Sex Ratio of Small Hive Beetles: The Role of Pupation and Adult Longevity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pupation Experiments
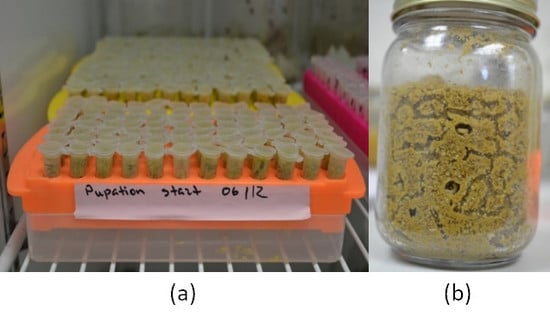
2.1.1. Individual Pupation
2.1.2. Mass Pupation
2.2. Sex Ratio Field vs. Laboratory
2.3. Statistical Analyses
3. Results
3.1. Pupation Experiments
3.2. Sex Ratio Field vs. Laboratory
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Girondot, M.; Pieau, C. Effects of sexual differences of age at maturity and survival on population sex ratio. Evol. Ecol. 1993, 7, 645–650. [Google Scholar] [CrossRef]
- Hamilton, W.D. Extraordinary Sex Ratios. Science 1967, 156, 477–488. [Google Scholar] [CrossRef]
- Page, R.E.; Metcalf, R.A. A Population investment sex ratio for the honey bee (Apis mellifera L.). Am. Nat. 1984, 124, 680–702. [Google Scholar] [CrossRef]
- West, S.; Reece, S.; Sheldon, B. Sex ratios. Heredity (Edinb.) 2002, 88, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, P.; Pirk, C.W.W.; Hepburn, R.P.; Elzen, P.J.; Baxter, J.R. Laboratory Rearing of Small Hive Beetles Aethina tumida (Coleoptera, Nitidulidae). J. Apic. Res. 2001, 40, 111–112. [Google Scholar] [CrossRef]
- Mürrle, T.; Neumann, P. Mass production of small hive beetles (Aethina tumida, Coleoptera: Nitidulidae). J. Apic. Res. 2004, 43, 144–145. [Google Scholar] [CrossRef]
- Ellis, J.D.; Hepburn, R.; Luckman, B.; Elzen, P.J. Effects of soil type, moisture, and density on pupation success of Aethina tumida (Coleoptera: Nitidulidae). Environ. Entom. Ecol. 2004, 33, 794–798. [Google Scholar] [CrossRef]
- Ellis, J.D.; Delaplane, K.S.; Hood, W.M. Small hive beetle (Aethina tumida Murray) weight, gross biometry, and sex proportion at three locations in the southeastern United States. Am. Bee J. 2002, 142, 520–522. [Google Scholar]
- Spiewok, S.; Neumann, P. Sex ratio and dispersal of small hive beetles. J. Apic. Res. 2012, 51, 216–217. [Google Scholar] [CrossRef]
- Lundie, A.E. The Small Hive Beetle, Aethina Tumida. Science Bulletin 220; Department of Agriculture and Forestry, Government Printer: Pretoria, South Africa, 1940.
- Al Toufailia, H.; Alves, D.A.; Bena, D.C.; Bento, J.M.S.; Iwanicki, N.S.A.; Cline, A.R.; Ellis, J.D.; Ratnieks, F.L.W. First record of small hive beetle, Aethina tumida Murray, in South America. J. Apic. Res. 2017, 56, 76–80. [Google Scholar] [CrossRef]
- Granato, A.; Zecchin, B.; Barrato, C.; Duquesne, V.; Negrisolo, E.; Chauzat, M.P.; Ribiere-Chabert, M.; Cattoli, G.; Mutinelli, F. Introduction of Aethina tumida (Coleoptera: Nitidulidae) in the regions of Calabria and Sicily (southern Italy). Apidologie 2017, 48, 194–203. [Google Scholar] [CrossRef]
- Lee, S.; Hong, K.-J.; Cho, Y.S.; Choi, Y.S.; Yoo, M.-S.; Lee, S. Review of the subgenus Aethina Erichson s. str. (Coleoptera: Nitidulidae: Nitidulinae) in Korea, reporting recent invasion of small hive beetle, Aethina tumida. J. Asia. Pac. Entomol. 2017, 20, 553–558. [Google Scholar] [CrossRef]
- Muli, E.; Kilonzo, J.; Sookar, P. Small Hive Beetle Infestations in Apis mellifera unicolor colonies in Mauritius Island, Mauritius. Bee World 2018, 95, 44–45. [Google Scholar] [CrossRef]
- Neumann, P.; Pettis, J.S.; Schäfer, M.O.S. Quo vadis Aethina tumida? Biology and control of small hive beetles. Apidologie 2016, 47, 427–466. [Google Scholar]
- Neumann, P.; Elzen, P.J. The biology of the small hive beetle (Aethina tumida, Coleoptera: Nitidulidae): Gaps in our knowledge of an invasive species. Apidologie 2004, 35, 3–229. [Google Scholar] [CrossRef]
- Gonthier, J.; Papach, A.; Straub, L.; Campbell, J.; Williams, G.R.; Neumann, P. Bees and flowers: How to feed an invasive beetle species. Ecol. Evol. in press.
- Ellis, J.D.; Neumann, P.; Hepburn, R.; Elzen, P.J. Longevity and Reproductive Success of Aethina tumida (Coleoptera: Nitidulidae) Fed Different Natural Diets. J. Econ. Entomol. 2002, 95, 902–907. [Google Scholar] [CrossRef]
- Peng, C.; Williams, R.N. Influence of Food on Development, Survival, Fecundity, Longevity, and Sex Ratio of Glischrochilus quadrisignatus (Coleoptera: Nitidulidae). Environ. Entomol. 1991, 20, 205–210. [Google Scholar] [CrossRef]
- House, C.M.; Simmons, L.W.; Kotiaho, J.S.; Tomkins, J.L.; Hunt, J. Sex ratio bias in the dung beetle Onthophagus taurus: Adaptive allocation or sex-specific offspring mortality? Evol. Ecol. 2011, 25, 363–372. [Google Scholar] [CrossRef]
- Lachowsky, L.E.; Reid, M.L. Developmental mortality increases sex-ratio bias of a size-dimorphic bark beetle. Ecol. Entomol. 2014, 39, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Oddie, K.R. Size matters: Competition between male and female great tit offspring. J. Anim. Ecol. 2000, 69, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, C.; Gotthard, K.; Nylin, S. Mating system and the evolution of sex-specific mortality rates in two nymphalid butterflies. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 1823–1828. [Google Scholar] [CrossRef]
- Mittwoch, U. Sex-determining mechanisms in animals. Trends Ecol. Evol. 1996, 11, 63–67. [Google Scholar] [CrossRef]
- Austad, S.N.; Fischer, K.E. Sex Differences in Lifespan. Cell Metab. 2016, 23, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Schmolke, M.D. A Study of Aethina tumida: The Small Hive Beetle; Project Report; University of Rhodesia: Harare, Zimbabwe, 1974. [Google Scholar]
- Neumann, P.; Evans, J.D.; Pettis, J.S.; Pirk, C.W.W.; Schäfer, M.O.; Tanner, G.; Ellis, J.D. Standard methods for small hive beetle research. J. Apic. Res. 2013, 52, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Arbogast, R.T.; Torto, B.; Willms, S.; Teal, P.E.A. Trophic habits of Aethina tumida (Coleoptera: Nitidulidae): Their adaptive significance and relevance to dispersal. Environ. Entomol. 2009, 38, 561–568. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papach, A.; Gonthier, J.; Williams, G.R.; Neumann, P. Sex Ratio of Small Hive Beetles: The Role of Pupation and Adult Longevity. Insects 2019, 10, 133. https://0-doi-org.brum.beds.ac.uk/10.3390/insects10050133
Papach A, Gonthier J, Williams GR, Neumann P. Sex Ratio of Small Hive Beetles: The Role of Pupation and Adult Longevity. Insects. 2019; 10(5):133. https://0-doi-org.brum.beds.ac.uk/10.3390/insects10050133
Chicago/Turabian StylePapach, Anna, Jérémy Gonthier, Geoffrey R. Williams, and Peter Neumann. 2019. "Sex Ratio of Small Hive Beetles: The Role of Pupation and Adult Longevity" Insects 10, no. 5: 133. https://0-doi-org.brum.beds.ac.uk/10.3390/insects10050133