Unraveling the Antioxidant Activity of 2R,3R-dihydroquercetin
Abstract
:1. Introduction
2. Results
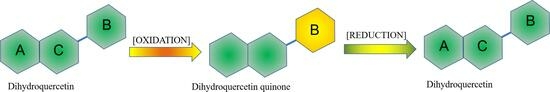
2.1. Oxidation of 2R,3R-DHQ by H2O2 and HRP Does Not Results in the Formation of Quercetin
2.2. Trapping the Quinone Formed in the Oxidation of 2R,3R-DHQ by GSH
2.3. Ascorbate Regenerates 2R,3R-DHQ Quinone to 2R,3R-DHQ
2.4. The HOMO/LUMO Energy and the Dual Descriptor of DHQ Quinone
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. 2R,3R-DHQ Oxidation
4.3. HPLC Analysis
4.4. LCMS-ESI-IT-TOF/MS and MS/MS Analysis
4.5. The HOMO/LUMO Energy and the Dual Descriptor of DHQ Quinone
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Zhang, M.; Haenen, G.R.M.M.; Vervoort, L.; Moalin, M. Flavonoids seen through the energy perspective. Int. J. Mol. Sci. 2021, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.L.E.; Brock, A.E.; F.Grant, F. Long range correlation in redox potential fluctuations signals energetic efficiency of bacterial Fe(II Oxidation). Sci. Rep. 2019, 9, 4018. [Google Scholar]
- Khongkarat, P.; Phuwapraisirisan, P.; Chanchao, C. Phytochemical content, especially spermidine derivatives, presenting antioxidant and antilipoxygenase activities in thai bee pollens. PeerJ 2022, 10, 13506. [Google Scholar] [CrossRef] [PubMed]
- Biro, P.A.; Thomas, F.; Ujvari, B.; Beckmann, C. A novel perspective suggesting high sustained energy expenditure may be net protective against cancer. Evol. Med. Public Health 2022, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Alshabi, A.M.; Shaikh, I.A. Antidiabetic and antioxidant potential of gardenia latifolia in type-2 diabetic rats fed with high-fat diet plus low-dose streptozotocin. Saudi Med. J. 2022, 43, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef]
- Benhar, M. Oxidants, Antioxidants and thiol redox switches in the control of regulated cell death pathways. Antioxidants 2020, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, A.; Maghiar, T.; Alaya, A.; Olah, N.; Turcus, V.; Pelea, D.; Totolici, B.D.; Neamtu, C.; Maghiar, A.M.; Mathe, E. A Comprehensive view on the quercetin impact on colorectal cancer. Molecules 2022, 27, 1873. [Google Scholar] [CrossRef]
- Terao, J. Potential role of quercetin glycosides as anti-Atherosclerotic food-derived factors for human health. Antioxidants 2023, 12, 258. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From theory to practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Yamaguchi, S.; Shirai, M.; Miyoshi, M.; Moon, J.H.; Oshima, S.; Inakuma, T.; Tsushida, T.; Kato, Y. Protection by quercetin and quercetin 3-O-beta-D-glucuronide of peroxynitrite-induced antioxidant consumption in human plasma low-density lipoprotein. Free Radic. Res. 2001, 35, 925–931. [Google Scholar] [CrossRef]
- Guo, X.D.; Zhang, D.Y.; Gao, X.J.; Parry, J.; Liu, K.; Liu, B.L.; Wang, M. Quercetin and quercetin-3-O-glucuronide are equally effective in ameliorating endothelial insulin resistance through inhibition of reactive oxygen species-associated inflammation. Mol. Nutr. Food Res. 2013, 57, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Anders, M.W. Putting bioactivation reactions to work: Targeting antioxidants to mitochondria. Chem. Biol. Interact. 2011, 192, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rogozhin, V.V.; Peretolchin, D.V. Kinetic regulations of dihydroquercetin oxidation with horseradish peroxide. Bioorg. Khim. 2009, 35, 640–645. [Google Scholar] [CrossRef]
- Biler, M.; Biedermann, D.; Valentová, K.; Křen, V.; Kubala, M. Quercetin and its analogues: Optical and acido-basic properties. Phys. Chem. Chem. Phys. 2017, 19, 26870–26879. [Google Scholar] [CrossRef]
- Sokolová, R.; Ramešová, Š.; Kocábová, J.; Kolivoška, V.; Degano, I.; Pitzalis, E. On the difference in decomposition of taxifolin and luteolin vs. fisetin and quercetin in aqueous media. Monats. Chem. 2016, 147, 1375–1383. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa, D.C.A.; Fuentes, J. Revisiting the oxidation of flavonoids: Loss, conservation or enhancement of their antioxidant properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Choudhary, N.; Tewari, D.; El-Demerdash, A.; Horbanczuk, O.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; et al. Quercetin: Total-scale literature landscape analysis of a valuable nutraceutical with numerous potential applications in the promotion of human and animal health—A review. Anim. Sci. Pap. Rep. 2021, 3, 39. [Google Scholar]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y.X. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Zhang, M.; Vervoort, L.; Moalin, M.; Mommers, A.; Douny, C.; den Hartog, G.J.M.; Haenen, G.R.M.M. The chemical reactivity of (-)-epicatechin quinone mainly resides in its B-ring. Free Radic. Biol. Med. 2018, 124, 31–39. [Google Scholar] [CrossRef]
- AW Boots, N.K.G.H. Oxidized quercetin reacts with thiols rather than with ascorbate: Implication for quercetin supplementation. Biochem. Bioph. Res. Commun. 2003, 308, 560–565. [Google Scholar] [CrossRef]
- Lin, L.; Wu, H.; Li, W.; Chen, W.; Lee, Y.; Wu, D.C.; Li, P.; Yeh, A. Kinetic studies of the oxidation of quercetin, rutin and taxifolin in the basic medium by (ethylenediaminetetraacetato) cobalt (III) complex. Inorg. Chem. Commun. 2010, 13, 633–635. [Google Scholar] [CrossRef]
- Li, Y.; Su, H.; Yin, Z.P.; Li, J.E.; Yuan, E.; Zhang, Q.F. Metabolism, tissue distribution and excretion of taxifolin in rat. Biomed. Pharmacother. 2022, 150, 112959. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, L.N.; Theander, O. Cis- and trans-dihydroquercetin glucosides from needles of Pinus sylvestris. Phytochemistry 1988, 27, 829–832. [Google Scholar] [CrossRef]
- Kiehlmann, E.; Li, E.P.M. Isomerization of dihydroquercetin. J. Nat. Product. 1995, 584, 450–455. [Google Scholar] [CrossRef]
- Osorio, E.; Pérez, E.G.; Areche, C.; Ruiz, L.M.; Cassels, B.K.; Flórez, E.; Tiznado, W.X. Why is quercetin a better antioxidant than taxifolin? Theoretical study of mechanisms involving activated forms. J. Mol. Model. 2013, 19, 2165–2172. [Google Scholar] [CrossRef]
- Chen, S.; Li, C. Detection and characterization of catechol quinone-derived protein adducts using biomolecular mass spectrometry. Front. Chem. 2019, 7, 571. [Google Scholar] [CrossRef]
- Stefan, B.; Thomas, R. Oxidation with a “Stopover”—Stable zwitterions as Intermediates in the oxidation of α-tocopherol (vitamin E) model compounds to their corresponding ortho-quinone methides. ChemistryOpen 2021, 10, 421–429. [Google Scholar]
- Daussin, F.N.; Heyman, E.; Burelle, Y. Effects of (-)-epicatechin on mitochondria. Nutr. Rev. 2021, 79, 25–41. [Google Scholar] [CrossRef]
- Apyari, V.V.; Furletov, A.A.; Kalinin, V.I.; Dmitrienko, S.G.; Zolotov, Y.A. A three-reagent “Green” paper-based analytical device for solid-phase spectrometric and colorimetric determination of dihydroquercetin. Sensors 2022, 22, 2893. [Google Scholar] [CrossRef]
- Liu, H.; Cao, M.; Jin, Y.; Jia, B.; Wang, L.; Dong, M.; Han, L.; Abankwah, J.; Liu, J.; Zhou, T.; et al. Network pharmacology and experimental validation to elucidate the pharmacological mechanisms of Bushen Huashi decoction against kidney stones. Front. Endocrinol. 2023, 14, 1031895. [Google Scholar] [CrossRef]
- Li, Z.; Moalin, M.; Zhang, M.; Vervoort, L.; Mommers, A.; Haenen, G.R.M.M. Delocalization of the unpaired electron in the quercetin radical: Comparison of experimental ESR data with DFT calculations. Int. J. Mol. Sci. 2020, 21, 2033. [Google Scholar] [CrossRef]
- Li, Z.; Moalin, M.; Zhang, M.; Vervoort, L.; Hursel, E.; Mommers, A.; Haenen, G.R.M.M. The flow of the redox energy in quercetin during its antioxidant activity in water. Int. J. Mol. Sci. 2020, 21, 6015. [Google Scholar] [CrossRef]
- Joyner, P.M. Protein adducts and protein oxidation as molecular mechanisms of flavonoid bioactivity. Molecules 2021, 26, 5102. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Hobbs, A.J. A comparison of the chemical biology of hydropersulfides (RSSH) with other protective biological antioxidants and nucleophiles. Nitric Oxide-Biol. Chem. 2021, 107, 46–57. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 5, 1–52. [Google Scholar] [CrossRef]
- Sthijns, M.M.J.P.; Schiffers, P.M.; Janssen, G.M.; Lemmens, K.J.A.; Ides, B.; Vangrieken, P.; Bouwman, F.G.; Mariman, E.C.; Pader, I.; Arnér, E.S.J.; et al. Rutin protects against H2O2-triggered impaired relaxation of placental arterioles and induces Nrf2-mediated adaptation in human umbilical vein endothelial cells exposed to oxidative stress. BBA-GEN Subj. 2017, 1861, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wen, E.; Upur, H.; Tian, S. High performance liquid chromatography-diode array detector method for the simultaneous determination of five compounds in the pulp and seed of sea buckthorn. Pharmacogn. Mag. 2017, 13, 136. [Google Scholar]
- Fan, L.; Qu, X.; Yi, T.; Peng, Y.; Jiang, M.; Miao, J.; Xiao, P. Metabolomics of the protective effect of ampelopsis grossedentata and its major active compound dihydromyricetin on the liver of high-fat diet hamster. Evid-Based Compl. Alt. 2020, 2020, 3472578. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Xu, F.; Li, H.; Wang, Y.; Li, F.; Shang, M.; Liu, G.; Wang, X.; Cai, S. Detection of 191 taxifolin metabolites and their distribution in rats using HPLC-ESI-IT-TOF-MSn. Molecules 2016, 21, 1209. [Google Scholar] [CrossRef]
- Young Jeong, J.; Atikul Islam, M.; Hong, J.H.; Hyeon Son, J.; Yeon Song, O.; Khan, N.; Jamila, N.; Kim, K.S. Determination of bioactive markers for the discrimination of syneilesis palmata and paris verticillata by high performance liquid chromatography (HPLC) with diode array detection (DAD) and ion trap time-of-flight mass spectrometry (IT-TOF-MS). Anal. Lett. 2021, 54, 2590–2599. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.E. Gaussian 09 Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009; Volume 32, pp. 5648–5652. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Tian, L.; Qinxue, C. Realization of conceptual density functional theory and information-theoretic approach in multiwfn program. In Conceptual Density Functional Theory; Wiley-Vch Gmbh: Weinheim, Germany, 2022; pp. 631–647. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, Z.; Wang, Y.; Li, C.; Zhang, M.; Chen, H.; Chen, W.; Zhong, Q.; Pei, J.; Chen, W.; et al. Unraveling the Antioxidant Activity of 2R,3R-dihydroquercetin. Int. J. Mol. Sci. 2023, 24, 14220. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms241814220
Xu Y, Li Z, Wang Y, Li C, Zhang M, Chen H, Chen W, Zhong Q, Pei J, Chen W, et al. Unraveling the Antioxidant Activity of 2R,3R-dihydroquercetin. International Journal of Molecular Sciences. 2023; 24(18):14220. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms241814220
Chicago/Turabian StyleXu, Yaping, Zhengwen Li, Yue Wang, Chujie Li, Ming Zhang, Haiming Chen, Wenxue Chen, Qiuping Zhong, Jianfei Pei, Weijun Chen, and et al. 2023. "Unraveling the Antioxidant Activity of 2R,3R-dihydroquercetin" International Journal of Molecular Sciences 24, no. 18: 14220. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms241814220