Electrochemical Activation and Its Prolonged Effect on the Durability of Bimetallic Pt-Based Electrocatalysts for PEMFCs
Abstract
:1. Introduction
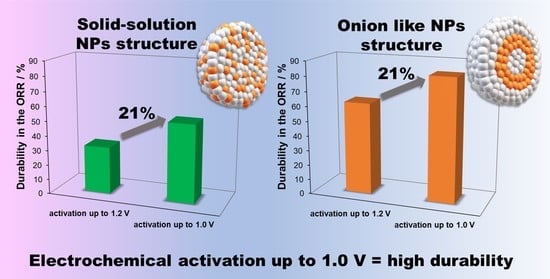
2. Results and Discussion
3. Materials and Methods
3.1. The Synthesis of the PtCu/C Catalysts
3.2. The Study of the Catalysts’ Structure
3.3. The Electrochemical Study of the Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, J.; Chen, M.; Zhao, Z.; Zhang, Z.; Ye, S.; Xu, S.; Wang, H.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P.; Ortiz-Herrera, J.; López-Chávez, E.; Solorza-Feria, O.; Medina, D. Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- Guo, N.; Xue, H.; Bao, A.; Wang, Z.; Sun, J.; Song, T.; Ge, X.; Zhang, W.; Huang, K.; He, F.; et al. Achieving Superior Electrocatalytic Performance by Surface Copper Vacancy Defects during Electrochemical Etching Process. Angew. Chem. Int. Ed. 2020, 59, 13778–13784. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; Farina, F.; Stievano, L.; Jones, D.J.; Rozière, J.; Cavaliere, S. Preparation of Ni@Pt core@shell conformal nanofibre oxygen reduction electrocatalysts via microwave-assisted galvanic displacement. Catal. Sci. Technol. 2019, 9, 6920–6928. [Google Scholar] [CrossRef]
- Wang, D.; Xin, H.L.; Hovden, R.; Wang, H.; Yu, Y.; Muller, D.A.; Disalvo, F.J.; Abruña, H.D. Structurally ordered intermetallic platinum–cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Sun, X.; Li, C.; Bai, J. Efficient electrocatalyst of Pt–Fe/CNFs for oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2020, 45, 15112–15120. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuttiyiel, K.A.; Adzic, R.R. Designing high performance Pt monolayer core–shell electrocatalysts for fuel cells. Curr. Opin. Electrochem. 2020, 21, 368–375. [Google Scholar] [CrossRef]
- Lyu, X.; Jia, Y.; Mao, X.; Li, D.; Li, G.; Zhuang, L.; Wang, X.; Yang, D.; Wang, Q.; Du, A.; et al. Gradient-Concentration Design of Stable Core–Shell Nanostructure for Acidic Oxygen Reduction Electrocatalysis. Adv. Mater. 2020, 32, e2003493. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Guterman, V.E.; Belenov, S.V.; Menshikov, V.S.; Tabachkova, N.Y.; Safronenko, O.I.; Moguchikh, E.A. Pt/C electrocatalysts based on the nanoparticles with the gradient structure. Int. J. Hydrogen Energy 2018, 43, 3676–3687. [Google Scholar] [CrossRef]
- Wu, J.; Gross, A.; Yang, H. Shape and Composition-Controlled Platinum Alloy Nanocrystals Using Carbon Monoxide as Reducing Agent. Nano Lett. 2011, 11, 798–802. [Google Scholar] [CrossRef]
- Bu, L.; Guo, S.; Zhang, X.; Shen, X.; Su, D.; Lu, G.; Zhu, X.; Yao, J.; Guo, J.; Huang, X. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis. Nat. Commun. 2016, 7, 11850. [Google Scholar] [CrossRef] [Green Version]
- Pavlets, A.; Alekseenko, A.; Tabachkova, N.Y.; Safronenko, O.; Nikulin, A.Y.; Alekseenko, D.; Guterman, V. A novel strategy for the synthesis of Pt–Cu uneven nanoparticles as an efficient electrocatalyst toward oxygen reduction. Int. J. Hydrogen Energy 2021, 46, 5355–5368. [Google Scholar] [CrossRef]
- Cai, X.; Lin, R.; Shen, D.; Zhu, Y. Gram-Scale Synthesis of Well-Dispersed Shape-Controlled Pt−Ni/C as High-Performance Catalysts for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2019, 11, 29689–29697. [Google Scholar] [CrossRef]
- Hu, B.; Yuan, J.; Zhang, J.; Shu, Q.; Guan, D.; Yang, G.; Zhou, W.; Shao, Z. High activity and durability of a Pt–Cu–Co ternary alloy electrocatalyst and its large-scale preparation for practical proton exchange membrane fuel cells. Compos. Part B Eng. 2021, 222, 109082. [Google Scholar] [CrossRef]
- Moriau, L.J.; Hrnjić, A.; Pavlišič, A.; Kamšek, A.R.; Petek, U.; Ruiz-Zepeda, F.; Šala, M.; Pavko, L.; Šelih, V.S.; Bele, M.; et al. Resolving the nanoparticles’ structure-property relationships at the atomic level: A study of Pt-based electrocatalysts. Iscience 2021, 24, 102102. [Google Scholar] [CrossRef]
- Jovanovič, P.; Šelih, V.S.; Šala, M.; Hočevar, S.B.; Pavlišič, A.; Gatalo, M.; Bele, M.; Ruiz-Zepeda, F.; Čekada, M.; Hodnik, N.; et al. Electrochemical in-situ dissolution study of structurally ordered, disordered and gold doped PtCu3 nanoparticles on carbon composites. J. Power Sources 2016, 327, 675–680. [Google Scholar] [CrossRef]
- Alekseenko, A.; Pavlets, A.; Belenov, S.; Safronenko, O.; Pankov, I.; Guterman, V. The electrochemical activation mode as a way to exceptional ORR performance of nanostructured PtCu/C materials. Appl. Surf. Sci. 2022, 595, 153533. [Google Scholar] [CrossRef]
- Pavlets, A.; Alekseenko, A.; Nikolskiy, A.; Kozakov, A.; Safronenko, O.; Pankov, I.; Guterman, V. Effect of the PtCu/C electrocatalysts initial composition on their activity in the de-alloyed state in the oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 30460–30471. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Y.; Xin, H.L.; Hovden, R.; Ercius, P.; Mundy, J.A.; Chen, H.; Richard, J.H.; Muller, D.A.; DiSalvo, F.J.; et al. Tuning Oxygen Reduction Reaction Activity via Controllable Dealloying: A Model Study of Ordered Cu3Pt/C Intermetallic Nanocatalysts. Nano Lett. 2012, 12, 5230–5238. [Google Scholar] [CrossRef]
- Belenov, S.; Alekseenko, A.; Pavlets, A.; Nevelskaya, A.; Danilenko, M. Architecture Evolution of Different Nanoparticles Types: Relationship between the Structure and Functional Properties of Catalysts for PEMFC. Catalysts 2022, 12, 638. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kim, D.-G.; Jang, I.; Kim, N.D.; Yoo, S.J.; Kim, P. Synthesis of hollow structured PtNi/Pt core/shell and Pt-only nanoparticles via galvanic displacement and selective etching for efficient oxygen reduction reaction. J. Ind. Eng. Chem. 2022, 111, 300–307. [Google Scholar] [CrossRef]
- Gatalo, M.; Moriau, L.; Petek, U.; Ruiz-Zepeda, F.; Šala, M.; Grom, M.; Galun, T.; Jovanovič, P.; Pavlišič, A.; Bele, M.; et al. CO-assisted ex-situ chemical activation of Pt-Cu/C oxygen reduction reaction electrocatalyst. Electrochimica Acta 2019, 306, 377–386. [Google Scholar] [CrossRef]
- Menshchikov, V.; Alekseenko, A.; Guterman, V.; Nechitailov, A.; Glebova, N.; Tomasov, A.; Spiridonova, O.; Belenov, S.; Zelenina, N.; Safronenko, O. Effective Platinum-Copper Catalysts for Methanol Oxidation and Oxygen Reduction in Proton-Exchange Membrane Fuel Cell. Nanomaterials 2020, 10, 742. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Yu, Y.; Zhu, J.; Liu, S.; Muller, D.A.; Abruña, H.D. Morphology and Activity Tuning of Cu3Pt/C Ordered Intermetallic Nanoparticles by Selective Electrochemical Dealloying. Nano Lett. 2015, 15, 1343–1348. [Google Scholar] [CrossRef]
- Gatalo, M.; Jovanovic, P.; Ruiz-Zepeda, F.; Pavlišič, A.; Robba, A.; Bale, M.; Dražič, G.; Gaberšček, M.; Hodnik, N. Insights into electrochemical dealloying of Cu out of Au-doped Pt-alloy nanoparticles at the sub-nano-scale. J. Electrochem. Sci. Eng. 2018, 8, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Barim, S.B.; Bozbag, S.E.; Deljoo, B.; Aindow, M.; Erkey, C. Highly Active Carbon Supported PtCu Electrocatalysts for PEMFCs by in situ Supercritical Deposition Coupled with Electrochemical Dealloying. Fuel Cells 2019, 20, 285–299. [Google Scholar] [CrossRef]
- Yang, T.; Pukazhselvan, D.; da Silva, E.; Santos, M.C.; Meng, L.; Ramasamy, D.; Jothi, S.; Graca, V.; Shi, S. Highly branched Pt Cu nanodandelion with high activity for oxygen reduction reaction. Int. J. Hydrogen Energy 2018, 44, 174–179. [Google Scholar] [CrossRef]
- Pizzutilo, E.; Geiger, S.; Grote, J.-P.; Mingers, A.; Mayrhofer, K.J.J.; Arenz, M.; Cherevko, S. On the Need of Improved Accelerated Degradation Protocols (ADPs): Examination of Platinum Dissolution and Carbon Corrosion in Half-Cell Tests. J. Electrochem. Soc. 2016, 163, F1510–F1514. [Google Scholar] [CrossRef] [Green Version]
- Impagnatiello, A.; Cerqueira, C.F.; Coulon, P.-E.; Morin, A.; Escribano, S.; Guétaz, L.; Clochard, M.-C.; Rizza, G. Degradation Mechanisms of Supported Pt Nanocatalysts in Proton Exchange Membrane Fuel Cells: An Operando Study through Liquid Cell Transmission Electron Microscopy. ACS Appl. Energy Mater. 2020, 3, 2360–2371. [Google Scholar] [CrossRef]
- Khalakhan, I.; Waidhas, F.; Brummel, O.; Vorokhta, M.; Kúš, P.; Yakovlev, Y.V.; Bertram, M.; Dopita, M.; Matolínová, I.; Libuda, J.; et al. Nanoscale Morphological and Structural Transformations of PtCu Alloy Electrocatalysts during Potentiodynamic Cycling. J. Phys. Chem. C 2018, 122, 21974–21982. [Google Scholar] [CrossRef]
- Pavlets, A.; Alekseenko, A.; Menshchikov, V.; Belenov, S.; Volochaev, V.; Pankov, I.; Safronenko, O.; Guterman, V. Influence of Electrochemical Pretreatment Conditions of PtCu/C Alloy Electrocatalyst on Its Activity. Nanomaterials 2021, 11, 1499. [Google Scholar] [CrossRef]
- Ghavidel, M.R.Z.; Monteverde Videla, A.H.A.; Specchia, S.; Easton, E.B. The relationship between the structure and ethanol oxidation activity of Pt-Cu/C alloy catalysts. Electrochim. Acta 2017, 230, 58–72. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, Y.; Liu, J.; Tang, Y.; Wu, C.; Chen, Y. Ordered Mesoporous Carbon Confined Highly Dispersed PtCo Alloy for the Oxygen Reduction Reaction: The Effect of Structure and Composition on Performance. Ind. Eng. Chem. Res. 2021, 60, 14728–14736. [Google Scholar] [CrossRef]
- Pryadchenko, V.V.; Srabionyan, V.V.; Kurzin, A.A.; Bulat, N.V.; Shemet, D.B.; Avakyan, L.A.; Belenov, S.V.; Volochaev, V.A.; Zizak, I.; Guterman, V.E.; et al. Bimetallic PtCu core-shell nanoparticles in PtCu/C electrocatalysts: Structural and electrochemical characterization. Appl. Catal. A Gen. 2016, 525, 226–236. [Google Scholar] [CrossRef]
- DOE Durability Working Group. Rotating Disk-Electrode Aqueous Electrolyte Accelerated Stress Tests for PGM Electrocatalyst/Support Durability Evaluation. Available online: https://www.energy.gov/sites/prod/files/2015/08/f25/fcto_dwg_pgm_electrocatalyst_support_aqueous_ast.pdf (accessed on 25 December 2022).
- Zhu, H.; Cai, Y.; Wang, F.; Gao, P.; Cao, J. Scalable Preparation of the Chemically Ordered Pt–Fe–Au Nanocatalysts with High Catalytic Reactivity and Stability for Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2018, 10, 22156–22166. [Google Scholar] [CrossRef]
- Stariha, S.; Macauley, N.; Sneed, B.T.; Langlois, D.; More, K.L.; Mukundan, R.; Borup, R.L. Recent Advances in Catalyst Accelerated Stress Tests for Polymer Electrolyte Membrane Fuel Cells. J. Electrochem. Soc. 2018, 165, F492–F501. [Google Scholar] [CrossRef]
- Chen, Z.; Liao, Y.; Chen, S. Facile synthesis of platinum-copper aerogels for the oxygen reduction reaction. Energy Mater. 2022, 2, 200033. [Google Scholar] [CrossRef]





| Sample | Mass Fraction of Pt, % | Mass Fraction of Metal, % | Atomic Composition (XRF) |
|---|---|---|---|
| S1 | 19.0 | 27.1 | Pt43Cu57 |
| S2 | 20.4 | 25.1 | Pt56Cu44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlets, A.; Pankov, I.; Alekseenko, A. Electrochemical Activation and Its Prolonged Effect on the Durability of Bimetallic Pt-Based Electrocatalysts for PEMFCs. Inorganics 2023, 11, 45. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11010045
Pavlets A, Pankov I, Alekseenko A. Electrochemical Activation and Its Prolonged Effect on the Durability of Bimetallic Pt-Based Electrocatalysts for PEMFCs. Inorganics. 2023; 11(1):45. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11010045
Chicago/Turabian StylePavlets, Angelina, Ilya Pankov, and Anastasia Alekseenko. 2023. "Electrochemical Activation and Its Prolonged Effect on the Durability of Bimetallic Pt-Based Electrocatalysts for PEMFCs" Inorganics 11, no. 1: 45. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11010045