Synthesis and Antiparasitic Activity of New Conjugates—Organic Drugs Tethered to Trithiolato-Bridged Dinuclear Ruthenium(II)–Arene Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.1.1. Synthesis of the Trithiolato-Bridged Diruthenium Intermediates
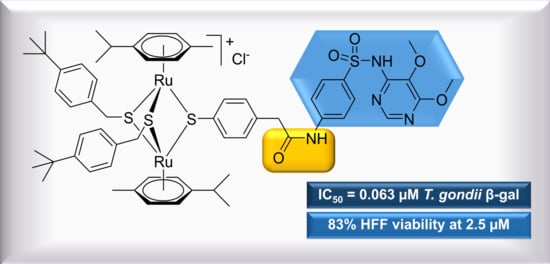
2.1.2. Conjugates with Sulfa-Drugs (Dapsone, Sulfamethoxazole, Sulfadiazine, Sulfadoxine)
2.1.3. Conjugates with Triclosan and Metronidazole
2.1.4. Conjugates with Ciprofloxacin
2.1.5. Conjugates with Menadione
2.1.6. X-ray Crystallography
| Compound | 9 |
|---|---|
| Formula | C60H74ClN3O4Ru2S4·3CH3CH2OH·CHCl3 |
| F.W. (g·mol−1) | 1524.62 |
| Temperature (K) | 110.2(5) |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a (Å) | 14.13470(10) |
| b (Å) | 24.4657(2) |
| c (Å) | 20.7272(2) |
| α (°) | 90 |
| β (°) | 100.0580(10) |
| γ (°) | 90 |
| V (Å3) | 7057.62(10) |
| Z | 4 |
| Dcalc (g·cm−3) | 1.435 |
| µ (mm-1) | 6.38 |
| F(000) | 3168 |
| Crystal size (mm3) | 0.2 × 0.075 × 0.05 |
| Θ range for data collection (°) | 5.64 to 154.266 |
| Index ranges | |
| h | −17/12 |
| k | −30/30 |
| l | −26/25 |
| Reflns. collected | 56,233 |
| Independent reflns. | 14,528 |
| [Rint = 0.0442, Rsigma = 0.0350] | |
| Data/restraints/parameters | 14,528/2/803 |
| GoodF2 | 1.047 |
| R1 [I ≥ 2σ(I)] | 0.0564 |
| wR2 | 0.1597 |
| R1 [all data] | 0.0613 |
| wR2 | 0.1645 |
| Largest diff. peak/hole (Å−3) | 3.23/−1.47 |
| Complex 9 | Complex J | |
|---|---|---|
| Ru-S | Ru(1)-S(1) 2.3749(10) Ru(1)-S(2) 2.3927(10) Ru(1)-S(3) 2.3973(10) Ru(2)-S(1) 2.3884(10) Ru(2)-S(2) 2.3931(11) Ru(2)-S(3) 2.3869(10) | Ru(1)-S(1) 2.3878(9) Ru(1)-S(2) 2.4023(9) Ru(1)-S(3) 2.3813(8) Ru(2)-S(1) 2.3992(9) Ru(2)-S(2) 2.3991(8) Ru(2)-S(3) 2.3882(8) |
| Ru-η6 | Ru(1)-cent(C21-C26) Ru(2)-cent(C31-C36) | Ru(1)-cent(C1-C6) 1.708 Ru(2)-cent(C11-C16) 1.709 |
| S-Ru-S | S(1)-Ru(2)-S(2) 76.29(4) S(1)-Ru(2)-S(3) 74.94(4) S(2)-Ru(2)-S(3) 77.32(4) S(1)-Ru(1)-S(2) 76.56(3) S(1)-Ru(1)-S(3) 75.00(4) S(2)-Ru(1)-S(3) 77.13(4) | S(1)-Ru(1)-S(2) 74.95(3) S(1)-Ru(1)-S(3) 77.72(3) S(2)-Ru(1)-S(3) 75.75(3) S(1)-Ru(2)-S(2) 74.81(3) S(1)-Ru(2)-S(3) 77.37(3) S(2)-Ru(2)-S(3) 75.68(3) |
| Ru-S-Ru | Ru(1)-S(1)-Ru(2) 89.45(3) Ru(1)-S(2)-Ru(2) 88.91(3) Ru(1)-S(3)-Ru(2) 88.95(4) | Ru(1)-S(1)-Ru(2) 89.27(3) Ru(1)-S(2)-Ru(2) 88.93(3) Ru(1)-S(3)-Ru(2) 89.68(3) |
| Ru-cent(S-S-S)-Ru | Ru(1)-cent(S1-S3)-Ru(2) 178.71 | Ru(1)-cent(S1-S3)-Ru(2) 177.30 |
| cent η6-cent(S-S-S)-cent η6 | cent(C24-C29)-cent(S1-S3)-cent(C72-C77) 177.74 | cent(C1-C6)-cent(S1-S3)-cent(C11-C16) 176.25 |
2.2. Assessment of the In Vitro Activity against the Apicomplexan Parasite Toxoplasma gondii
2.2.1. Primary Screening
Diruthenium Intermediates
Antimicrobial Drugs and Conjugates
2.2.2. Secondary Screening
3. Experimental
3.1. Chemistry
3.2. Crystal-Structure Determination
3.3. In Vitro Activity Assessment against T. gondii Tachyzoites and HFF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hartinger, C.G.; Metzler-Nolte, N.; Dyson, P.J. Challenges and opportunities in the development of organometallic anticancer drugs. Organometallics 2012, 31, 5677–5685. [Google Scholar] [CrossRef]
- Lin, K.; Zhao, Z.Z.; Bo, H.B.; Hao, X.J.; Wang, J.Q. Applications of ruthenium complex in tumor diagnosis and therapy. Front. Pharmacol. 2018, 9, 1323. [Google Scholar] [CrossRef] [Green Version]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: Current clinical uses, cytotoxicity studies and future perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sadler, P.J. Advances in the design of organometallic anticancer complexes. J. Organomet. Chem. 2017, 839, 5–14. [Google Scholar] [CrossRef]
- Trondl, R.; Heffeter, P.; Kowol, C.R.; Jakupec, M.A.; Berger, W.; Keppler, B.K. NKP-1339, the first ruthenium-based anticancer drug on the edge to clinical application. Chem. Sci. 2014, 5, 2925–2932. [Google Scholar] [CrossRef] [Green Version]
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, Two Iconic Ruthenium Anticancer Drug Candidates Face-to-Face: A Case Story in Medicinal Inorganic Chemistry. Molecules 2019, 24, 1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coverdale, J.P.C.; Laroiya-McCarron, T.; Romero-Canelón, I. Designing ruthenium anticancer drugs: What have we learnt from the key drug candidates? Inorganics 2019, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Alessio, E. Thirty years of the drug candidate NAMI-A and the myths in the field of ruthenium anticancer compounds: A personal perspective. Eur. J. Inorg. Chem. 2016, 2017, 1549–1560. [Google Scholar] [CrossRef]
- Berndsen, R.H.; Weiss, A.; Abdul, U.K.; Wong, T.J.; Meraldi, P.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. Combination of ruthenium(II)-arene complex [Ru(eta(6)-p-cymene)Cl2(pta)] (RAPTA-C) and the epidermal growth factor receptor inhibitor erlotinib results in efficient angiostatic and antitumor activity. Sci. Rep. 2017, 7, 43005. [Google Scholar] [CrossRef]
- Weiss, A.; Berndsen, R.H.; Dubois, M.; Müller, C.; Schibli, R.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. In vivo anti-tumor activity of the organometallic ruthenium(II)-arene complex [Ru(η6-p-cymene)Cl2(pta)] (RAPTA-C) in human ovarian and colorectal carcinomas. Chem. Sci. 2014, 5, 4742–4748. [Google Scholar] [CrossRef] [Green Version]
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The development of RAPTA compounds for the treatment of tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Bergamo, A.; Masi, A.; Peacock, A.F.; Habtemariam, A.; Sadler, P.J.; Sava, G. In vivo tumour and metastasis reduction and in vitro effects on invasion assays of the ruthenium RM175 and osmium AFAP51 organometallics in the mammary cancer model. J. Inorg. Biochem. 2010, 104, 79–86. [Google Scholar] [CrossRef]
- Aird, R.E.; Cummings, J.; Ritchie, A.A.; Muir, M.; Morris, R.E.; Chen, H.; Sadler, P.J.; Jodrell, D.I. In vitro and in vivo activity and cross resistance profiles of novel ruthenium (II) organometallic arene complexes in human ovarian cancer. Br. J. Cancer 2002, 86, 1652–1657. [Google Scholar] [CrossRef] [Green Version]
- Suss-Fink, G. Arene ruthenium complexes as anticancer agents. Dalton Trans. 2010, 39, 1673–1688. [Google Scholar] [CrossRef] [PubMed]
- Golbaghi, G.; Castonguay, A. Rationally designed ruthenium complexes for breast cancer therapy. Molecules 2020, 25, 265. [Google Scholar] [CrossRef] [Green Version]
- Ong, Y.C.; Roy, S.; Andrews, P.C.; Gasser, G. Metal compounds against neglected tropical diseases. Chem. Rev. 2019, 119, 730–796. [Google Scholar] [CrossRef]
- Gambino, D.; Otero, L. Design of prospective antiparasitic metal-based compounds including selected organometallic cores. Inorg. Chim. Acta 2018, 472, 58–75. [Google Scholar] [CrossRef]
- Laurent, Q.; Batchelor, L.K.; Dyson, P.J. Applying a Trojan horse strategy to ruthenium complexes in the pursuit of novel antibacterial agents. Organometallics 2018, 37, 915–923. [Google Scholar] [CrossRef]
- Mbaba, M.; Golding, T.M.; Smith, G.S. Recent advances in the biological investigation of organometallic platinum-group metal (Ir, Ru, Rh, Os, Pd, Pt) complexes as antimalarial agents. Molecules 2020, 25, 5276. [Google Scholar] [CrossRef]
- Dkhar, L.; Sawkmie, M.; Ka-Ot, A.L.; Joshi, S.R.; Kaminsky, W.; Kollipara, M.R. Cp and indenyl ruthenium complexes containing dithione derivatives: Synthesis, antibacterial and antifungal study. J. Organomet. Chem. 2020, 923, 121418. [Google Scholar] [CrossRef]
- Su, W.; Tang, Z.; Li, P. Development of arene ruthenium antitumor complexes. Mini. Rev. Med. Chem. 2016, 16, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Cherioux, F.; Thomas, C.M.; Therrien, B.; Suss-Fink, G. Dendritic systems based on dinuclear ruthenium or rhodium units generating peripheral catalytic sites. Chem. Eur. J. 2002, 8, 4377–4382. [Google Scholar] [CrossRef]
- Gras, M.; Therrien, B.; Suss-Fink, G.; Zava, O.; Dyson, P.J. Thiophenolato-bridged dinuclear areneruthenium complexes: A new family of highly cytotoxic anticancer agents. Dalton Trans. 2010, 39, 10305–10313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, F.; Furrer, J.; Ibao, A.-F.; Süss-Fink, G.; Therrien, B.; Zava, O.; Baquie, M.; Dyson, P.J.; Stěpnička, P. Highly cytotoxic trithiophenolatodiruthenium complexes of the type [(η6-p-MeC6H4Pri)2Ru2(SC6H4-p-X)3]+: Synthesis, molecular structure, electrochemistry, cytotoxicity, and glutathione oxidation potential. J. Biol. Inorg. Chem. 2012, 17, 951–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, F.; Paul, L.E.H.; Furrer, J.; Therrien, B.; Süss-Fink, G. Highly cytotoxic diruthenium trithiolato complexes of the type [(η6-p-MeC6H4Pri)2Ru2(μ2-SR)3]+: Synthesis, characterization, molecular structure and in vitro anticancer activity. New J. Chem. 2013, 37, 3503–3511. [Google Scholar] [CrossRef]
- Furrer, J.; Suss-Fink, G. Thiolato-bridged dinuclear arene ruthenium complexes and their potential as anticancer drugs. Coord. Chem. Rev. 2016, 309, 36–50. [Google Scholar] [CrossRef]
- Giannini, F.; Furrer, J.; Suss-Fink, G.; Clavel, C.M.; Dyson, P.J. Synthesis, characterization and in vitro anticancer activity of highly cytotoxic trithiolato diruthenium complexes of the type [(η(6)-p-(MeC6H4Pr)-Pr-i)(2)Ru-2(μ(2)-SR1)(2)(μ(2)-SR2)](+) containing different thiolato bridges. J. Organomet. Chem. 2013, 744, 41–48. [Google Scholar] [CrossRef]
- Muthna, D.; Tomsik, P.; Havelek, R.; Kohlerova, R.; Kasilingam, V.; Cermakova, E.; Stibal, D.; Rezacova, M.; Suss-Fink, G. In-vitro and in-vivo evaluation of the anticancer activity of diruthenium-2, a new trithiolato arene ruthenium complex [(η6-p-MeC6H4Pri)2Ru2(μ-S-p-C6H4OH)3]Cl. Anticancer Drugs 2016, 27, 643–650. [Google Scholar] [CrossRef]
- Tomšík, P.; Muthná, D.; Řezáčová, M.; Mičuda, S.; Ćmielová, J.; Hroch, M.; Endlicher, R.; Červinková, Z.; Rudolf, E.; Hann, S.; et al. [(p-MeC6H4Pri)2Ru2(SC6H4-p-But)3]Cl (diruthenium-1), a dinuclear arene ruthenium compound with very high anticancer activity: An in vitro and in vivo study. J. Organomet. Chem. 2015, 782, 42–51. [Google Scholar] [CrossRef]
- Basto, A.P.; Muller, J.; Rubbiani, R.; Stibal, D.; Giannini, F.; Suss-Fink, G.; Balmer, V.; Hemphill, A.; Gasser, G.; Furrer, J. Characterization of the activities of dinuclear thiolato-bridged arene ruthenium complexes against Toxoplasma gondii. Antimicrob. Agents Chemother. 2017, 61, e01031-17. [Google Scholar] [CrossRef] [Green Version]
- Basto, A.P.; Anghel, N.; Rubbiani, R.; Muller, J.; Stibal, D.; Giannini, F.; Suss-Fink, G.; Balmer, V.; Gasser, G.; Furrer, J.; et al. Targeting of the mitochondrion by dinuclear thiolato-bridged arene ruthenium complexes in cancer cells and in the apicomplexan parasite Neospora caninum. Metallomics 2019, 11, 462–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelk, J.; Balmer, V.; Stibal, D.; Giannini, F.; Suss-Fink, G.; Butikofer, P.; Furrer, J.; Hemphill, A. Anti-parasitic dinuclear thiolato-bridged arene ruthenium complexes alter the mitochondrial ultrastructure and membrane potential in Trypanosoma brucei bloodstream forms. Exp. Parasitol. 2019, 205, 107753. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.C.; Goulart, C.; Hayward, J.A.; Kupz, A.; Miller, C.M.; van Dooren, G.G. Control of human toxoplasmosis. Int. J. Parasitol. 2021, 51, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Alday, P.H.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Dev. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef] [Green Version]
- Milne, G.; Webster, J.P.; Walker, M. Toxoplasma gondii: An Underestimated Threat? Trends Parasitol. 2020, 36, 959–969. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical perspective, animal models, and current clinical practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Bierbach, U. Metal-containing pharmacophores in molecularly targeted anticancer therapies and diagnostics. Eur. J. Inorg. Chem. 2017, 12, 1561–1572. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Guo, Z. Functionalization of platinum complexes for biomedical applications. Acc. Chem. Res. 2015, 48, 2622–2631. [Google Scholar] [CrossRef]
- Chellan, P.; Sadler, P.J. Enhancing the activity of drugs by conjugation to organometallic fragments. Chem. Eur. J. 2020, 26, 8676–8688. [Google Scholar] [CrossRef]
- Stibal, D.; Therrien, B.; Suss-Fink, G.; Nowak-Sliwinska, P.; Dyson, P.J.; Cermakova, E.; Rezacova, M.; Tomsik, P. Chlorambucil conjugates of dinuclear p-cymene ruthenium trithiolato complexes: Synthesis, characterization and cytotoxicity study in vitro and in vivo. J. Biol. Inorg. Chem. 2016, 21, 443–452. [Google Scholar] [CrossRef]
- Giannini, F.; Bartoloni, M.; Paul, L.E.H.; Suss-Fink, G.; Reymond, J.L.; Furrer, J. Cytotoxic peptide conjugates of dinuclear arene ruthenium trithiolato complexes. Medchemcomm 2015, 6, 347–350. [Google Scholar] [CrossRef]
- Desiatkina, O.; Paunescu, E.; Mosching, M.; Anghel, N.; Boubaker, G.; Amdouni, Y.; Hemphill, A.; Furrer, J. Coumarin-tagged dinuclear trithiolato-bridged ruthenium(II)arene complexes: Photophysical properties and antiparasitic activity. ChemBioChem 2020, 21, 2818–2835. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.J.; Zach, S.J.; Wang, X.; Larson, J.J.; Judge, A.K.; Davis, L.A.; Vennerstrom, J.L.; Davis, P.H. Clinically available medicines demonstrating anti-toxoplasma activity. Antimicrob. Agents Chemother. 2015, 59, 7161–7169. [Google Scholar] [CrossRef] [Green Version]
- Radke, J.B.; Burrows, J.N.; Goldberg, D.E.; Sibley, L.D. Evaluation of current and emerging antimalarial medicines for Inhibition of Toxoplasma gondii growth in vitro. ACS Infect. Dis. 2018, 4, 1264–1274. [Google Scholar] [CrossRef]
- Wei, H.X.; Wei, S.S.; Lindsay, D.S.; Peng, H.J. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS ONE 2015, 10, e0138204. [Google Scholar] [CrossRef] [PubMed]
- Shammaa, A.M.; Powell, T.G.; Benmerzouga, I. Adverse outcomes associated with the treatment of Toxoplasma infections. Sci. Rep. 2021, 11, 1035. [Google Scholar] [CrossRef]
- Konstantinovic, N.; Guegan, H.; Stajner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019, 15, e00036. [Google Scholar] [CrossRef]
- Mansour, A.M.; Radacki, K. Experimental and DFT studies of sulfadiazine ‘piano-stool’ Ru(II) and Rh(III) complexes. Rsc Adv. 2020, 10, 10673–10680. [Google Scholar] [CrossRef]
- Chellan, P.; Avery, V.M.; Duffy, S.; Triccas, J.A.; Nagalingam, G.; Tam, C.; Cheng, L.W.; Liu, J.; Land, K.M.; Clarkson, G.J.; et al. Organometallic conjugates of the drug sulfadoxine for combatting antimicrobial resistance. Chem. Eur. J. 2018, 24, 10078–10090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotzé, T.J.; Duffy, S.; Avery, V.M.; Jordaan, A.; Warner, D.F.; Loots, L.; Smith, G.S.; Chellan, P. Synthesis and antimicrobial study of organoiridium amido-sulfadoxine complexes. Inorg. Chim. Acta 2020, 517, 120175. [Google Scholar] [CrossRef]
- McLeod, R.; Muench, S.P.; Rafferty, J.B.; Kyle, D.E.; Mui, E.J.; Kirisits, M.J.; Mack, D.G.; Roberts, C.W.; Samuel, B.U.; Lyons, R.E.; et al. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of apicomplexan Fab I. Int. J. Parasitol. 2001, 31, 109–113. [Google Scholar] [CrossRef]
- Martins-Duarte, E.S.; Carias, M.; Vommaro, R.; Surolia, N.; de Souza, W. Apicoplast fatty acid synthesis is essential for pellicle formation at the end of cytokinesis in Toxoplasma gondii. J. Cell. Sci. 2016, 129, 3320–3331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muench, S.P.; Stec, J.; Zhou, Y.; Afanador, G.A.; McPhillie, M.J.; Hickman, M.R.; Lee, P.J.; Leed, S.E.; Auschwitz, J.M.; Prigge, S.T.; et al. Development of a triclosan scaffold which allows for adaptations on both the A- and B-ring for transport peptides. Bioorg. Med. Chem. Lett. 2013, 23, 3551–3555. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Muench, S.P.; Zhou, Y.; Afanador, G.A.; Mui, E.J.; Fomovska, A.; Lai, B.S.; Prigge, S.T.; Woods, S.; Roberts, C.W.; et al. Design, synthesis, and biological activity of diaryl ether inhibitors of Toxoplasma gondii enoyl reductase. Bioorg. Med. Chem. Lett. 2013, 23, 2035–2043. [Google Scholar] [CrossRef] [Green Version]
- Stec, J.; Fomovska, A.; Afanador, G.A.; Muench, S.P.; Zhou, Y.; Lai, B.S.; El Bissati, K.; Hickman, M.R.; Lee, P.J.; Leed, S.E.; et al. Modification of triclosan scaffold in search of improved inhibitors for enoyl-acyl carrier protein (ACP) reductase in Toxoplasma gondii. ChemMedChem 2013, 8, 1138–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, B.U.; Hearn, B.; Mack, D.; Wender, P.; Rothbard, J.; Kirisits, M.J.; Mui, E.; Wernimont, S.; Roberts, C.W.; Muench, S.P.; et al. Delivery of antimicrobials into parasites. Proc. Natl. Acad. Sci. USA 2003, 100, 14281–14286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Zawawy, L.A.; El-Said, D.; Mossallam, S.F.; Ramadan, H.S.; Younis, S.S. Triclosan and triclosan-loaded liposomal nanoparticles in the treatment of acute experimental toxoplasmosis. Exp. Parasitol. 2015, 149, 54–64. [Google Scholar] [CrossRef]
- El-Zawawy, L.A.; El-Said, D.; Mossallam, S.F.; Ramadan, H.S.; Younis, S.S. Preventive prospective of triclosan and triclosan-liposomal nanoparticles against experimental infection with a cystogenic ME49 strain of Toxoplasma gondii. Acta Trop. 2015, 141, 103–111. [Google Scholar] [CrossRef]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An update on metabolism, structure-cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; Lopez Contreras, L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar] [CrossRef]
- Chew, W.K.; Segarra, I.; Ambu, S.; Mak, J.W. Significant reduction of brain cysts caused by Toxoplasma gondii after treatment with spiramycin coadministered with metronidazole in a mouse model of chronic toxoplasmosis. Antimicrob. Agents Chemother. 2012, 56, 1762–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, L.D.; Tocher, J.H.; Dyson, T.M.; Edwards, D.I.; Tocher, D.A. Studies on DNA damage and induction of SOS repair by novel multifunctional bioreducible compounds. II. A metronidazole adduct of a ruthenium-arene compound. Anti-Cancer Drug Des. 1992, 7, 3–14. [Google Scholar]
- Martins-Duarte, E.S.; Dubar, F.; Lawton, P.; da Silva, C.F.; Soeiro, M.D.C.; de Souza, W.; Biot, C.; Vommaro, R.C. Ciprofloxacin derivatives affect parasite cell division and increase the survival of mice infected with Toxoplasma gondii. PLoS ONE 2015, 10, e0125705. [Google Scholar] [CrossRef] [PubMed]
- Dubar, F.; Wintjens, R.; Martins-Duarte, E.S.; Vommaro, R.C.; de Souza, W.; Dive, D.; Pierrot, C.; Pradines, B.; Wohlkonig, A.; Khalife, J.; et al. Ester prodrugs of ciprofloxacin as DNA-gyrase inhibitors: Synthesis, antiparasitic evaluation and docking studies. Medchemcomm 2011, 2, 430–435. [Google Scholar] [CrossRef]
- Koloczek, P.; Skorska-Stania, A.; Cierniak, A.; Sebastian, V.; Komarnicka, U.K.; Plotek, M.; Kyziol, A. Polymeric micelle-mediated delivery of half-sandwich ruthenium(II) complexes with phosphanes derived from fluoroloquinolones for lung adenocarcinoma treatment. Eur. J. Pharm. Biopharm. 2018, 128, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Ude, Z.; Romero-Canelon, I.; Twamley, B.; Hughes, D.F.; Sadler, P.J.; Marmion, C.J. A novel dual-functioning ruthenium(II)-arene complex of an anti-microbial ciprofloxacin derivative—Anti-proliferative and anti-microbial activity. J. Inorg. Biochem. 2016, 160, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Turel, I.; Kljun, J.; Perdih, F.; Morozova, E.; Bakulev, V.; Kasyanenko, N.; Byl, J.A.W.; Osheroff, N. First ruthenium organometallic complex of antibacterial agent ofloxacin. Crystal structure and interactions with DNA. Inorg. Chem. 2010, 49, 10750–10752. [Google Scholar] [CrossRef] [Green Version]
- Scholer, N.; Krause, K.; Kayser, O.; Muller, R.H.; Borner, K.; Hahn, H.; Liesenfeld, O. Atovaquone nanosuspensions show excellent therapeutic effect in a new murine model of reactivated toxoplasmosis. Antimicrob. Agents Chemother. 2001, 45, 1771–1779. [Google Scholar] [CrossRef] [Green Version]
- Muller, J.; Aguado-Martinez, A.; Ortega-Mora, L.M.; Moreno-Gonzalo, J.; Ferre, I.; Hulverson, M.A.; Choi, R.; McCloskey, M.C.; Barrett, L.K.; Maly, D.J.; et al. Development of a murine vertical transmission model for Toxoplasma gondii oocyst infection and studies on the efficacy of bumped kinase inhibitor (BKI)-1294 and the naphthoquinone buparvaquone against congenital toxoplasmosis. J. Antimicrob. Chemother. 2017, 72, 2334–2341. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.A.; Oliveira, A.B.; Gualberto, S.A.; Vitor, R.W. Activity of natural and synthetic naphthoquinones against Toxoplasma gondii, in vitro and in murine models of infection. Parasite 2002, 9, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gormley, P.D.; Pavesio, C.E.; Minnasian, D.; Lightman, S. Effects of drug therapy on Toxoplasma cysts in an animal model of acute and chronic disease. Investig. Ophthalmol. Visual Sci. 1998, 39, 1171–1175. [Google Scholar]
- Srivastava, I.K.; Rottenberg, H.; Vaidya, A.B. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J. Biol. Chem. 1997, 272, 3961–3966. [Google Scholar] [CrossRef] [Green Version]
- Spoerlein-Guettler, C.; Mahal, K.; Schobert, R.; Biersack, B. Ferrocene and (arene)ruthenium(II) complexes of the natural anticancer naphthoquinone plumbagin with enhanced efficacy against resistant cancer cells and a genuine mode of action. J. Inorg. Biochem. 2014, 138, 64–72. [Google Scholar] [CrossRef]
- Tabrizi, L.; Chiniforoshan, H. Ruthenium(II) p-cymene complexes of naphthoquinone derivatives as antitumor agents: A structure-activity relationship study. J. Organomet. Chem. 2016, 822, 211–220. [Google Scholar] [CrossRef]
- Kubanik, M.; Kandioller, W.; Kim, K.; Anderson, R.F.; Klapproth, E.; Jakupec, M.A.; Roller, A.; Sohnel, T.; Keppler, B.K.; Hartinger, C.G. Towards targeting anticancer drugs: Ruthenium(II)-arene complexes with biologically active naphthoquinone-derived ligand systems. Dalton Trans. 2016, 45, 13091–13103. [Google Scholar] [CrossRef] [PubMed]
- Kandioller, W.; Balsano, E.; Meier, S.M.; Jungwirth, U.; Goschl, S.; Roller, A.; Jakupec, M.A.; Berger, W.; Keppler, B.K.; Hartinger, C.G. Organometallic anticancer complexes of lapachol: Metal centre-dependent formation of reactive oxygen species and correlation with cytotoxicity. Chem. Commun. 2013, 49, 3348–3350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackl, C.M.; Schoenhacker-Alte, B.; Klose, M.H.M.; Henke, H.; Legina, M.S.; Jakupec, M.A.; Berger, W.; Keppler, B.K.; Bruggemann, O.; Teasdale, I.; et al. Synthesis and in vivo anticancer evaluation of poly(organo) phosphazene-based metallodrug conjugates. Dalton Trans. 2017, 46, 12114–12124. [Google Scholar] [CrossRef] [Green Version]
- Chérioux, F.; Thomas, C.M.; Monnier, T.; Süss-Fink, G. Specific reactivity of SH versus OH functions towards dinuclear arene ruthenium units: Synthesis of cationic complexes of the type [(arene)2Ru2(SR)3]+. Polyhedron 2003, 22, 543–548. [Google Scholar] [CrossRef]
- Păunescu, E.; Boubaker, G.; Desiatkina, O.; Anghel, N.; Amdouni, Y.; Hemphill, A.; Furrer, J. The quest of the best—A SAR study of trithiolato-bridged dinuclear ruthenium(II)-arene compounds presenting antiparasitic properties. Manuscript in preparation. 2021. [Google Scholar]
- Roy, B.; Dutta, S.; Choudhary, A.; Basak, A.; Dasgupta, S. Design, synthesis and RNase A inhibition activity of catechin and epicatechin and nucleobase chimeric molecules. Bioorg. Med. Chem. Lett. 2008, 18, 5411–5414. [Google Scholar] [CrossRef]
- Zabarska, N.; Stumper, A.; Rau, S. CuAAC click reactions for the design of multifunctional luminescent ruthenium complexes. Dalton Trans. 2016, 45, 2338–2351. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, J.; Saczewski, J. Modifications of quinolones and fluoroquinolones: Hybrid compounds and dual-action molecules. Monatsh. Chem. 2018, 149, 1199–1245. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Harmuth, S.; Barth, E.R.; Wurm, E.; Fobbe, R.; Sickmann, A.; Krumm, C.; Tiller, J.C. Conjugation of ciprofloxacin with poly(2-oxazoline)s and polyethylene glycol via end groups. Bioconjugate Chem. 2015, 26, 1950–1962. [Google Scholar] [CrossRef]
- Cilibrizzi, A.; Fedorova, M.; Collins, J.; Leatherbarrow, R.; Woscholski, R.; Vilar, R. A tri-functional vanadium(iv) complex to detect cysteine oxidation. Dalton Trans. 2017, 46, 6994–7004. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, N.; Yurek-George, A.; Ganesan, A. Rapid deprotection of N-Boc amines by TFA combined with freebase generation using basic ion-exchange resins. Mol. Diversity 2005, 9, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Kongkathip, N.; Kongkathip, B.; Siripong, P.; Sangma, C.; Luangkamin, S.; Niyomdecha, M.; Pattanapa, S.; Plyavirlyagul, S.; Kongsaeree, P. Potent antitumor activity of synthetic 1,2-naphthoquinones and 1,4-naphthoquinones. Bioorg. Med. Chem. 2003, 11, 3179–3191. [Google Scholar] [CrossRef]
- Wang, S.H.; Lo, C.Y.; Gwo, Z.H.; Lin, H.J.; Chen, L.G.; Kuo, C.D.; Wu, J.Y. Synthesis and biological evaluation of lipophilic 1,4-naphthoquinone derivatives against human cancer cell lines. Molecules 2015, 20, 11994–12015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, B.S.; Ravi, K.; Verma, A.K.; Fatima, K.; Hasanain, M.; Singh, A.; Sarkar, J.; Luqman, S.; Chanda, D.; Negi, A.S. Synthesis of pharmacologically important naphthoquinones and anticancer activity of 2-benzyllawsone through DNA topoisomerase-II inhibition. Bioorg. Med. Chem. 2017, 25, 1364–1373. [Google Scholar] [CrossRef]
- Salmon-Chemin, L.; Buisine, E.; Yardley, V.; Kohler, S.; Debreu, M.A.; Landry, V.; Sergheraert, C.; Croft, S.L.; Krauth-Siegel, R.L.; Davioud-Charvet, E. 2-and 3-Substituted 1,4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from Trypanosoma cruzi: Synthesis and correlation between redox cycling activities and in vitro cytotoxicity. J. Med. Chem. 2001, 44, 548–565. [Google Scholar] [CrossRef]
- Davioud-Charvet, E.; Delarue, S.; Biot, C.; Schwobel, B.; Boehme, C.C.; Mussigbrodt, A.; Maes, L.; Sergheraert, C.; Grellier, P.; Schirmer, R.H.; et al. A prodrug form of a Plasmodium falciparum glutathione reductase inhibitor conjugated with a 4-anilinoquinoline. J. Med. Chem. 2001, 44, 4268–4276. [Google Scholar] [CrossRef]
- Biot, C.; Bauer, H.; Schirmer, R.H.; Davioud-Charvet, E. 5-Substituted tetrazoles as bioisosteres of carboxylic acids. Bioisosterism and mechanistic studies on glutathione reductase inhibitors as antimalarials. J. Med. Chem. 2004, 47, 5972–5983. [Google Scholar] [CrossRef] [PubMed]
- Biot, C.; Dessolin, J.; Grellier, P.; Davioud-Charvet, E. Double-drug development against antioxidant enzymes from Plasmodium falciparum. Redox Rep. 2003, 8, 280–283. [Google Scholar] [CrossRef]
- Paunescu, E.; Soudani, M.; Clavel, C.M.; Dyson, P.J. Varying the metal to ethacrynic acid ratio in ruthenium(ii)/osmium(ii)-p-cymene conjugates. J. Inorg. Biochem. 2017, 175, 198–207. [Google Scholar] [CrossRef]
- Paunescu, E.; McArthur, S.; Soudani, M.; Scopelliti, R.; Dyson, P.J. Nonsteroidal anti-inflammatory-organometallic anticancer compounds. Inorg. Chem. 2016, 55, 1788–1808. [Google Scholar] [CrossRef]
- Studer, V.; Anghel, N.; Desiatkina, O.; Felder, T.; Boubaker, G.; Amdouni, Y.; Ramseier, J.; Hungerbuhler, M.; Kempf, C.; Heverhagen, J.T.; et al. Conjugates containing two and three trithiolato-bridged dinuclear ruthenium(II)-arene units as in vitro antiparasitic and anticancer agents. Pharmaceuticals 2020, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Stibal, D.; Suss-Fink, G.; Therrien, B. Crystal structure of (μ-4-hy-droxy-benzene-thiol-ato-κ(2) S:S)bis-(μ-phenyl-methane-thiol-ato-κ(2) S:S)bis-[(η(6)-1-isopropyl-4-methyl-benzene)-ruthenium(II)] tetra-fluorido-borate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2015, 71, 1174–1176. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.J.; Nyirongo, S.K.; Mukaka, M.; Zijlstra, E.E.; Plowe, C.V.; Molyneux, M.E.; Ward, S.A.; Winstanley, P.A. Sulfadoxine-pyrimethamine-based combinations for malaria: A randomised blinded trial to compare efficacy, safety and selection of resistance in Malawi. PLoS ONE 2008, 3, e1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terlouw, D.J.; Nahlen, B.L.; Courval, J.M.; Kariuki, S.K.; Rosenberg, O.S.; Oloo, A.J.; Kolczak, M.S.; Hawley, W.A.; Lal, A.A.; Kuile, F.O. Sulfadoxine-pyrimethamine in treatment of malaria in Western Kenya: Increasing resistance and underdosing. Antimicrob. Agents Chemother. 2003, 47, 2929–2932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, P.J.; Thigpen, M.C.; Parise, M.E.; Newman, R.D. Safety and toxicity of sulfadoxine/pyrimethamine: Implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf. 2007, 30, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Deloron, P.; Bertin, G.; Briand, V.; Massougbodji, A.; Cot, M. Sulfadoxine/pyrimethamine intermittent preventive treatment for malaria during pregnancy. Emerg. Infect. Dis. 2010, 16, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Giannini, F.; Suss-Fink, G.; Furrer, J. Efficient oxidation of cysteine and glutathione catalyzed by a dinuclear areneruthenium trithiolato anticancer complex. Inorg. Chem. 2011, 50, 10552–10554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxford Diffraction. CrysAlisPro (Version 1.171.40.37a); Oxford Diffraction Ltd.: Oxfordshire, UK, 2018. [Google Scholar]
- Macchi, P.; Bürgi, H.-B.; Chimpri, A.S.; Hauser, J.; Gál, Z. Low-energy contamination of Mo microsource X-ray radiation: Analysis and solution of the problem. J. Appl. Cryst. 2011, 44, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Barna, F.; Debache, K.; Vock, C.A.; Kuster, T.; Hemphill, A. In vitro effects of novel ruthenium complexes in Neospora caninum and Toxoplasma gondii tachyzoites. Antimicrob. Agents Chemother. 2013, 57, 5747–5754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFadden, D.C.; Seeber, F.; Boothroyd, J.C. Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob. Agents Chemother. 1997, 41, 1849–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, J.; Aguado-Martinez, A.; Manser, V.; Balmer, V.; Winzer, P.; Ritler, D.; Hostettler, I.; Arranz-Solis, D.; Ortega-Mora, L.; Hemphill, A. Buparvaquone is active against Neospora caninum in vitro and in experimentally infected mice. Int. J. Parasitol. Drugs Drug. Resist. 2015, 5, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]















| Compound | Contact D-H∙∙∙A | Distance (Å) | Angle (°) | ||
|---|---|---|---|---|---|
| D-H | H∙∙∙A | D∙∙∙A | D-H∙∙∙A | ||
| 9 | N52-H52…O41 | 0.860 | 2.162 | 2.786 | 129.22 |
| N42-H42…Cl1 | 0.859 | 2.407 | 3.254 | 169.15 | |
| Compound | HFF Viability (%) | T. gondii β-gal Growth (%) | ||
|---|---|---|---|---|
| 0.1 µM | 1 µM | 0.1 µM | 1 µM | |
| Ruthenium intermediates | ||||
| 2 a | 91 ± 4 | 73 ± 1 | 114 ± 2 | 110 ± 2 |
| 3 a | 76 ± 6 | 46 ± 6 | 66 ± 14 | 2 ± 0 |
| 4 a | 74 ± 2 | 48 ± 1 | 57 ± 1 | 2 ± 0 |
| 6 a | 97 ± 4 | 61 ± 6 | 115 ± 4 | 85 ± 5 |
| 7 | 71 ± 2 | 46 ± 6 | 52 ± 13 | 3 ± 1 |
| Conjugates with sulfa-drugs | ||||
| Dapsone | 92 ± 4 | 103 ± 3 | 77 ± 4 | 42 ± 0 |
| 8 | 104 ± 1 | 91 ± 2 | 148 ± 2 | 36 ± 2 |
| Sulfamethoxazole | 93 ± 3 | 102 ± 5 | 78 ± 5 | 75 ± 7 |
| 9 | 90 ± 12 | 63 ± 7 | 83 ± 8 | 77 ± 3 |
| Sulfadiazine | 101 ± 2 | 33 ± 3 | 57 ± 5 | 70 ± 5 |
| 10 | 113 ± 1 | 93 ± 2 | 72 ± 3 | 0 ± 0 |
| Sulfadoxine | 97 ± 3 | 104 ± 0 | 111 ± 3 | 83 ± 2 |
| 11 | 100 ± 3 | 100 ± 8 | 116 ± 1 | 11 ± 1 |
| Conjugates with triclosan and metronidazole | ||||
| Triclosan | 99 ± 1 | 97 ± 1 | 80 ± 2 | 71 ± 2 |
| 12 | 100 ± 2 | 103 ± 1 | 76 ± 6 | 66 ± 12 |
| Metronidazole | 101 ± 2 | 100 ± 1 | 115 ± 8 | 116 ± 6 |
| 13 | 115 ± 2 | 93 ± 1 | 101 ± 7 | 1 ± 0 |
| 14 | 98 ± 3 | 97 ± 2 | 115 ±7 | 92 ± 1 |
| 15 | 116 ± 1 | 99 ± 1 | 98 ± 2 | 1 ± 0 |
| Conjugates with ciprofloxacin | ||||
| Ciprofloxacin | 101 ± 1 | 99 ± 0 | 82 ± 3 | 84 ± 3 |
| 18 | 92 ± 0 | 89 ± 0 | 94 ± 2 | 102 ± 1 |
| 19 | 102 ± 2 | 94 ± 2 | 68 ± 4 | 21 ± 3 |
| Conjugates with menadione | ||||
| Menadione | 117 ± 3 | 101 ± 4 | 103 ± 7 | 50 ± 2 |
| 20 | 105 ± 3 | 94 ± 2 | 101 ± 8 | 107 ± 3 |
| 21 | 109 ± 2 | 95 ± 1 | 86 ± 10 | 84 ± 5 |
| 22 | 110 ± 3 | 87 ± 1 | 83 ± 4 | 92 ± 1 |
| 24 | 95 ± 1 | 92 ± 2 | 65 ± 4 | 3 ± 0 |
| 26 | 101 ± 2 | 102 ± 1 | 89 ± 16 | 90 ± 7 |
| 27 | 100 ± 2 | 100 ± 3 | 164 ± 4 | 92 ± 3 |
| 28 | 98 ± 3 | 92 ± 2 | 71 ± 6 | 46 ± 1 |
| Compound | T. gondii β-gal IC50 (µM) | [LS; LI] b | SE c | HFF Viability at 2.5 µM (%) d | SD e |
|---|---|---|---|---|---|
| Pyrimethamine | 0.326 | [0.396; 0.288] | 0.052 | 99 | 6 |
| Ruthenium intermediates | |||||
| 2a | 0.181 | [1.482; 0.274] | 0.954 | 99 | 2 |
| 4a | 0.153 | [0.185; 0.127] | 0.049 | 51 | 5 |
| Conjugates with sulfa-drugs | |||||
| 10 | 0.524 | [0.562; 0.488] | 0.069 | 62 | 1 |
| 11 | 0.063 | [0.072; 0.055] | 0.136 | 83 | 0 |
| Conjugates with triclosan and metronidazole | |||||
| 13 | 0.152 | [0.181; 0.127] | 0.175 | 64 | 3 |
| 15 | 0.500 | [0.884; 0.284] | 0.568 | 102 | 2 |
| Conjugates with menadione | |||||
| 24 | 0.481 | [0.525; 0.441] | 0.086 | 32 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desiatkina, O.; Johns, S.K.; Anghel, N.; Boubaker, G.; Hemphill, A.; Furrer, J.; Păunescu, E. Synthesis and Antiparasitic Activity of New Conjugates—Organic Drugs Tethered to Trithiolato-Bridged Dinuclear Ruthenium(II)–Arene Complexes. Inorganics 2021, 9, 59. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9080059
Desiatkina O, Johns SK, Anghel N, Boubaker G, Hemphill A, Furrer J, Păunescu E. Synthesis and Antiparasitic Activity of New Conjugates—Organic Drugs Tethered to Trithiolato-Bridged Dinuclear Ruthenium(II)–Arene Complexes. Inorganics. 2021; 9(8):59. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9080059
Chicago/Turabian StyleDesiatkina, Oksana, Serena K. Johns, Nicoleta Anghel, Ghalia Boubaker, Andrew Hemphill, Julien Furrer, and Emilia Păunescu. 2021. "Synthesis and Antiparasitic Activity of New Conjugates—Organic Drugs Tethered to Trithiolato-Bridged Dinuclear Ruthenium(II)–Arene Complexes" Inorganics 9, no. 8: 59. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9080059