent OR scents OR fragrance OR fragrances OR smell OR aromatherapy OR aromatherapies OR volatile oils OR essential oils OR essential oil).
2.2. Data Extraction and Quality Assessment
3. Results
3.1. Quality Assessment
3.2. Essential Oil and Fragrant Compound Preparation
3.3. Administration Methods
3.4. Effects of Essential Oils and Fragrant Compounds on Appetite
3.4.1. Effects of Essential Oils on Appetite in Clinical Studies
3.4.2. Essential Oils and Fragrant Compounds with Appetite-Enhancing Effects
3.4.3. Essential Oils and Fragrant Compounds with Appetite-Reducing Effects
3.4.4. Essential Oils and Fragrant Compounds with No Effect on Appetite
3.4.5. Essential Oils and Fragrant Compounds with Varied Effects
3.5. Mechanisms of Action
3.5.1. Essential Oils and Fragrant Compounds Regulate Appetite-Related Neuropeptides and Leptin Resistance
3.5.2. Essential Oils and Fragrant Compounds Affect Autonomic Nervous Activity
3.5.3. Essential Oils and Fragrant Compounds Affect Appetite via Cognitive Regulation
4. Discussion
4.1. Routes of Administration, Doses, and Treatment Duration in Appetite Regulation
4.2. Chemical Properties in Appetite Regulation




4.3. Mechanisms of Action in Appetite Regulation
4.4. Quality of Selected Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freitas, A.; Albuquerque, G.; Silva, C.; Oliveira, A. Appetite-Related Eating Behaviours: An Overview of Assessment Methods, Determinants and Effects on Children’s Weight. Ann. Nutr. Metab. 2018, 73, 19–29. [Google Scholar] [CrossRef]
- Dao, M.C.; Thiron, S.; Messer, E.; Sergeant, C.; Sévigné, A.; Huart, C.; Rossi, M.; Silverman, I.; Sakaida, K.; Bel Lassen, P.; et al. Cultural Influences on the Regulation of Energy Intake and Obesity: A Qualitative Study Comparing Food Customs and Attitudes to Eating in Adults from France and the United States. Nutrients 2020, 13, 63. [Google Scholar] [CrossRef]
- Espinoza García, A.S.; Martínez Moreno, A.G.; Reyes Castillo, Z. The role of ghrelin and leptin in feeding behavior: Genetic and molecular evidence. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2021, 68, 654–663. [Google Scholar] [CrossRef]
- Hanemaayer, R.; Neufeld, H.T.; Anderson, K.; Haines, J.; Gordon, K.; Lickers, K.R.L.; Xavier, A.; Peach, L.; Peeters, M. Exploring the environmental determinants of food choice among Haudenosaunee female youth. BMC Public Health 2022, 22, 1156. [Google Scholar] [CrossRef]
- Campos, A.; Port, J.D.; Acosta, A. Integrative Hedonic and Homeostatic Food Intake Regulation by the Central Nervous System: Insights from Neuroimaging. Brain Sci. 2022, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Serrenho, D.; Santos, S.D.; Carvalho, A.L. The Role of Ghrelin in Regulating Synaptic Function and Plasticity of Feeding-Associated Circuits. Front. Cell. Neurosci. 2019, 13, 205. [Google Scholar] [CrossRef]
- Suzuki, K.; Simpson, K.A.; Minnion, J.S.; Shillito, J.C.; Bloom, S.R. The role of gut hormones and the hypothalamus in appetite regulation. Endocr. J. 2010, 57, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.C.; Berridge, K.C. Advances in the neurobiological bases for food ‘liking’ versus ‘wanting’. Physiol. Behav. 2014, 136, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Higgs, S.; Spetter, M.S.; Thomas, J.M.; Rotshtein, P.; Lee, M.; Hallschmid, M.; Dourish, C.T. Interactions between metabolic, reward and cognitive processes in appetite control: Implications for novel weight management therapies. J. Psychopharmacol. 2017, 31, 1460–1474. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Ogunseye, K.O.; DeBenedictis, J.N.; Peris, J.; Kasper, J.M.; Hommel, J.D. Glutamatergic projections from homeostatic to hedonic brain nuclei regulate intake of highly palatable food. Sci. Rep. 2020, 10, 22093. [Google Scholar] [CrossRef] [PubMed]
- Puckett, L.; Grayeb, D.; Khatri, V.; Cass, K.; Mehler, P. A Comprehensive Review of Complications and New Findings Associated with Anorexia Nervosa. J. Clin. Med. 2021, 10, 2555. [Google Scholar] [CrossRef]
- Balasundaram, P.; Santhanam, P. Eating Disorders; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Melchior, C.; Desprez, C.; Riachi, G.; Leroi, A.M.; Déchelotte, P.; Achamrah, N.; Ducrotté, P.; Tavolacci, M.P.; Gourcerol, G. Anxiety and Depression Profile Is Associated With Eating Disorders in Patients With Irritable Bowel Syndrome. Front. Psychiatry 2019, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Lowes, K.L.; Clarke, P.J.F.; Hoiles, K.J.; Shu, C.Y.; Watson, H.J.; Dunlop, P.D.; Egan, S.J. The relationships between perfectionism, anxiety and depression across time in paediatric eating disorders. Eat. Behav. 2019, 34, 101305. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.; Moessner, M.; Bauer, S. Depression, Anxiety and Eating Disorder-Related Impairment: Moderators in Female Adolescents and Young Adults. Int. J. Environ. Health. Res. 2021, 18, 2779. [Google Scholar] [CrossRef] [PubMed]
- Nuffer, W. Chapter 5-Pharmacologic Agents Chapter for Abdominal Obesity. In Nutrition in the Prevention and Treatment of Abdominal Obesity, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 51–66. [Google Scholar]
- Sharma, S.; Aware, K.S.; Hatware, K.; Patil, K. Chemistry, Analysis, Pharmacokinetics and Pharmacodynamics Aspects of Lorcaserin, a Selective Serotonin 5-HT2C Receptor Agonist: An Update. Mini Rev. Med. Chem. 2020, 20, 768–778. [Google Scholar] [CrossRef]
- Kim, K.-K. Mechanisms of action and clinical applications of anti-obesity drugs currently available in Korea. J. Korean Med. Assoc. 2019, 62, 588–597. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Kurniawan, A. Appetite problem in cancer patients: Pathophysiology, diagnosis, and treatment. Cancer Treat. Res. Commun. 2021, 27, 100336. [Google Scholar] [CrossRef]
- McCullough, M.C.; Namias, N.; Schulman, C.; Gomez, E.; Manning, R.; Goldberg, S.; Pizano, L.; Ward, G.C. Incidence of hepatic dysfunction is equivalent in burn patients receiving oxandrolone and controls. J. Burn. Care. Res. 2007, 28, 412–420. [Google Scholar] [CrossRef]
- Yeh, S.S.; Schuster, M.W. Megestrol acetate in cachexia and anorexia. Int. J. Nanomed. 2006, 1, 411–416. [Google Scholar] [CrossRef]
- Schneider, E.; Higgs, S.; Dourish, C.T. Lisdexamfetamine and binge-eating disorder: A systematic review and meta-analysis of the preclinical and clinical data with a focus on mechanism of drug action in treating the disorder. Eur. Neuropsychopharmacol. 2021, 53, 49–78. [Google Scholar] [CrossRef]
- Sohel, A.J.; Shutter, M.C.; Molla, M. Fluoxetine; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Liang, J.; Zhang, Y.; Chi, P.; Liu, H.; Jing, Z.; Cao, H.; Du, Y.; Zhao, Y.; Qin, X.; Zhang, W.; et al. Essential oils: Chemical constituents, potential neuropharmacological effects and aromatherapy-A review. Pharmacol. Res.-Mod. Chin. Med. 2023, 6, 100210. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Malik, S. Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Springer International Publishing: New York, NY, USA, 2019. [Google Scholar]
- Hou, T.; Sana, S.S.; Li, H.; Xing, Y.; Nanda, A.; Netala, V.R.; Zhang, Z. Essential oils and its antibacterial, antifungal and anti-oxidant activity applications: A review. Food Biosci. 2022, 47, 101716. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Fung, T.K.H.; Lau, B.W.M.; Ngai, S.P.C.; Tsang, H.W.H. Therapeutic Effect and Mechanisms of Essential Oils in Mood Disorders: Interaction between the Nervous and Respiratory Systems. Int. J. Mol. Sci. 2021, 22, 4844. [Google Scholar] [CrossRef]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation Aromatherapy via Brain-Targeted Nasal Delivery: Natural Volatiles or Essential Oils on Mood Disorders. Front. Pharmacol. 2022, 13, 860043. [Google Scholar] [CrossRef]
- Jia, Y.; Zou, J.; Wang, Y.; Zhang, X.; Shi, Y.; Liang, Y.; Guo, D.; Yang, M. Action mechanism of Roman chamomile in the treatment of anxiety disorder based on network pharmacology. J. Food Biochem. 2021, 45, e13547. [Google Scholar] [CrossRef]
- Zhong, Y.; Zheng, Q.; Hu, P.; Huang, X.; Yang, M.; Ren, G.; Du, Q.; Luo, J.; Zhang, K.; Li, J.; et al. Sedative and hypnotic effects of compound Anshen essential oil inhalation for insomnia. BMC Complement. Altern. Med. 2019, 19, 306. [Google Scholar] [CrossRef]
- Ress, N.B.; Hailey, J.R.; Maronpot, R.R.; Bucher, J.R.; Travlos, G.S.; Haseman, J.K.; Orzech, D.P.; Johnson, J.D.; Hejtmancik, M.R. Toxicology and carcinogenesis studies of microencapsulated citral in rats and mice. Toxicol. Sci. 2003, 71, 198–206. [Google Scholar] [CrossRef]
- Yamamoto, T.; Inui, T.; Tsuji, T. The odor of Osmanthus fragrans attenuates food intake. Sci. Rep. 2013, 3, 8. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Niu, J.; Yan, P.; Yao, L.; He, W.; Wang, M.; Li, H.; Cao, L.; Li, X.; Shi, X.; et al. The effectiveness and safety of acupuncture for depression: An overview of meta-analyses. Complement. Ther. Med. 2020, 50, 102202. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Niijima, A.; Tanida, M.; Horii, Y.; Maeda, K.; Nagai, K. Olfactory stimulation with scent of lavender oil affects autonomic nerves, lipolysis and appetite in rats. Neurosci. Lett. 2005, 383, 188–193. [Google Scholar] [CrossRef]
- Shen, J.; Niijima, A.; Tanida, M.; Horii, Y.; Maeda, K.; Nagai, K. Olfactory stimulation with scent of grapefruit oil affects autonomic nerves, lipolysis and appetite in rats. Neurosci. Lett. 2005, 380, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Schöne, F.; Vetter, A.; Hartung, H.; Bergmann, H.; Biertümpfel, A.; Richter, G.; Müller, S.; Breitschuh, G. Effects of essential oils from fennel (Foeniculi aetheroleum) and caraway (Carvi aetheroleum) in pigs. J. Anim. Physiol. Anim. Nutr. 2006, 90, 500–510. [Google Scholar] [CrossRef]
- Chaves, A.V.; Stanford, K.; Gibson, L.L.; McAllister, T.A.; Benchaar, C. Effects of carvacrol and cinnamaldehyde on intake, rumen fermentation, growth performance, and carcass characteristics of growing lambs. Anim. Feed. Sci. Technol. 2008, 145, 396–408. [Google Scholar] [CrossRef]
- Munakata, M.; Kobayashi, K.; Niisato-Nezu, J.; Tanaka, S.; Kakisaka, Y.; Ebihara, T.; Ebihara, S.; Haginoya, K.; Tsuchiya, S.; Onuma, A. Olfactory Stimulation Using Black Pepper Oil Facilitates Oral Feeding in Pediatric Patients Receiving Long-Term Enteral Nutrition. Tohoku J. Exp. Med. 2008, 214, 327–332. [Google Scholar] [CrossRef]
- Nakamura, A.; Suzuki, T.; Taniguchi, D.; Matsuda, A.; Tobeta, M.; Nakamura, T. Odour of limonene affects feeding behaviour in the blowfly, Phormia regina. Neurosci. Lett. 2008, 446, 36–39. [Google Scholar] [CrossRef]
- Suanarunsawat, T.; Ayutthaya, W.D.N.; Songsak, T.; Rattanamahaphoom, J. Anti-lipidemic actions of essential oil extracted from Ocimum sanctum L. leaves in rats fed with high cholesterol diet. J. Appl. Biomed. 2009, 7, 45–53. [Google Scholar] [CrossRef]
- Asnaashari, S.; Delazar, A.; Habibi, B.; Vasfi, R.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Essential Oil from Citrus aurantifolia Prevents Ketotifen-induced Weight-gain in Mice. Phytother. Res. 2010, 24, 1893–1897. [Google Scholar] [CrossRef]
- Khodambashi, E.N.; Samie, A.; Rahmani, H.R.; Ruiz-Feria, C.A. The effect of peppermint essential oil and fructooligosaccharides, as alternatives to virginiamycin, on growth performance, digestibility, gut morphology and immune response of male broilers. Anim. Feed. Sci. Technol. 2012, 175, 57–64. [Google Scholar] [CrossRef]
- Batubara, I.; Suparto, I.H.; Sadiah, S.; Matsuoka, R.; Mitsunaga, T. Effect of Zingiber zerumbet essential oils and zerumbone inhalation on body weight of Sprague Dawley rat. Pak. J. Biol. Sci. 2013, 16, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Caldas, G.F.R.; Araujo, A.V.; Albuquerque, G.S.; Silva-Neto, J.D.; Costa-Silva, J.H.; de Menezes, I.R.A.; Leite, A.C.L.; da Costa, J.G.M.; Wanderley, A.G. Repeated-Doses Toxicity Study of the Essential Oil of Hyptis martiusii Benth. (Lamiaceae) in Swiss Mice. Evid.-Based Complement Altern. Med. 2013, 2013, 11. [Google Scholar]
- Batubara, I.; Suparto, I.H.; Sa’diah, S.; Matsuoka, R.; Mitsunaga, T. Effects of Inhaled Citronella Oil and Related Compounds on Rat Body Weight and Brown Adipose Tissue Sympathetic Nerve. Nutrients 2015, 7, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Escobar, F.M.; Sabini, M.C.; Cariddi, L.N.; Sabini, L.I.; Manas, F.; Cristofolini, A.; Bagnis, G.; Gallucci, M.N.; Cavaglieri, L.R. Safety assessment of essential oil from Minthostachys verticillata (Griseb.) Epling (peperina): 90-Days oral subchronic toxicity study in rats. Regul. Toxicol. Pharmacol. 2015, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khosravinia, H. Effects of Satureja khuzistanica essential oils in drinking water on mortality, production performance, water intake, and organ weights in broiler chickens reared under heat stress condition. Int. J. Biometeorol. 2015, 59, 1711–1719. [Google Scholar] [CrossRef]
- Dahham, S.S.; Hassan, L.E.A.; Ahamed, M.B.K.; Majid, A.S.A.; Majid, A.; Zulkepli, N.N. In vivo toxicity and antitumor activity of essential oils extract from agarwood (Aquilaria crassna). BMC Complement. Altern. Med. 2016, 16, 236. [Google Scholar] [CrossRef]
- Firmin, M.W.; Gillette, A.L.; Hobbs, T.E.; Wu, D. Effects of olfactory sense on chocolate craving. Appetite 2016, 105, 700–704. [Google Scholar] [CrossRef]
- Ogawa, K.; Ito, M. Appetite-Enhancing Effects of Curry Oil. Biol. Pharm. Bull. 2016, 39, 1559–1563. [Google Scholar] [CrossRef]
- Ogawa, K.; Ito, M. Appetite-enhancing Effects of trans-Cinnamaldehyde, Benzylacetone and 1-Phenyl-2-butanone by Inhalation. Planta Med. 2016, 82, 84–88. [Google Scholar] [CrossRef]
- Ogawa, K.; Ito, M. Appetite-Enhancing Effects: The Influence of Concentrations of Benzylacetone and trans-Cinnamaldehyde and Their Inhalation Time, as Well as the Effect of Aroma, on Body Weight in Mice. Biol. Pharm. Bull. 2016, 39, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, M.G.; Passetti, R.A.C.; Torrecilhas, J.A.; Mottin, C.; Vital, A.C.P.; Guerrero, A.; Sañudo, C.; del Mar Campo, M.; Prado, I.N. Essential oils in the diet of young bulls: Effect on animal performance, digestibility, temperament, feeding behaviour and carcass characteristics. Anim. Feed Sci. Technol. 2017, 234, 274–283. [Google Scholar] [CrossRef]
- Walia, K.; Arguello, H.; Lynch, H.; Leonard, F.C.; Grant, J.; Yearsley, D.; Kelly, S.; Duffy, G.; Gardiner, G.E.; Lawlor, P.G. Effect of strategic administration of an encapsulated blend of formic acid, citric acid, and essential oils on Salmonella carriage, seroprevalence, and growth of finishing pigs. Prev. Vet. Med. 2017, 137, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, S.H.; Bi, Q.; Liang, L.; Wang, Y.F.; Yang, X.X.; Gu, W.; Yu, J. Volatile Oil from Amomi Fructus Attenuates 5-Fluorouracil-Induced Intestinal Mucositisd. Front. Pharmacol. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, N.; Afsharmanesh, M.; Salarmoini, M.; Ebrahimnejad, H.; Bitaraf, A. Effect of pennyroyal, savory and thyme essential oils on Japanese quail physiology. Heliyon 2018, 4, E00881. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.R.; Ansari, H.S. Anti-Obesity Effect of Arq Zeera and Its Main Components Thymol and Cuminaldehyde in High Fat Diet Induced Obese Rats. Drug. Res. 2018, 68, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Tashima, A.; Sadakata, M.; Morinaga, O. Appetite-enhancing effects of vanilla flavours such as vanillin. J. Nat. Med. 2018, 72, 798–802. [Google Scholar] [CrossRef]
- Chapman, C.E.; Ort, S.B.; Aragona, K.M.; Cabral, R.G.; Erickson, P.S. Effect of cinnamaldehyde on feed intake, rumen fermentation, and nutrient digestibility, in lactating dairy cows1. J. Anim. Sci. 2019, 97, 1819–1827. [Google Scholar] [CrossRef]
- Coelho-de-Souza, A.N.; Rocha, M.; Oliveira, K.A.; Vasconcelos, Y.A.G.; Santos, E.C.; Silva-Alves, K.S.; Diniz, L.R.L.; Ferreira-da-Silva, F.W.; Oliveira, A.C.; Ponte, E.L.; et al. Volatile oil of Croton zehntneri per oral sub-acute treatment offers small toxicity: Perspective of therapeutic use. Rev. Bras. Farm. 2019, 29, 228–233. [Google Scholar] [CrossRef]
- Mekonnen, A.; Tesfaye, S.; Christos, S.G.; Dires, K.; Zenebe, T.; Zegeye, N.; Shiferaw, Y.; Lulekal, E. Evaluation of Skin Irritation and Acute and Subacute Oral Toxicity of Lavandula angustifolia Essential Oils in Rabbit and Mice. J. Toxicol. 2019, 2019, 8. [Google Scholar] [CrossRef]
- Ogawa, K.; Ito, M. Appetite-enhancing effects of nutmeg oil and structure-activity relationship of habituation to phenylpropanoids. J. Nat. Med. 2019, 73, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Cho, J.; Boo, C.G.; Youn, M.Y.; Pan, J.H.; Kim, J.K.; Shin, E.-C. Inhalation of Patchouli (Pogostemon Cablin Benth.) Essential Oil Improved Metabolic Parameters in Obesity-Induced Sprague Dawley Rats. Nutrients 2020, 12, 2077. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Honda, M.; Tanigawa, A.; Hatase, A.; Ito, A.; Higa, Y.; Morinaga, O. Appetite-enhancing effects of inhaling cinnamon, clove, and fennel essential oils containing phenylpropanoid analogues. J. Nat. Med. 2020, 74, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Rossini, C.; Rodrigo, F.; Davyt, B.; Umpierrez, M.L.; Gonzalez, A.; Garrido, P.M.; Cuniolo, A.; Porrini, L.P.; Eguaras, M.J.; Porrini, M.P. Sub-lethal effects of the consumption of Eupatorium buniifolium essential oil in honeybees. PLoS ONE 2020, 15, 19. [Google Scholar] [CrossRef]
- Yokoyama, I.; Nakai, Y.; Suzuki, Y.; Ohata, M.; Komiya, Y.; Nagasao, J.; Arihara, K. DMHF (2,5-dimethyl-4-hydroxy-3(2H)-furanone), a volatile food component generated by the Maillard reaction, promotes appetite and changes gene expression in the rat brain through inhalation. J. Food Sci. 2020, 85, e0241666. [Google Scholar] [CrossRef]
- Canche-Colli, C.; Estrella-Maldonado, H.; Medina-Medina, L.A.; Moo-Valle, H.; Calvo-Irabien, L.M.; Chan-Vivas, E.; Rodriguez, R.; Canto, A. Effect of yeast and essential oil-enriched diets on critical determinants of health and immune function in Africanized Apis mellifera. PeerJ 2021, 9, 24. [Google Scholar] [CrossRef]
- Lu, V.; Bastaki, M.; Api, A.M.; Aubanel, M.; Bauter, M.; Cachet, T.; Demyttenaere, J.; Diop, M.M.; Harman, C.L.; Hayashi, S.M.; et al. Dietary administration of beta-ionone epoxide to Sprague-Dawley rats for 90 days. Curr. Res. Toxicol. 2021, 2, 192–201. [Google Scholar] [CrossRef]
- Rafferty, C.; Lamont, B.B. Plant Tannins and Essential Oils Have an Additive Deterrent Effect on Diet Choice by Kangaroos. Forests 2021, 12, 1639. [Google Scholar] [CrossRef]
- Torki, M.; Mohebbifar, A.; Mohammadi, H. Effects of supplementing hen diet with Lavandula angustifolia and/or Mentha spicata essential oils on production performance, egg quality and blood variables of laying hens. Vet. Med. Sci. 2021, 7, 184–193. [Google Scholar] [CrossRef]
- Zhang, K.L.; Lei, N.; Li, M.; Li, J.J.; Li, C.J.; Shen, Y.; Guo, P.X.; Xiong, L.; Xie, Y.H. Cang-Ai Volatile Oil Ameliorates Depressive Behavior Induced by Chronic Stress Through IDO-Mediated Tryptophan Degradation Pathway. Front. Psychiatry 2021, 12, 12. [Google Scholar] [CrossRef]
- Boaventura, T.P.; dos Santos, F.A.C.; de Sena Souza, A.; Batista, F.S.; Júlio, G.S.C.; Luz, R.K. Thymol and linalool chemotypes of the essential oil of Thymus vulgaris (thyme) as anesthetic for Colossoma macropomum: Physiology and feed consumption. Aquaculture 2022, 554, 738161. [Google Scholar] [CrossRef]
- Haze, S.; Sakai, K.; Gozu, Y. Effects of fragrance inhalation on sympathetic activity in normal adults. Jpn. J. Pharmacol. 2002, 90, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Russo, B.; Menduni, M.; Borboni, P.; Picconi, F.; Frontoni, S. Autonomic Nervous System in Obesity and Insulin-Resistance-The Complex Interplay between Leptin and Central Nervous System. Int. J. Mol. Sci. 2021, 22, 5187. [Google Scholar] [CrossRef] [PubMed]
- Groh, C.; Rössler, W. Analysis of Synaptic Microcircuits in the Mushroom Bodies of the Honeybee. Insects 2020, 11, 43. [Google Scholar] [CrossRef]
- Yan, L.; Wang, J.P.; Kim, H.J.; Meng, Q.W.; Ao, X.; Hong, S.M.; Kim, I.H. Influence of essential oil supplementation and diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, meat quality and fecal noxious gas content in grower–finisher pigs. Livest. Sci. 2010, 128, 115–122. [Google Scholar] [CrossRef]
- Abu Isha, A.A.; Abd El-Hamid, A.; Ziena, H.; Ahmed, H. Effect of spearmint (mentha spicata) on productive and physiological parameters of broiler chicks. Egypt. Poult. Sci. J. 2018, 38, 815–829. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Eedara, B.B.; Alabsi, W.; Encinas-Basurto, D.; Polt, R.; Ledford, J.G.; Mansour, H.M. Inhalation Delivery for the Treatment and Prevention of COVID-19 Infection. Pharmaceutics 2021, 13, 1077. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Abbas, F.; Vinberg, F. Transduction and Adaptation Mechanisms in the Cilium or Microvilli of Photoreceptors and Olfactory Receptors From Insects to Humans. Front. Cell. Neurosci. 2021, 15, 662453. [Google Scholar] [CrossRef]
- Harrold, M.W.; Zavod, R.M. Basic Concepts in Medicinal Chemistry. Drug Dev. Ind. Pharm. 2014, 40, 988. [Google Scholar] [CrossRef]
- Kar, S.; Gupta, P.; Gupta, J. Essential Oils: Biological Activity Beyond Aromatherapy. Nat. Prod. Sci. 2018, 24, 139–147. [Google Scholar] [CrossRef]
- Grigalunas, M.; Brakmann, S.; Waldmann, H. Chemical Evolution of Natural Product Structure. J. Am. Chem. Soc. 2022, 144, 3314–3329. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Li, X.J.; Zhang, H.Y. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009, 10, 194–200. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Kong, L.; Yuan, T.; Li, W.; Zhang, W.; Hou, B.; Lu, Y.; Du, G. The strategies and techniques of drug discovery from natural products. Pharmacol. Ther. 2020, 216, 107686. [Google Scholar] [CrossRef]
- Fongang Fotsing Yannick, S.; Bankeu Kezetas Jean, J. Terpenoids as Important Bioactive Constituents of Essential Oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; Mozaniel Santana de, O., Wanessa Almeida da, C., Sebastião Gomes, S., Eds.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and medicinal uses of terpenes. In Medicinal Plants; Springer: New York, NY, USA, 2019; pp. 333–359. [Google Scholar]
- Kang, A.; Lee, T.S. Chapter 2-Secondary Metabolism for Isoprenoid-based Biofuels. In Biotechnology for Biofuel Production and Optimization; Eckert, C.A., Trinh, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–71. [Google Scholar]
- Volcho, K.P.; Anikeev, V.I. Chapter 3-Environmentally Benign Transformations of Monoterpenes and Monoterpenoids in Supercritical Fluids. In Supercritical Fluid Technology for Energy and Environmental Applications; Anikeev, V., Fan, M., Eds.; Elsevier: Boston, UK, 2014; pp. 69–87. [Google Scholar]
- Chizzola, R. Regular Monoterpenes and Sesquiterpenes (Essential Oils). In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2973–3008. [Google Scholar]
- Deno, N.C. Basic principles of organic chemistry. J. Chem. Educ. 1965, 42, 177. [Google Scholar] [CrossRef]
- Li, Q.; Kang, C. Mechanisms of Action for Small Molecules Revealed by Structural Biology in Drug Discovery. Int. J. Mol. Sci. 2020, 21, 5262. [Google Scholar] [CrossRef]
- Speight, J.G. Chapter 1-Chemistry and Chemical Technology. In Handbook of Industrial Hydrocarbon Processes; Speight, J.G., Ed.; Gulf Professional Publishing: Boston, UK, 2011; pp. 1–41. [Google Scholar]
- Kramer, S.D.; Lombardi, D.; Primorac, A.; Thomae, A.V.; Wunderli-Allenspach, H. Lipid-bilayer permeation of drug-like compounds. Chem. Biodivers. 2009, 6, 1900–1916. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. 5-Aromatic Compounds. In Principles of Organic Chemistry; Ouellette, R.J., Rawn, J.D., Eds.; Elsevier: Boston, UK, 2015; pp. 133–162. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic. Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Smith, K.L.; Gardiner, J.V.; Ward, H.L.; Kong, W.M.; Murphy, K.G.; Martin, N.M.; Ghatei, M.A.; Bloom, S.R. Overexpression of CART in the PVN Increases Food Intake and Weight Gain in Rats. Obesity 2008, 16, 2239–2244. [Google Scholar] [CrossRef]
- Hunter, R.G.; Bellani, R.; Bloss, E.; Costa, A.; Romeo, R.D.; McEwen, B.S. Regulation of CART mRNA by stress and corticosteroids in the hippocampus and amygdala. Brain Res. 2007, 1152, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cha, S.H.; Chohnan, S.; Lane, M.D. Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proc. Natl. Acad. Sci. USA 2003, 100, 12624–12629. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-F.; Hsieh, C.-T.; Hsieh, T.-J.; Chang, F.-R.; Wang, C.-K. In vitro anti-diabetic effect and chemical component analysis of 29 essential oils products. J. Food Drug Anal. 2015, 23, 124–129. [Google Scholar] [CrossRef]
- Sun, R.; Xiao, R.; Lv, P.; Guo, F.; Gong, Y.; Yan, M. Pink Lotus Essential Oil and Alleviates on Free Fatty Acid Induced Steatosis in HepG2 Cells via PI3K/Akt and NF-κB Pathways. J. Oleo. Sci. 2022, 71, 95–104. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Chen, P.N.; Yu, C.H.; Kuo, D.Y. Central dopamine action modulates neuropeptide-controlled appetite via the hypothalamic PI3K/NF-κB-dependent mechanism. Genes Brain. Behav. 2014, 13, 784–793. [Google Scholar] [CrossRef]
- Kudo, M.; Yoshitomi, H.; Momoo, M.; Suguro, S.; Yamagishi, Y.; Gao, M. Evaluation of the Effects and Mechanism of L-Citrulline on Anti-obesity by Appetite Suppression in Obese/Diabetic KK-Ay Mice and High-Fat Diet Fed SD Rats. Biol. Pharm. Bull. 2017, 40, 524–530. [Google Scholar] [CrossRef]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef]
- Guzmán, A.; Hernández-Coronado, C.G.; Rosales-Torres, A.M.; Hernández-Medrano, J.H. Leptin regulates neuropeptides associated with food intake and GnRH secretion. Ann. Endocrinol. 2019, 80, 38–46. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef]
- Caron, A.; Dungan Lemko, H.M.; Castorena, C.M.; Fujikawa, T.; Lee, S.; Lord, C.C.; Ahmed, N.; Lee, C.E.; Holland, W.L.; Liu, C.; et al. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. Elife 2018, 7, e33710. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.J.; Qi, Y.; Enriquez, R.F.; Clarke, I.; Ip, C.K.; Wee, N.; Baldock, P.A.; Herzog, H. Energy partitioning between fat and bone mass is controlled via a hypothalamic leptin/NPY relay. Int. J. Obes. Suppl. 2020, 44, 2149–2164. [Google Scholar] [CrossRef] [PubMed]
- Mainardi, M.; Pizzorusso, T.; Maffei, M. Environment, Leptin Sensitivity, and Hypothalamic Plasticity. Neural Plast. 2013, 2013, 438072. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K.; Haynes, W.G.; Morgan, D.A.; Mark, A.L. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension 2003, 41, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.K.; Sharma, D.; Ding, X.; Lin, S.; Marra, F.; Merlin, D.; Anania, F.A. Concomitant Activation of the JAK/STAT, PI3K/AKT, and ERK Signaling Is Involved in Leptin-Mediated Promotion of Invasion and Migration of Hepatocellular Carcinoma Cells. Cancer Res. 2007, 67, 2497–2507. [Google Scholar] [CrossRef]
- Banks, W.A. Role of the blood-brain barrier in the evolution of feeding and cognition. Ann. N. Y. Acad. Sci. 2012, 1264, 13–19. [Google Scholar] [CrossRef]
- Waxenbaum, J.A.; Reddy, V.; Varacallo, M. Anatomy, Autonomic Nervous System; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2019. [Google Scholar]
- Lundgren, O. Sympathetic input into the enteric nervous system. Gut 2000, 47 (Suppl. 4), iv33–iv35. [Google Scholar] [CrossRef]
- Noyes, F.R.; Barber-Westin, S.D. 40-Diagnosis and Treatment of Complex Regional Pain Syndrome. In Noyes’ Knee Disorders: Surgery, Rehabilitation, Clinical Outcomes, 2nd ed.; Noyes, F.R., Barber-Westin, S.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1122–1160. [Google Scholar]
- LeBouef, T.; Yaker, Z.; Whited, L. Physiology, Autonomic Nervous System; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef]
- Montagrin, A.; Martins-Klein, B.; Sander, D.; Mather, M. Effects of hunger on emotional arousal responses and attention/memory biases. Emotion 2021, 21, 148–158. [Google Scholar] [CrossRef]
- El Hadi, H.; Di Vincenzo, A.; Vettor, R.; Rossato, M. Food Ingredients Involved in White-to-Brown Adipose Tissue Conversion and in Calorie Burning. Front. Physiol. 2018, 9, 1954. [Google Scholar] [CrossRef]
- Gaspar, R.C.; Pauli, J.R.; Shulman, G.I.; Muñoz, V.R. An update on brown adipose tissue biology: A discussion of recent findings. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E488–E495. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, B.; Li, M.; Speakman, J.R. Switching on the furnace: Regulation of heat production in brown adipose tissue. Mol. Asp. Med. 2019, 68, 60–73. [Google Scholar] [CrossRef]
- Davidson, T.L.; Jones, S.; Roy, M.; Stevenson, R.J. The Cognitive Control of Eating and Body Weight: It’s More Than What You “Think”. Front. Psychol. 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Higgs, S. Memory for recent eating and its influence on subsequent food intake. Appetite 2002, 39, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Whitelock, V.; Nouwen, A.; van den Akker, O.; Higgs, S. The role of working memory sub-components in food choice and dieting success. Appetite 2018, 124, 24–32. [Google Scholar] [CrossRef]
- Yeomans, M.R.; Milton, M.R.; Chambers, L. Additive effects of sensory-enhanced satiety and memory for recent eating on appetite. Appetite 2017, 117, 335–341. [Google Scholar] [CrossRef]
- Reichelt, A.C.; Westbrook, R.F.; Morris, M.J. Integration of reward signalling and appetite regulating peptide systems in the control of food-cue responses. Br. J. Pharmacol. 2015, 172, 5225–5238. [Google Scholar] [CrossRef]
- Rolls, E.T. Understanding the mechanisms of food intake and obesity. Obes. Rev. 2007, 8 (Suppl. 1), 67–72. [Google Scholar] [CrossRef]
- Weltens, N.; Zhao, D.; Van Oudenhove, L. Where is the comfort in comfort foods? Mechanisms linking fat signaling, reward, and emotion. Neurogastroenterol. Motil. 2014, 26, 303–315. [Google Scholar] [CrossRef]
- Maffei, M.; Giordano, A. Leptin, the brain and energy homeostasis: From an apparently simple to a highly complex neuronal system. Rev. Endocr. Metab. Disord. 2022, 23, 87–101. [Google Scholar] [CrossRef]
- Zeithamova, D.; Bowman, C.R. Generalization and the hippocampus: More than one story? Neurobiol. Learn. Mem. 2020, 175, 107317. [Google Scholar] [CrossRef]
- Cenquizca, L.A.; Swanson, L.W. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J. Comp. Neurol. 2006, 497, 101–114. [Google Scholar] [CrossRef]
- Kishi, T.; Tsumori, T.; Ono, K.; Yokota, S.; Ishino, H.; Yasui, Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J. Comp. Neurol. 2000, 419, 205–222. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of Lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, L.; Feng, L.; Yao, L. The anxiolytic effect of essential oil of Cananga odorata exposure on mice and determination of its major active constituents. Phytomedicine 2016, 23, 1727–1734. [Google Scholar] [CrossRef]
- Lv, X.; Feng, Y.; Ma, R.; Tang, Y.; Li, Y.; Cui, D.; Wu, Y. Effects of Peppermint Essential Oil on Learning and Memory Ability in APP/PS1 Transgenic Mice. Molecules 2022, 27, 2051. [Google Scholar] [CrossRef]
- Aso, Y.; Hattori, D.; Yu, Y.; Johnston, R.M.; Iyer, N.A.; Ngo, T.-T.B.; Dionne, H.; Abbott, L.F.; Axel, R.; Tanimoto, H.; et al. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 2014, 3, e04577. [Google Scholar] [CrossRef] [PubMed]
- Dubnau, J.; Grady, L.; Kitamoto, T.; Tully, T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature 2001, 411, 476–480. [Google Scholar] [CrossRef]
- Coccurello, R. Anhedonia in depression symptomatology: Appetite dysregulation and defective brain reward processing. Behav. Brain Res. 2019, 372, 112041. [Google Scholar] [CrossRef]
- McCaughey, S.A.; Tordoff, M.G. Magnesium appetite in the rat. Appetite 2002, 38, 29–38. [Google Scholar] [CrossRef]
- Jerlhag, E. PRECLINICAL STUDY: Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict. Biol. 2008, 13, 358–363. [Google Scholar] [CrossRef] [PubMed]


| No. | Authors | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | Score | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ress et al., 2003 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 6 | [33] |
| 2 | Shen et al., 2005 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 7 | [37] |
| 3 | Shen et al., 2005 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 6 | [38] |
| 4 | Schöne et al., 2006 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 4 | [39] |
| 5 | Chaves et al., 2008 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 6 | [40] |
| 6 | Munakata et al., 2008 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 4 | [41] |
| 7 | Nakamura et al., 2008 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 7 | [42] |
| 8 | Suanarunsawat et al., 2009 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 | [43] |
| 9 | Asnaashari et al., 2010 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 | [44] |
| 10 | Khodambashi et al., 2012 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 7 | [45] |
| 11 | Batubara et al., 2013 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 7 | [46] |
| 12 | Caldas et al., 2013 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 | [47] |
| 13 | Yamamoto et al., 2013 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 7 | [34] |
| 14 | Batubara et al., 2015 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 | [48] |
| 15 | Escobar et al., 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 | [49] |
| 16 | Khosravinia, 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 7 | [50] |
| 17 | Dahham et al., 2016 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 7 | [51] |
| 18 | Firmin et al., 2016 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 5 | [52] |
| 19 | Ogawa and Ito, 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 | [53] |
| 20 | Ogawa and Ito, 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | [54] |
| 21 | Ogawa and Ito, 2016 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 | [55] |
| 22 | Ornaghi et al., 2017 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 | [56] |
| 23 | Walia et al., 2017 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | [57] |
| 24 | Zhang et al., 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 8 | [58] |
| 25 | Dehghani et al., 2018 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 6 | [59] |
| 26 | Haque and Ansari, 2018 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | [60] |
| 27 | Ogawa et al., 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 | [61] |
| 28 | Chapman et al., 2019 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 7 | [62] |
| 29 | Coelho-de-Souza et al., 2019 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 | [63] |
| 30 | Mekonnen et al., 2019 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 | [64] |
| 31 | Ogawa and Ito, 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 | [65] |
| 32 | Hong et al., 2020 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 8 | [66] |
| 33 | Ogawa et al., 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 | [67] |
| 34 | Rossini et al., 2020 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 5 | [68] |
| 35 | Yokoyama et al., 2020 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 8 | [69] |
| 36 | Canche-Colli et al., 2021 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 7 | [70] |
| 37 | Lu et al., 2021 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 7 | [71] |
| 38 | Rafferty and Lamont, 2021 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 6 | [72] |
| 39 | Torki et al., 2021 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 7 | [73] |
| 40 | Zhang et al., 2021 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 | [74] |
| 41 | Boaventura et al., 2022 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 | [75] |
| No. | Essential Oil | Major Compounds | Route of Administration | Dose | Duration | Effects on Appetite | Mechanism | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Black pepper essential oil | n/a | Intranasal | 100 µL/filter paper stick | 1 min | Increase | n/a | [41] |
| 2 | Vanilla essential oil | n/a | Inhalation | Two drops (~0.1 mL)/petri dish | n/a | Increase | n/a | [52] |
| 3 | Slique Essence | n/a | Inhalation | Two drops (~0.1 mL)/petri dish | n/a | Decrease | n/a | [52] |
| No. | Essential Oil | Major Compounds | Route of Administration | Dose | Duration | Species | Mechanism | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Lavender essential oil | Linalool 1,8-Cineole (Eucalyptol) Camphor | Inhalation | 100,000× dilution in water | 15 min/day, 33 days | Wistar rats | Decrease WAT, BAT, and adrenal SNA; increase gastric PSNA | [37] |
| 2 | Fennel essential oil | trans-Anethole Fenchone | Mix with diet | 100 mg/kg of diet | 3 weeks | Pietrain × (Landrace × Large White) pigs | n/a | [39] |
| 3 | Zingiber zerumbet essential oil | Zerumbone | Inhalation | 100× dilution in water | 5 weeks | Sprague Dawley rats | Decrease BAT SNA | [46] |
| 4 | Curry essential oil | trans-Anethole 2-Methyl-3-phenylpropanal | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [53] |
| 5 | Cinnamon essential oil | trans-Cinnamaldehyde trans-2-Methoxycinnamaldehyde | Mix with diet | 7% (w/w) of diet | 15 days | Crossbred bulls | n/a | [56] |
| 6 | Clove essential oil | Eugenol Eugenol acetate | Mix with diet | 3.5% (w/w) of diet | 15 days | Crossbred bulls | n/a | [56] |
| 7 | Amomum villosum Lour. essential oil | Bornyl acetate Camphor | Oral | 8, 16, 32 mg/kg | 12 days | Sprague Dawley rats | n/a | [58] |
| 8 | Nutmeg essential oil | Sabinene α-Pinene | Inhalation | 7.4 × 10−7 mg/cm3 | 1 h | ddY mice | n/a | [65] |
| 9 | Cinnamon essential oil | trans-Cinnamaldehyde trans-2-Methoxycinnamaldehyde | Inhalation | 4.5 × 10−4~4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 10 | Clove essential oil | Eugenol Eugenol acetate | Inhalation | 4.5 × 10−4~4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 11 | Fennel essential oil | trans-Anethole Fenchone | Inhalation | 4.5 × 10−4~4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 12 | Cang-ai essential oil | Eugenol 1,8-Cineole | Oral | 4.6 μg/kg/day | 28 days | Sprague Dawley rats | n/a | [74] |
| No. | Fragrant Compounds | Route of Administration | Dose | Duration | Model | Mechanism | References |
|---|---|---|---|---|---|---|---|
| 1 | Linalool | Inhalation | 5000× dilution in water | 33 days | Wistar rats | Decrease adrenal SNA, increase gastric PSNA | [37] |
| 2 | Zerumbone | Inhalation | 100× dilution in water | 5 weeks | Sprague Dawley rats | Decrease BAT SNA | [46] |
| 3 | Eugenol | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [53] |
| 4 | Mixture of trans-cinnamaldehyde, eugenol, trans-anethole (1:2.6:5.6) | Inhalation | 4.5 × 10−5 mg/cage | 1 h | ddY mice | n/a | [53] |
| 5 | trans-Anethole | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [53] |
| 6 | trans-Cinnamaldehyde | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [53] |
| 7 | 1-Phenyl-2-butanone | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | Increase NPY mRNA expression | [54] |
| 8 | Benzylacetone | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | Increase NPY mRNA expression | [54] |
| 9 | trans-Cinnamaldehyde | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | Increase NPY mRNA expression | [54] |
| 10 | Bornyl acetate | Oral | 2, 4, 8 mg/kg | 12 days | Sprague Dawley rats | n/a | [58] |
| 11 | Ethyl vanillin | Inhalation | 4.5 × 10−4~4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [61] |
| 12 | Eugenol | Inhalation | 4.5 × 10−4~2.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [61] |
| 13 | Vanillin | Inhalation | 4.5 × 10−5~4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [61] |
| 14 | Benzylacetone | Inhalation | 7.4 × 10−8~7.4 × 10−2 mg/cm3 | 5~60 min | ddY mice | n/a | [65] |
| 15 | Methyl eugenol | Inhalation | 7.4 × 10−9 mg/cm3 | 1 h | ddY mice | n/a | [65] |
| 16 | Myristicin | Inhalation | 7.4 × 10−9 mg/cm3 | 1 h | ddY mice | n/a | [65] |
| 17 | Vanillylacetone | Inhalation | 7.4 × 10−10~7.4 × 10−7 mg/cm3 | 1 h | ddY mice | n/a | [65] |
| 18 | 2-Methoxycinnamaldehyde | Inhalation | 4.5 × 10−5~4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [67] |
| 19 | 3-Phenylpropionaldehyde | Inhalation | 4.5 × 10−4~4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 20 | Benzyl benzoate | Inhalation | 4.5 × 10−6~2.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 21 | Benzylacetone | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [67] |
| 22 | Cinnamyl acetate | Inhalation | 4.5 × 10−5~4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [67] |
| 23 | Eugenol | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [67] |
| 24 | Eugenol acetate | Inhalation | 1.1 × 10−3~4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 25 | Methyl salicylate | Inhalation | 4.5 × 10−4~2.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 26 | Mixture of eugenol and eugenol acetate (2:1) | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [67] |
| 27 | p-Anisketone | Inhalation | 4.5 × 10−4~2.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 28 | trans-Anethole | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [67] |
| 29 | trans-Cinnamaldehyde | Inhalation | 4.5 × 10−4~4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 30 | trans-Cinnamyl alcohol | Inhalation | 4.5 × 10−4~4.5 × 10−2 mg/cage | 1 h | ddY mice | n/a | [67] |
| 31 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone (DMHF) | Inhalation | 5.7 mg/L water | 6 weeks | Wistar rats | Increase mRNA expression of Cartpt, and Agt | [69] |
| No. | Essential Oil | Major Compounds | Route of Administration | Dose | Duration | Species | Mechanism | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Grapefruit essential oil | Limonene | Inhalation | 15 min, 3 times/week for 6 weeks | Wistar rats, C57BL/6J mice | Increase WAT, BAT, and adrenal SNA; decrease gastric PSNA | [38] | |
| 2 | Caraway (Carvi aetheroleum) essential oil | Limonene Carvone | Mix with diet | 100 mg/kg of diet | 3 weeks | Pietrain × (Landrace × Large White) pigs | n/a | [39] |
| 3 | Citrus aurantifolia essential oil | D-Limonene α-Terpineol | Subcutaneous injection | 125, 250, 500 mg/kg | 45 days | Mice | n/a | [44] |
| 4 | Peppermint essential oil | (L)-menthol (L)-menthone | Mix with diet | 400, 500 mg/kg | 6 weeks | Ross 308 Broilers | n/a | [45] |
| 5 | Osmanthus fragrans essential oil | n/a | Inhalation | 100 μL/filter paper | 23 days | Wistar rats | Decrease mRNA expression of AgRP and NPY, increase mRNA expression of CART and POMC | [34] |
| 6 | FormaXOL | n/a | Mix with diet | 4 kg/ton diet | 28 days | Pigs | n/a | [57] |
| 7 | Thyme essential oil | Thymol Durenol | Mix with diet | 400 ppm | 35 days | Japanese quail | n/a | [59] |
| 8 | Arq zeera (Trachyspermum ammi L., Zingiber officinale Roxb., Carum carvi L., and Cuminum cyminum L) | Oral | 7.75 mg/kg | Twice per day for 4 weeks | Wistar rats | Lower the elevated serum leptin level (reduced leptin resistance) in obese rats | [60] | |
| 9 | Croton zehntneri essential oil | trans-Anethole Estragole | Oral | 250 mg/kg | 10 weeks | Wistar rats | n/a | [63] |
| 10 | Patchouli essential oil | α-Patchoulene β-Patchoulene | Inhalation | 0.3% or 1% | 30 min for 6 or 12 weeks | Sprague Dawley rats | Lower the elevated serum leptin level (reduced leptin resistance) in obese rats | [66] |
| 11 | Pine essential oil | n/a | Mix with diet | 2% (w/w) of diet | n/a | Western grey kangaroos | n/a | [72] |
| No. | Fragrant Compounds | Route of Administration | Dose | Duration | Model | Mechanism | References |
|---|---|---|---|---|---|---|---|
| 1 | Citral | Mix with diet | 3900, 7800, 15,600, 31,300 ppm | 14 weeks | F344/N rats | n/a | [33] |
| 2 | Limonene | Inhalation | 5000× dilution in water | 6 weeks | Wistar rats, C57BL/6J mice | Increase adrenal SNA, decrease gastric PSNA | [38] |
| 3 | D-Limonene | Inhalation | Flow rate: 200 mL/min | 10 min | Blowflies | n/a | [42] |
| 4 | β-Citronellol | Inhalation | 100× dilution in water | 35 days | Sprague Dawley rats | Increase BAT SNA | [48] |
| 5 | Thymol | Oral | 12 mg/kg | Twice per day for 4 weeks | Wistar rats | Lower the elevated serum leptin level (reduced leptin resistance) in obese rats | [60] |
| 6 | trans-Anethole | Oral | 250 mg/kg | 10 weeks | Wistar rats | n/a | [63] |
| 7 | 1,8-Cineole | Mix with diet | 2% (w/w) of diet | n/a | Western grey kangaroos | n/a | [72] |
| No. | Essential Oil | Major Compounds | Route of Administration | Dose | Duration | Species | Mechanism | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Ocimum sanctum L. essential oil | Eugenol Methyl eugenol | Mix with diet | 80 µL/kg bw/day | 3 weeks | Wistar rats | n/a | [43] |
| 2 | Hyptis martiusii Benth. essential oil | 1,8-Cineole δ-3-Carene | Oral | 100, 500 mg/kg | 30 days | Swiss mice | n/a | [47] |
| 3 | Citronella essential oil | R-Citronellal Neryl acetate | Inhalation | 100× dilution in water | Sprague Dawley rats | n/a | [48] | |
| 4 | Minthostachys verticillata essential oil | Pulegone Menthone | Mix with diet | 1, 4, 7 g/kg feed | 90 days | Wistar rats | n/a | [49] |
| 5 | Satureja khuzistanica essential oil | Carvacrol p-Cymene | Mix with water | 200, 300, 400, and 500 mg/L in water | 42 days | Arian broiler chicks | n/a | [50] |
| 6 | Aquilaria crassna essential oil | n/a | Oral | 100, 500 mg/kg | 28 days | Swiss mice | n/a | [51] |
| 7 | Ginger essential oil | Geranial Neral | Inhalation | 4.5 × 10−4 mg/cage | 1h | ddY mice | n/a | [53] |
| 8 | Geranium essential oil | n/a | Inhalation | 4.5 × 10−3 mg/cage | 1h | ddY mice | n/a | [54] |
| 9 | Pennyroyal essential oil | Pulegone 3,3′-dimenthol | Mix with diet | 200, 300, and 400 ppm | 35 days | Japanese quail | n/a | [59] |
| 10 | Savory essential oil | Thymol 4,4′-diapophytoene | Mix with diet | 200, 300, and 400 ppm | 35 days | Japanese quail | n/a | [59] |
| 11 | Lavender essential oil | Linalool 1,8-Cineole (Eucalyptol) Camphor | Oral | 2000 mg/kg | 21 days | Swiss albino mice | n/a | [64] |
| 12 | Eupatorium buniifolium essential oil | α-pinene (E)-β-guaiene | Oral | 300, 3000, 6000 ppm | 12 days | Apis mellifera bees | n/a | [68] |
| 13 | Carvacrol essential oil from Lippia graveolens | Carvacrol p-Cymene | Mix with diet | 1% (w/w) of diet | 9~12 days | Apis mellifera bees | n/a | [70] |
| 14 | Sesquiterpenes essential oil from Lippia graveolens | β-Caryophyllene α-Humulene | Mix with diet | 1% (w/w) of diet | 9~12 days | Apis mellifera bees | n/a | [70] |
| 15 | Thymol essential oil from Lippia graveolens | Thymol β-Caryophyllene | Mix with diet | 1% (w/w) of diet | 9~12 days | Apis mellifera bees | n/a | [70] |
| 16 | Lavender essential oil | Linalool 1,8-Cineole (Eucalyptol) Camphor | Mix with diet | 250 mg/kg diet | 14 weeks | Lohmann LSL-Lite laying hens | n/a | [73] |
| 17 | Mentha spicata essential oil | Carvone Limonene | Mix with diet | 250 mg/kg diet | 14 weeks | Lohmann LSL-Lite laying hens | n/a | [73] |
| 18 | Linalool essential oil from Thymus vulgaris | Linalool Carvacrol | Mixed in aquarium | 100 mg/L | n/a | Colossoma macropomum | n/a | [75] |
| 19 | Thymol essential oil from Thymus vulgaris | Thymol p-Cymene | Mixed in aquarium | 50 mg/L | n/a | Colossoma macropomum | n/a | [75] |
| No. | Fragrant Compounds | Route of Administration | Dose | Duration | Model | Mechanism | References |
|---|---|---|---|---|---|---|---|
| 1 | Carvacrol | Mix with diet | 0.2 g/kg | 7 days | Canadian Arcott lambs | n/a | [40] |
| 2 | Cinnamaldehyde | Mix with diet | 0.2 g/kg | 7 days | Canadian Arcott lambs | n/a | [40] |
| 3 | R-Citronellal | Inhalation | 100× dilution in water | 35 days | Sprague Dawley rats | n/a | [48] |
| 4 | Estragole | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [53] |
| 5 | Safrole | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [53] |
| 6 | β-Caryophyllene | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [53] |
| 7 | (R)-Linalool | Inhalation | 4.5 × 10−5 mg/cage | 1 h | ddY mice | n/a | [54] |
| 8 | 6-Methyl-5-hepten-2-one | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [54] |
| 9 | Benzaldehyde | Inhalation | 4.5 × 10−5 mg/cage | 1 h | ddY mice | n/a | [54] |
| 10 | Benzylacetone | Intraperitoneal injection | 0.01–1 μg/kg | 1 h | ddY mice | n/a | [54] |
| 11 | Butylbenzene | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | n/a | [54] |
| 12 | Cuminaldehyde | Oral | 6 mg/kg | Twice per day for 4 weeks | Wistar rats | n/a | [60] |
| 13 | Eugenol | Intraperitoneal injection | 0.01–1 μg/kg | 1 h | ddY mice | n/a | [61] |
| 14 | Isoeugenol | Inhalation | 4.5 × 10−5–4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [61] |
| 15 | Safrole | Inhalation | 4.5 × 10−5–4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [61] |
| 16 | Vanillin | Intraperitoneal injection | 0.01–1 μg/kg | 1 h | ddY mice | n/a | [61] |
| 17 | Cinnamaldehyde | Mix with diet | 2, 4 mg/kg of bw | 19 days | Holsteinian dairy cows | n/a | [62] |
| 18 | Elemicin | Inhalation | 7.4 × 10−10–7.4 × 10−8 mg/cm3 | 1 h | ddY mice | n/a | [65] |
| 19 | Estragole | Inhalation | 4.5 × 10−5–4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 20 | Coumarin | Inhalation | 4.5 × 10−5–4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 21 | p-Anisaldehyde | Inhalation | 4.5 × 10−5–4.5 × 10−3 mg/cage | 1 h | ddY mice | n/a | [67] |
| 22 | β-Ionone epoxide | Mix with diet | 20, 40, 80 mg/kg bw/day | 90 days | Sprague Dawley rats | n/a | [71] |
| No. | Essential Oil | Major Compounds | Route of Administration | Dose | Duration | Species | Effect on Appetite | Mechanism | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Lavender essential oil | Linalool 1,8-Cineole (Eucalyptol) Camphor | Inhalation | 100,000× dilution in water | 15 min/day, 33 days | Wistar rats | Increase | Decrease WAT, BAT, and adrenal SNA; increase gastric PSNA | [37] |
| Oral | 2000 mg/kg | 21 days | Swiss albino mice | No effect | n/a | [64] | |||
| Mix with diet | 250 mg/kg diet | 14 weeks | Lohmann LSL-Lite laying hens | No effect | n/a | [73] |
| No. | Fragrant Compounds | Route of Administration | Dose | Duration | Model | Effects on Appetite | Mechanism | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Benzylacetone | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | Increase | Increase NPY mRNA expression | [54] |
| Intraperitoneal injection | 0.01–1 μg/kg | 1 h | ddY mice | No effect | n/a | [54] | ||
| Inhalation | 7.4 × 10−8–7.4 × 10−2 mg/cm3 | 5–60 min | ddY mice | Increase | n/a | [55] | ||
| Inhalation | 7.4 × 10−8–7.4 × 10−2 mg/cm3 | 5–60 min | ddY mice | Increase | n/a | [65] | ||
| Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | Increase | n/a | [67] | ||
| 2 | Eugenol | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | Increase | n/a | [53] |
| Inhalation | 4.5 × 10−4–2.5 × 10−3 mg/cage | 1 h | ddY mice | Increase | n/a | [61] | ||
| Intraperitoneal injection | 0.01–1 μg/kg | 1 h | ddY mice | No effect | n/a | [61] | ||
| Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | Increase | n/a | [67] | ||
| 3 | trans-Anethole | Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | Increase | n/a | [53] |
| Oral | 250 mg/kg | 10 weeks | Wistar rats | Decrease | n/a | [63] | ||
| Inhalation | 4.5 × 10−4 mg/cage | 1 h | ddY mice | Increase | n/a | [67] | ||
| 4 | Vanillin | Inhalation | 4.5 × 10−5–4.5 × 10−3 mg/cage | 1 h | ddY mice | Increase | n/a | [61] |
| Intraperitoneal injection | 0.01–1 μg/kg | 1 h | ddY mice | No effect | n/a | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.P.K.; Tran, K.N.; Nguyen, L.T.H.; Shin, H.-M.; Yang, I.-J. Effects of Essential Oils and Fragrant Compounds on Appetite: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7962. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24097962
Nguyen NPK, Tran KN, Nguyen LTH, Shin H-M, Yang I-J. Effects of Essential Oils and Fragrant Compounds on Appetite: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(9):7962. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24097962
Chicago/Turabian StyleNguyen, Nhi Phuc Khanh, Khoa Nguyen Tran, Ly Thi Huong Nguyen, Heung-Mook Shin, and In-Jun Yang. 2023. "Effects of Essential Oils and Fragrant Compounds on Appetite: A Systematic Review" International Journal of Molecular Sciences 24, no. 9: 7962. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24097962