2.2. Magnetic Properties
The magnetic properties of the polycrystalline Mn
2−xCo
xSb (
x = 0.1, 0.15, 0.2) samples were studied by isofield measurements. Since we are interested in the magnetically ordered phases, we have used the low-temperature option of the vibrating sample magnetometer. The temperature-dependent magnetization curves (
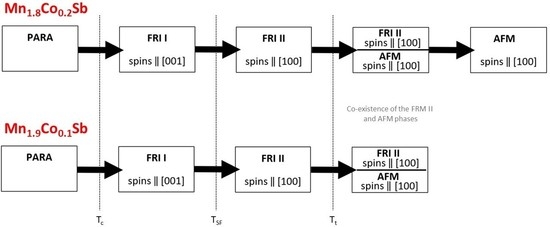
Figure 1) exhibit similar features for all three compositions. At low temperatures we observe a constant magnetization, which increases towards higher temperatures. The temperature dependence features an inflection point for all three compositions between 100 K and 200 K, and then a maximum between 250 K and 325 K. The magnetization is decreased at temperatures approaching 380 K, but the behavior is clearly not paramagnetic. The different response depending on the temperature protocol can be due to small differences in the microstructure of the powder and also due to the metastability. The magnetization increase in the low-temperature region becomes more pronounced with increasing Co content. For all three curves, we observe distinct differences between the cooling and the heating curves. To obtain the accurate phase transition temperatures, the extrema of first derivatives of the magnetization curves d
M/d
T were determined (
Figures S1–S3). In
Table 1, we compare the transition temperatures for different temperature protocols.
The phase transition from the FRI into an AFM state does not occur in pure Mn
2Sb. A thermal hysteresis between the cooling and heating cycles in Mn
2−xCo
xSb (
x = 0.1, 0.15, 0.2) indicates the first-order phase transition. No correlation between the Co content and
Tt was found in this study (
Figure S5).
TSF are slightly higher, while
Tt are slightly lower than the temperatures reported in the literature (
Figures S4 and S5). Again, this can be due to instrumental discrepancies or deviations in the chemical compositions. No correlation between the Co content and the width of the thermal hysteresis (
Thys) was observed. With
Thys = 23 K, Mn
1.85Co
0.15Sb shows the broadest hysteresis, followed by Mn
1.9Co
0.1Sb with
Thys = 17 K. Surprisingly, Mn
1.8Co
0.2Sb shows a very narrow thermal hysteresis with
Thys = 3 K. Nevertheless, this compound is the only one that exhibits a magnetization of ~0 Am
2/kg in the low-temperature region, which indicates the occurrence of AFM phases.
Measurements on an oriented Mn
1.9Co
0.1Sb single crystal provide better insight into the anisotropy of the system. In a constant magnetic field of 0.01 T, we observe distinct anisotropy for the fields perpendicular or parallel to the
c direction (
Figure 2). At low temperatures, the easy axis of magnetization lies within the
a-
b plane. Around 180 K, the response goes through a local minimum and then increases slightly up to 230 K and finally decreases towards 0 at higher temperatures. The response parallel to
c changes in the other way. From a small residual moment below 50 K, the moment rises up to 230 K. It then decreases and is essentially constant up to 380 K, the highest temperature we have measured. From the ratio
we clearly identify the spin flip transition at 230 K for this sample. This observation is in contrast to the results from the powder magnetization measurements. The transition appears at lower temperature and also the second transition to the AF phase is suppressed. This might be understood as the
x = 0.1 composition is close to the phase boundary, where the transition into an AFM phase is lost. While the nominal composition is
x = 0.1, the actual Co content could be slightly smaller for the single crystal.
2.3. Neutron Powder Diffraction and Magnetic Structure
Neutron powder diffractograms were collected at five temperatures (50 K, 200 K, room temperature (RT), 350 K, and 550 K) for each Mn2−xCoxSb (x = 0.1, 0.15, 0.2) compound to determine the nuclear and magnetic structures. Due to restrictions in the available beamtime, the measurement for Mn1.85Co0.15Sb started with a heating cycle, followed by a cooling cycle, while Mn1.8Co0.2Sb and Mn1.9Co0.1Sb were measured the other way around. The measured diffractograms for Mn1.85Co0.15Sb show only very diffuse magnetic reflections with a low signal-to-noise ratio. Unfortunately, the data quality is not sufficient for further magnetic structure refinements. This is presumably since the magnetic ordering for Mn1.85Co0.15Sb on cooling was not fully completed and the thermodynamic equilibrium was not reached yet. The important role of the thermal history is thus evidenced by this observation.
The neutron powder diffractograms measured at 550 K contain only the scattering from the nuclear structure and correspond to the paramagnetic state of the compounds. To check the Co incorporation on both crystallographic sites, equal amounts of Co were distributed on both Mn positions initially. The atomic coordinates and the isotropic thermal displacement parameters of Mn1/Co1 and Mn2/Co2 were restricted to be equal, while the occupancy parameters were refined. The refinements would always show negative values for Co2. This confirms the absence of Co atoms on the Mn2 site for all compounds, which was also claimed by several authors [
1,
3,
6,
14]. A refinement of the Co occupancy on the Mn1 site led to the result that the stoichiometric and the refined occupancies agree with each other within one standard deviation (
Table S10). The final fit for Mn
1.8Co
0.2Sb is shown in
Figure 3. The refined lattice parameters, unit cell volumes, atomic coordinates, isotropic thermal displacements for Mn
1.8Co
0.2Sb and Mn
1.9Co
0.1Sb as well as the final fit for Mn
1.9Co
0.1Sb are given in the
Supplementary Materials (
Tables S5–S9,
Figures S7 and S11).
In the neutron powder diffractograms measured at 350 K and at RT magnetic scattering contributions from the ferrimagnetic phases are present. The same is true for the Mn
1.9Co
0.1Sb at 200 K. Ferrimagnetic phases are characterized by an overlap of nuclear and magnetic peaks. As seen in
Figure 1, the isofield measurements confirm the presence of a global magnetic moment for Mn
1.9Co
0.1Sb and Mn
1.8Co
0.2Sb within this temperature range. From all the Shubnikov groups deduced via irreducible representations of the nuclear space group with
Jana2006 [
15,
16,
17], only four of them allow a global magnetic moment:
P4/
nm’m’,
Cm’me’,
Pmm’n’ and
P2
’/
n’. In contrast to the other three Shubnikov groups, more than one parameter is needed to describe the magnetic moments of the atoms for the symmetry
P2
’/
n’. In the refinement, strong correlations between these parameters were observed, most probably indicating that the monoclinic symmetry is too low. Therefore, refinements in this magnetic space group were discarded. Magnetic refinements with the remaining Shubnikov groups (
P4/
nm’m’,
Cm’me’, Pmm’n’) were tested to find the best model. Apart from the information obtained from the magnetization measurements, the R-values (
Table 2 and
Table S11) and the difference curves of the magnetic structure refinements were carefully examined to deduce the correct model. Of the three possible magnetic space groups, only
P4/
nm’m’ has the magnetic moments aligned parallel to the
c axis, which according to the magnetization measurements on the single crystal (
Figure 2) corresponds to an arrangement in the FRI-I state. In the Shubnikov groups
Cm’me’ and
Pmm’n’, the magnetic moments are aligned within the
a-
b plane. Models in these magnetic space groups could therefore correspond to the FRI-II state described in the literature.
Considering
TSF determined from the temperature-dependent magnetization measurements (
Figure 1 and
Table 1), some further assumptions can be made. Mn
1.8Co
0.2Sb should be in the FRI-I state (
P4/
nm’m’) at 350 K, while at RT it should rather correspond either to the
Cm’me’ or
Pmm’n’ symmetries. For Mn
1.9Co
0.1Sb, the determined spin flip transition is between 275 and 280 K. According to this, the 350 K and RT measurements should correspond to the FRI-I state. In this case,
P4/
nm’m’ is the only choice of a Shubnikov group. At 200 K, below the
TSF,
Cm’me’ or
Pmm’n’ would be the better choices for the magnetic space group.
The magnetic structure refinements of the Mn
1.8Co
0.2Sb diffractograms at 350 K and RT are shown in
Figure 4 and
Figure 5, respectively. The magnetic structure refinements of the Mn
1.9Co
0.1Sb diffractograms at 350 K, RT, and 200 K are provided in the Appendix (
Figures S8–S10).
Additional peaks are clearly visible for Mn
1.8Co
0.2Sb at 200 K and 50 K (
Figure 6a,b), as well as for Mn
1.9Co
0.1Sb at 50 K. Apart from this, the diffractograms show a reduced intensity of several low-θ Bragg peaks. These observations point to the existence of AFM structures. The additional reflections can be indexed assuming a doubling of the
c lattice parameter [
12]. Consequently, a propagation vector (0 0 ½) was introduced into the magnetic structure refinement, as proposed by [
9]. All possible resulting magnetic symmetries are characterized by a global magnetic moment of 0, confirming the antiferromagnetic nature of the order. For Mn
1.8Co
0.2Sb at 200 and 50 K, only two magnetic models (
C[c]mce and
P[c]mcn), are plausible, as they are the only ones indexing all additional peaks in the diffraction patterns. In the first one, the magnetic moments lie within the
a-
b plane, in the second one the moments lie parallel to the
a direction.
For the data at 50 K of Mn
1.8Co
0.2Sb the magnetic peaks show high intensities and the determination of the magnetic structure was straightforward, as the model in
P[c]mcn leads to better agreement factors (
Table 3) then the one in
C[c]mce. However, in the 200 K diffractogram, the magnetic reflections have very low intensities and it became clear that none of the two magnetic models (
C[c]mce and
P[c]mcn) resulted in a satisfactory fit. As the intensities of the magnetic peaks associated with the antiferromagnetic ordering are very low, we assumed a co-existence of the FRI-II phase with the AFM phase. Metastable frozen FRI-II phases at low temperatures due to kinetic arrest were also reported by [
8,
13]. A refinement considering both phases led indeed to an acceptable fit. In the refinement the profile parameters, the
a lattice parameter and the isotropic thermal displacement parameters of both phases, were restricted to be equal. The
c lattice parameter of the FRI-II phase was set to be ½
c of the AFM phase and the z-coordinates of the Mn2 and Sb1 in the AFM phase were restricted to be
2z of the corresponding atoms in the FRI-II phase. This way, only three additional parameters (Mx0 of Mn1/Co1, Mx0 of Mn2 and the volume fraction of phase 2) were introduced when compared to a one-phase refinement of the antiferromagnetic phase alone. The refined volume fractions clearly show that the ferrimagnetic phase is still dominating in the sample (V
AFM/V
FRI = 0.18/0.82). This model is in good agreement with the residual magnetization observed in the temperature-dependent magnetization measurements of the polycrystalline samples (
Figure 1).
The additional magnetic reflections in Mn
1.9Co
0.1Sb at 50 K are also weak and we used a similar two-phase refinement model, as already described for Mn
1.8Co
0.2Sb at 200 K, assuming a co-existence of an FRI-II and AFM phase (
Figure S11). The ferrimagnetic phase is clearly dominating (V
AFM/V
FRI = 0.21/0.79) and the result is in very good agreement with both observations from the macroscopic magnetization measurements (
Figure 1). Yet, it should be noted that the diffractograms for Mn
1.9Co
0.1Sb and Mn
1.8Co
0.2Sb measured at 50 K show a peak at about 2θ~27°, that is not indexed with the chosen model. The presence of possible impurities MnSb, Co, Mn, or Sb was checked, but none of these phases explains this reflection.
Table 3 shows the refined magnetic moments on the Mn1 and Mn2 sites at all measured temperatures. The moments are visualized in
Figure 7. The magnetic moments always point into opposite directions [
3,
13] in all magnetic states. Only in the FRI-I state (
P4/
nm’m’) the spins are aligned parallel to the
c axis, in the remaining states the spins are parallel to the
a axis (
Figure 7).
The macroscopic measurements show similar residual magnetizations for Mn
1.9Co
0.1Sb at 50 K and for Mn
1.8Co
0.2Sb at 200 K (
Figure 1), which correspond to about 2.25–2.5 Am
2/kg. This is in full accordance with the assumption that at these temperatures the FRI-II phase co-exists with the AFM phase for both compounds. The drop of magnetization for Mn
1.8Co
0.2Sb is pronounced at
Tt, which agrees with the observation from the neutron powder diffraction studies at 50 K. Here, only the AFM phase is observed. The very small remaining net residual magnetization below
Tt for this compound might be related to a very small amount of impurity. A hint for the occurrence of these impurities might be the unindexed peak at 2θ~27°, which is visible in the diffractograms of both compounds at 50 K. However, the exact origin of this peak remains unclear as it cannot be attributed to any of the likely impurity phases (Co, Mn, and Sb or any of the corresponding binary phases).
The interatomic distances of Mn
1.9Co
0.1Sb and Mn
1.8Co
0.2Sb resulting from the refinements are plotted against the temperature in
Figure 8. The distances Mn1/Co1–Sb and Mn1/Co1–Mn2 increase abruptly between 200 K and 50 K, where the magnetic phase transition from the FRI into the AFM state occurs. For the remaining interatomic distances, no significant changes are observed.