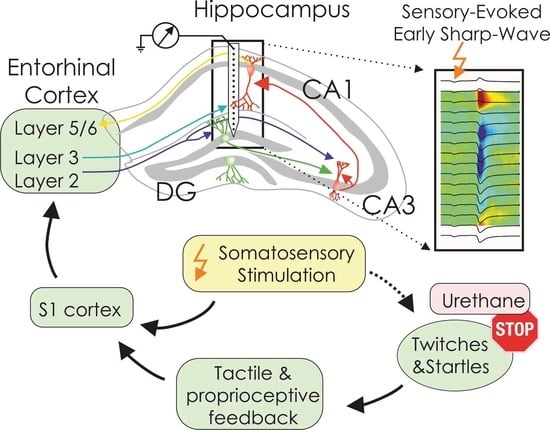
Somatosensory-Evoked Early Sharp Waves in the Neonatal Rat Hippocampus
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cossart, R.; Khazipov, R. How development sculpts hippocampal circuits and function. Physiol. Rev. 2022, 102, 343–378. [Google Scholar] [CrossRef]
- Leinekugel, X.; Khazipov, R.; Cannon, R.; Hirase, H.; Ben Ari, Y.; Buzsaki, G. Correlated bursts of activity in the neonatal hippocampus in vivo. Science 2002, 296, 2049–2052. [Google Scholar] [CrossRef]
- Marguet, S.L.; Le-Schulte, V.T.; Merseburg, A.; Neu, A.; Eichler, R.; Jakovcevski, I.; Ivanov, A.; Hanganu-Opatz, I.L.; Bernard, C.; Morellini, F.; et al. Treatment during a vulnerable developmental period rescues a genetic epilepsy. Nat. Med. 2015, 21, 1436–1444. [Google Scholar] [CrossRef]
- Mohns, E.J.; Blumberg, M.S. Synchronous bursts of neuronal activity in the developing hippocampus: Modulation by active sleep and association with emerging gamma and theta rhythms. J. Neurosci. 2008, 28, 10134–10144. [Google Scholar] [CrossRef]
- Mohns, E.J.; Karlsson, K.A.; Blumberg, M.S. Developmental emergence of transient and persistent hippocampal events and oscillations and their association with infant seizure susceptibility. Eur. J. Neurosci. 2007, 26, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Valeeva, G.; Janackova, S.; Nasretdinov, A.; Rychkova, V.; Makarov, R.; Holmes, G.L.; Khazipov, R.; Lenck-Santini, P.P. Emergence of Coordinated Activity in the Developing Entorhinal-Hippocampal Network. Cereb. Cortex 2019, 29, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 2015, 25, 1073–1188. [Google Scholar] [CrossRef]
- Karlsson, K.A.; Mohns, E.J.; di Prisco, G.V.; Blumberg, M.S. On the co-occurrence of startles and hippocampal sharp waves in newborn rats. Hippocampus 2006, 16, 959–965. [Google Scholar] [CrossRef]
- Leprince, E.; Dard, R.F.; Mortet, S.; Filippi, C.; Giorgi-Kurz, M.; Bourboulou, R.; Lenck-Santini, P.P.; Picardo, M.A.; Bocchio, M.; Baude, A.; et al. Extrinsic control of the early postnatal CA1 hippocampal circuits. Neuron 2023, 111, 888–902.e8. [Google Scholar] [CrossRef]
- Dard, R.F.; Leprince, E.; Denis, J.; Rao Balappa, S.; Suchkov, D.; Boyce, R.; Lopez, C.; Giorgi-Kurz, M.; Szwagier, T.; Dumont, T.; et al. The rapid developmental rise of somatic inhibition disengages hippocampal dynamics from self-motion. eLife 2022, 11, e78116. [Google Scholar] [CrossRef] [PubMed]
- Inacio, A.R.; Nasretdinov, A.; Lebedeva, J.; Khazipov, R. Sensory feedback synchronizes motor and sensory neuronal networks in the neonatal rat spinal cord. Nat. Commun. 2016, 7, 13060. [Google Scholar] [CrossRef] [PubMed]
- Petersson, P.; Waldenstrom, A.; Fahraeus, C.; Schouenborg, J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature 2003, 424, 72–75. [Google Scholar] [CrossRef]
- Akhmetshina, D.; Nasretdinov, A.; Zakharov, A.; Valeeva, G.; Khazipov, R. The Nature of the Sensory Input to the Neonatal Rat Barrel Cortex. J. Neurosci. 2016, 36, 9922–9932. [Google Scholar] [CrossRef] [PubMed]
- Dooley, J.C.; Glanz, R.M.; Sokoloff, G.; Blumberg, M.S. Self-Generated Whisker Movements Drive State-Dependent Sensory Input to Developing Barrel Cortex. Curr. Biol. 2020, 30, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Khazipov, R.; Sirota, A.; Leinekugel, X.; Holmes, G.L.; Ben Ari, Y.; Buzsaki, G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 2004, 432, 758–761. [Google Scholar] [CrossRef]
- McVea, D.A.; Mohajerani, M.H.; Murphy, T.H. Voltage-sensitive dye imaging reveals dynamic spatiotemporal properties of cortical activity after spontaneous muscle twitches in the newborn rat. J. Neurosci. 2012, 32, 10982–10994. [Google Scholar] [CrossRef]
- Colonnese, M.T.; Kaminska, A.; Minlebaev, M.; Milh, M.; Bloem, B.; Lescure, S.; Moriette, G.; Chiron, C.; Ben-Ari, Y.; Khazipov, R. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron 2010, 67, 480–498. [Google Scholar] [CrossRef]
- Khazipov, R.; Milh, M. Early patterns of activity in the developing cortex: Focus on the sensorimotor system. Semin. Cell Dev. Biol. 2018, 76, 120–129. [Google Scholar] [CrossRef]
- Khazipov, R.; Luhmann, H.J. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006, 29, 414–418. [Google Scholar] [CrossRef]
- Luhmann, H.J.; Khazipov, R. Neuronal activity patterns in the developing barrel cortex. Neuroscience 2018, 368, 256–267. [Google Scholar] [CrossRef]
- Mohns, E.J.; Blumberg, M.S. Neocortical activation of the hippocampus during sleep in infant rats. J. Neurosci. 2010, 30, 3438–3449. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G.; Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Bellistri, E.; Aguilar, J.; Brotons-Mas, J.R.; Foffani, G.; de la Prida, L.M. Basic properties of somatosensory-evoked responses in the dorsal hippocampus of the rat. J. Physiol. 2013, 591, 2667–2686. [Google Scholar] [CrossRef]
- Brankack, J.; Buzsaki, G. Hippocampal responses evoked by tooth pulp and acoustic stimulation: Depth profiles and effect of behavior. Brain Res. 1986, 378, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Deadwyler, S.A.; West, M.O.; Robinson, J.H. Entorhinal and septal inputs differentially control sensory-evoked responses in the rat dentate gyrus. Science 1981, 211, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Krupic, J. Do hippocampal pyramidal cells respond to nonspatial stimuli? Physiol. Rev. 2021, 101, 1427–1456. [Google Scholar] [CrossRef]
- Pereira, A.; Ribeiro, S.; Wiest, M.; Moore, L.C.; Pantoja, J.; Lin, S.C.; Nicolelis, M.A. Processing of tactile information by the hippocampus. Proc. Natl. Acad. Sci. USA 2007, 104, 18286–18291. [Google Scholar] [CrossRef]
- Vinogradova, O.S. Hippocampus as comparator: Role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus 2001, 11, 578–598. [Google Scholar] [CrossRef]
- Valeeva, G.; Nasretdinov, A.; Rychkova, V.; Khazipov, R. Bilateral Synchronization of Hippocampal Early Sharp Waves in Neonatal Rats. Front. Cell. Neurosci. 2019, 13, 29. [Google Scholar] [CrossRef]
- Minlebaev, M.; Colonnese, M.; Tsintsadze, T.; Sirota, A.; Khazipov, R. Early gamma oscillations synchronize developing thalamus and cortex. Science 2011, 334, 226–229. [Google Scholar] [CrossRef]
- Tiriac, A.; Uitermarkt, B.D.; Fanning, A.S.; Sokoloff, G.; Blumberg, M.S. Rapid whisker movements in sleeping newborn rats. Curr. Biol. 2012, 22, 2075–2080. [Google Scholar] [CrossRef]
- Yang, J.W.; An, S.; Sun, J.J.; Reyes-Puerta, V.; Kindler, J.; Berger, T.; Kilb, W.; Luhmann, H.J. Thalamic Network Oscillations Synchronize Ontogenetic Columns in the Newborn Rat Barrel Cortex. Cereb. Cortex 2013, 23, 1299–1316. [Google Scholar] [CrossRef]
- Yang, J.W.; Hanganu-Opatz, I.L.; Sun, J.J.; Luhmann, H.J. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J. Neurosci. 2009, 29, 9011–9025. [Google Scholar] [CrossRef]
- Unichenko, P.; Yang, J.W.; Luhmann, H.J.; Kirischuk, S. Glutamatergic system controls synchronization of spontaneous neuronal activity in the murine neonatal entorhinal cortex. Pflug. Arch. 2015, 467, 1565–1575. [Google Scholar] [CrossRef]
- Bragin, A.; Jando, G.; Nadasdy, Z.; van Landeghem, M.; Buzsaki, G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J. Neurophysiol. 1995, 73, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Doischer, D.; Hosp, J.A.; Yanagawa, Y.; Obata, K.; Jonas, P.; Vida, I.; Bartos, M. Postnatal differentiation of basket cells from slow to fast signaling devices. J. Neurosci. 2008, 28, 12956–12968. [Google Scholar] [CrossRef] [PubMed]
- Khazipov, R.; Minlebaev, M.; Valeeva, G. Early gamma oscillations. Neuroscience 2013, 250, 240–252. [Google Scholar] [CrossRef]
- Le, M.C.; Monyer, H. GABAergic interneurons shape the functional maturation of the cortex. Neuron 2013, 77, 388–405. [Google Scholar]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal GABAergic Inhibitory Interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef]
- Ben Ari, Y.; Gaiarsa, J.L.; Tyzio, R.; Khazipov, R. GABA: A Pioneer Transmitter That Excites Immature Neurons and Generates Primitive Oscillations. Physiol. Rev. 2007, 87, 1215–1284. [Google Scholar] [CrossRef]
- Murata, Y.; Colonnese, M.T. GABAergic interneurons excite neonatal hippocampus in vivo. Sci. Adv. 2020, 6, eaba1430. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.; Zhang, C.; Marguet, S.L.; Herrmann, T.; Flossmann, T.; Hinsch, R.; Rahmati, V.; Guenther, M.; Frahm, C.; Urbach, A.; et al. A limited role of NKCC1 in telencephalic glutamatergic neurons for developing hippocampal network dynamics and behavior. Proc. Natl. Acad. Sci. USA 2021, 118, e2014784118. [Google Scholar] [CrossRef] [PubMed]
- Donato, F.; Jacobsen, R.I.; Moser, M.B.; Moser, E.I. Stellate cells drive maturation of the entorhinal-hippocampal circuit. Science 2017, 355, eaai8178. [Google Scholar] [CrossRef] [PubMed]
- Kasyanov, A.M.; Safiulina, V.F.; Voronin, L.L.; Cherubini, E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 3967–3972. [Google Scholar] [CrossRef]
- Durand, G.M.; Kovalchuk, Y.; Konnerth, A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature 1996, 381, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Groc, L.; Petanjek, Z.; Gustafsson, B.; Ben Ari, Y.; Hanse, E.; Khazipov, R. In vivo blockade of neural activity alters dendritic development of neonatal CA1 pyramidal cells. Eur. J. Neurosci. 2002, 16, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Khazipov, R.; Leinekugel, X.; Caillard, O.; Gaïarsa, J.-L. GABAA, NMDA and AMPA receptors: A developmentally regulated ‘ménage a trois’. Trends Neurosci. 1997, 20, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Leinekugel, X.; Medina, I.; Khalilov, I.; Ben-Ari, Y.; Khazipov, R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABAA and NMDA receptors in the neonatal hippocampus. Neuron 1997, 18, 243–255. [Google Scholar] [CrossRef]
- Khazipov, R.; Zaynutdinova, D.; Ogievetsky, E.; Valeeva, G.; Mitrukhina, O.; Manent, J.B.; Represa, A. Atlas of the Postnatal Rat Brain in Stereotaxic Coordinates. Front. Neuroanat. 2015, 9, 161. [Google Scholar] [CrossRef]
- Freeman, J.A.; Nicholson, C. Experimental optimization of current source-density technique for anuran cerebellum. J. Neurophysiol. 1975, 38, 369–382. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gainutdinov, A.; Shipkov, D.; Sintsov, M.; Fabrizi, L.; Nasretdinov, A.; Khazipov, R.; Valeeva, G. Somatosensory-Evoked Early Sharp Waves in the Neonatal Rat Hippocampus. Int. J. Mol. Sci. 2023, 24, 8721. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24108721
Gainutdinov A, Shipkov D, Sintsov M, Fabrizi L, Nasretdinov A, Khazipov R, Valeeva G. Somatosensory-Evoked Early Sharp Waves in the Neonatal Rat Hippocampus. International Journal of Molecular Sciences. 2023; 24(10):8721. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24108721
Chicago/Turabian StyleGainutdinov, Azat, Dmitrii Shipkov, Mikhail Sintsov, Lorenzo Fabrizi, Azat Nasretdinov, Roustem Khazipov, and Guzel Valeeva. 2023. "Somatosensory-Evoked Early Sharp Waves in the Neonatal Rat Hippocampus" International Journal of Molecular Sciences 24, no. 10: 8721. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24108721