Adhesion Performance in the Eggs of the Philippine Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Morphology
2.3. Detachment Force Measurements
2.4. Surface Preparation
2.4.1. Glass
2.4.2. Epoxy Resin
2.5. Statistical Analysis
3. Results
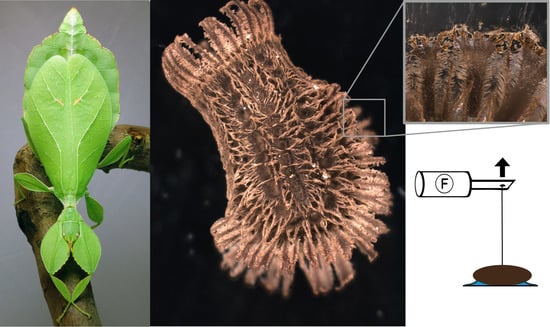
3.1. Egg Morphology
3.2. Pinnae Behavior and Adhesive Secretion
3.3. Egg Attachment
4. Discussion
4.1. Attachment Mechanism
4.2. Influence of Substrate Roughness
4.3. Influence of Surface Chemistry
4.4. Glue Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bedford, G.O. Biology and ecology of the Phasmatodea. Annu. Rev. Entomol. 1978, 23, 125–149. [Google Scholar] [CrossRef]
- Robertson, J.A.; Bradler, S.; Whiting, M.F. Evolution of oviposition techniques in stick and leaf insects (Phasmatodea). Front. Ecol. Evol. 2018, 6, 216. [Google Scholar] [CrossRef] [Green Version]
- Hennemann, F.H.; Conle, O.V.; Gottardo, M.; Bresseel, J. On certain species of the genus Phyllium Illiger, 1798, with proposals for an intra-generic systematization and the descriptions of five new species from the Philippines and Palawan (Phasmatodea: Phylliidae: Phylliinae: Phylliini). Zootaxa 2009, 2322, 1–83. [Google Scholar] [CrossRef]
- Cumming, R.T.; Bank, S.; Le Tirant, S.; Bradler, S. Notes on the leaf insects of the genus Phyllium of Sumatra and Java, Indonesia, including the description of two new species with purple coxae (Phasmatodea, Phylliidae). ZooKeys 2020, 913, 89. [Google Scholar] [CrossRef]
- Büscher, T.H.; Grohmann, C.; Bradler, S.; Gorb, S.N. Tarsal attachment pads in Phasmatodea (Hexapoda: Insecta). Zoologica 2019, 164, 1–94. [Google Scholar]
- Wedmann, S.; Bradler, S.; Rust, J. The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior. Proc. Natl. Acad. Sci. USA 2007, 104, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Béthoux, O.; Bradler, S.; Jacques, F.M.B.; Cui, Y.; Ren, D. Under cover at pre-angiosperm times: A cloaked phasmatodean insect from the Early Cretaceous Jehol biota. PLoS ONE 2014, 9, e91290. [Google Scholar] [CrossRef]
- Buckley, T.R.; Attanayake, D.; Bradler, S. Extreme convergence in stick insect evolution: Phylogenetic placement of the Lord Howe Island tree lobster. Proc. R. Soc. B 2009, 276, 1055–1062. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.D.; Soltis, D.E.; Soltis, P.S. The age and diversification of the angiosperms re-revisited. Am. J. Bot. 2010, 97, 1296–1303. [Google Scholar] [CrossRef]
- Magallón, S.; Castillo, A. Angiosperm diversification through time. Am. J. Bot. 2009, 96, 349–365. [Google Scholar] [CrossRef]
- Buckley, T.R.; Attanayake, D.; Nylander, J.A.A.; Bradler, S. The phylogenetic placement and biogeographical origins of the New Zealand stick insects (Phasmatodea). Syst. Entomol. 2010, 35, 207–225. [Google Scholar] [CrossRef]
- Bradler, S.; Cliquennois, N.; Buckley, T.R. Single origin of the Mascarene stick insects: Ancient radiation on sunken islands? BMC Evol. Biol. 2015, 15, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, J.; Bresseel, J.; Constant, J.; Kneubühler, B.; Leubner, F.; Michalik, P.; Bradler, S. Extreme convergence in egg-laying strategy across insect orders. Sci. Rep. 2015, 5, 7825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, S.; Letsch, H.; Bank, S.; Buckley, T.R.; Donath, A.; Liu, S.; Machida, R.; Meusemann, K.; Misof, B.; Podsiadlowski, L.; et al. Old World and New World Phasmatodea: Phylogenomics resolve the evolutionary history of stick and leaf insects. Front. Ecol. Evol. 2019, 7, 345. [Google Scholar] [CrossRef] [Green Version]
- Sellick, J. The range of egg capsule morphology within the phasmatodea and its relevance to the taxonomy of the order. Ital. J. Zool. 1997, 64, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Bradler, S. The Phasmatodea Tree of Life: Surprising facts and open questions in the evolution of stick and leaf insects. Entomol. Heute 2015, 27, 1–23. [Google Scholar]
- Bußhardt, P.; Wolf, H.; Gorb, S.N. Adhesive and frictional properties of tarsal attachment pads in two species of stick insects (Phasmatodea) with smooth and nubby euplantulae. Zoology 2012, 115, 135–141. [Google Scholar] [CrossRef]
- Büscher, T.H.; Gorb, S.N. Subdivision of the neotropical Prisopodinae Brunner von Wattenwyl, 1893 based on features of tarsal attachment pads (Insecta, Phasmatodea). ZooKeys 2017, 645, 1–11. [Google Scholar] [CrossRef]
- Büscher, T.H.; Gorb, S.N. Complementary effect of attachment devices in stick insects (Phasmatodea). J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef]
- Büscher, T.H.; Buckley, T.R.; Grohmann, C.; Gorb, S.N.; Bradler, S. The evolution of tarsal adhesive microstructures in stick and leaf insects (Phasmatodea). Front. Ecol. Evol. 2018, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Büscher, T.H.; Kryuchkov, M.; Katanaev, V.L.; Gorb, S.N. Versatility of Turing patterns potentiates rapid evolution in tarsal attachment microstructures of stick and leaf insects (Phasmatodea). J. R. Soc. Interface 2018, 15, 20180281. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, U. A review of the different types of egglaying in the Phasmida in relation to the shape of the eggs and with a discussion on their taxonomic importance (Insecta). Biol. Zentralblatt 1983, 102, 587–602. [Google Scholar]
- Sellick, J.T.C. Descriptive terminology of the phasmid egg capsule, with an extended key to the phasmid genera based on egg structure. Syst. Entomol. 1997, 22, 97–122. [Google Scholar] [CrossRef]
- Bradler, S.; Buckley, T.R. Biodiversity of Phasmatodea. In Insect Biodiversity: Science and Society II; Foottit, R.G., Adler, P.H., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 281–313. [Google Scholar] [CrossRef]
- Moore, P.D. How to get carried away. Nature 1993, 361, 304–305. [Google Scholar] [CrossRef]
- Stanton, A.O.; Dias, D.A.; O’Hanlon, J.C. Egg dispersal in the Phasmatodea: Convergence in chemical signalling strategies between plants and animals? J. Chem. Ecol. 2015, 41, 689–695. [Google Scholar] [CrossRef]
- Compton, S.G.; Ware, A.B. Ants disperse the elaiosome-bearing eggs of an african stick insect. Psyche 1991, 98, 207–213. [Google Scholar] [CrossRef]
- Hughes, L.; Westoby, M. Capitula on stick insects and elaiosomes on seeds: Convergent adaptations for burial by ants. Funct. Ecol. 1992, 6, 642–648. [Google Scholar] [CrossRef]
- Windsor, D.M.; Trapnell, D.W.; Amat, G. The egg capitulum of a Neotropical walkingstick, Calynda biscuspis, induces aboveground egg dispersal by the ponerine ant, Ectatomma ruidum. J. Inst. Behav. 1996, 9, 353–367. [Google Scholar] [CrossRef]
- Traveset, A.; Robertson, A.W.; Rodríguez-Pérez, J. A review on the role of endozoochory in seed germination. In Seed Dispersal: Theory and Its Application in a Changing World; Dennis, A.J., Schupp, E.W., Green, R.J., Westcott, D.A., Eds.; CABI: Wallingford, UK, 2007; pp. 78–103. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Kovalev, A.E.; Gorb, S.N. Slipping vs sticking: Water-dependent adhesive and frictional properties of Linum usitatissimum L. seed mucilaginous envelope and its biological significance. Acta Biomater. 2015, 17, 152–159. [Google Scholar] [CrossRef]
- Shelomi, M. Phasmid eggs do not survive digestion by Quails and Chickens. J. Orthoptera Res. 2011, 20, 159–162. [Google Scholar] [CrossRef]
- Suetsugu, K.; Funaki, S.; Takahashi, A.; Ito, K.; Yokoyama, T. Potential role of bird predation in the dispersal of otherwise flightless stick insects. Ecology 2018, 99, 1504–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-H.; Chu, Y.-I. The morphological study of the egg shell of the Tsuda’s giant stick insect Megacrania alpheus Westwood. NTU Phytopathol. Entomol. 1982, 9, 98–109. [Google Scholar]
- Ushirokita, M. Eggs of stick insect drifting in the wake of screw pine’s seed. Insectarium 1998, 35, 108–115. [Google Scholar]
- Kobayashi, S.; Usui, R.; Nomoto, K.; Ushirokita, M.; Denda, T.; Izawa, M. Does egg dispersal occur via the ocean in the stick insect Megacrania tsudai (Phasmida: Phasmatidae)? Ecol. Res. 2014, 29, 1025–1032. [Google Scholar] [CrossRef]
- Kobayashi, S.; Usui, R.; Nomoto, K.; Ushirokita, M.; Denda, T.; Izawa, M. Population dynamics and the effects of temperature on the eggs of the seawater-dispersed stick insect Megacrania tsudai (Phasmida: Phasmatidae). Zool. Stud. 2016, 55, 20. [Google Scholar] [CrossRef]
- Dehgan, B.; Yuen, C.K.K.H. Seed morphology in relation to dispersal, evolution; propagation of Cycas, L. Bot. Gaz. 1983, 144, 412–418. [Google Scholar] [CrossRef]
- Nakanishi, H. Dispersal ecology of the maritime plants in the Ryukyu Islands, Japan. Ecol. Res. 1988, 3, 163–173. [Google Scholar] [CrossRef]
- Cumming, R.T.; Leong, J.V.; Lohman, D.J. Leaf insects from Luzon, Philippines, with descriptions of four new species, the new genus Pseudomicrophyllium, and redescription of Phyllium (Phyllium) geryon Gray, 1843,(Phasmida: Phylliidae). Zootaxa 2017, 4365, 101–131. [Google Scholar] [CrossRef]
- Cumming, R.T. A new species of Phyllium (Phyllium) Illiger, 1798 from Mindanao, Philippines (Phasmida, Phylliidae). Zootaxa 2017, 4303, 297–300. [Google Scholar] [CrossRef]
- Cumming, R.T.; Le Tirant, S.; Hennemann, F.H. A new leaf insect from Obi Island (Wallacea, Indonesia) and description of a new subgenus within Phyllium Illiger, 1798 (Phasmatodea: Phylliidae: Phylliinae). Faunitaxys 2019, 7, 1–9. [Google Scholar]
- Clark, J.T. The eggs of leaf insects (Insecta: Phasmida). Zool. J. Linn. Soc. 1978, 63, 249–258. [Google Scholar] [CrossRef]
- Al Bitar, L.; Gorb, S.N.; Zebitz, C.P.W.; Voigt, D. Egg adhesion of the codling moth Cydia pomonella L. (Lepidoptera, Tortricidae) to various substrates: I. Leaf surfaces of different apple cultivars. Arthropod Plant Interact. 2012, 6, 471–488. [Google Scholar] [CrossRef]
- Al Bitar, L.; Gorb, S.N.; Zebitz, C.P.W.; Voigt, D. Egg adhesion of the codling moth Cydia pomonella L. (Lepidoptera, Tortricidae) to various substrates: II. Fruit surfaces of different apple cultivars. Arthropod Plant Interact. 2014, 8, 57–77. [Google Scholar] [CrossRef]
- Voigt, D.; Gorb, S.N. Egg attachment of the asparagus beetle Crioceris asparagi to the crystalline waxy surface of Asparagus officinalis. Proc. R. Soc. B 2010, 277, 895–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fordyce, J.A.; Nice, C.C. Variation in butterfly egg adhesion: Adaptation to local host plant senescence characteristics? Ecol. Lett. 2003, 6, 23–27. [Google Scholar] [CrossRef]
- Li, D.; Huson, M.G.; Graham, L.D. Proteinaceous adhesive secretions from insects; in particular the egg attachment glue of Opodiphthera sp. moths. Arch. Insect Biochem. 2008, 69, 85–105. [Google Scholar] [CrossRef]
- Yoshida, K.; Nagata, M. Adhesive strength of the glue substances in the colleterial glands of the silkmoth, Bombyx mori. J. Seric. Sci. Jpn. 1997, 66, 453–456. [Google Scholar] [CrossRef]
- Yago, M.; Mitamura, T.; Abe, S.; Hashimoto, S. Adhesive strength of glue-like substances from the colleterial glands of Antheraea yamamai and Rhodinia fugax. Int. J. Wild Silkmoth Silk 2001, 6, 11–15. [Google Scholar]
- England, M.W.; Sato, T.; Yagihashi, M.; Hozumi, A.; Gorb, S.N.; Gorb, E.V. Surface roughness rather than surface chemistry essentially affects insect adhesion. Beilstein J. Nanotechnol. 2016, 7, 1471–1479. [Google Scholar] [CrossRef] [Green Version]
- Habenicht, G. Kleben: Grundlagen, Technologien, Anwendung, 4th ed.; Springer: Berlin, Germany, 2002. [Google Scholar]
- Cogley, T.P.; Anderson, J.R.; Weintraub, J. Ultrastructure and function of the attachment organ of warble fly eggs (Diptera: Oestridae: Hypodermatinae). Int. J. Insect Morphol. Embryol. 1981, 10, 7–18. [Google Scholar] [CrossRef]
- Cogley, T.P.; Cogley, M.C. Morphology of the eggs of the human bot fly, Dermatobia hominis (L. Jr.) (Diptera: Cuterebridae) and their adherence to the transport carrier. Int. J. Insect Morph. Embryol. 1989, 18, 239–248. [Google Scholar] [CrossRef]
- Hinton, H.E. Biology of Insect Eggs; Pergamon Press: Oxford, UK, 1981. [Google Scholar] [CrossRef]
- Margaritis, L.H. Structure and physiology of eggshell. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 153–230. [Google Scholar]
- Miller, P.L. Oviposition behaviour and eggshell structure in some libellulid dragonflies, with particular reference to Brachythemis lacustris (Kirby) and Orthetrum coerulescens (Fabricius)(Anisoptera). Odonatologica 1987, 16, 361–374. [Google Scholar]
- Ivey, R.K.; Bailey, J.C.; Stark, B.P.; Lentz, D.L. A preliminary report of egg chorion features in dragonflies (Anisoptera). Odonatologica 1988, 17, 393–399. [Google Scholar]
- Trueman, J.W.H. Egg chorionic structures in Corduliidae and Libellulidae (Anisoptera). Odonatologica 1991, 20, 441–452. [Google Scholar]
- Sahlén, G. Ultrastructure of the eggshell and micropylar apparatus in Somatochlora metallica (Vander, L.), Orthetrum cancellatum (L.) and Sympetrum sanguineum (Müll.) (Anisoptera: Corduliidae, Libellulidae). Odonatologica 1994, 23, 255–269. [Google Scholar]
- Sahlén, G. Eggshell ultrastructure in Onychogomphus forcipatus unguiculatus (Vander Linden) (Odonata: Gomphidae). Int. J. Insect Morphol. Embryol. 1995, 24, 281–286. [Google Scholar] [CrossRef]
- Andrew, R.J.; Tembhare, D.B. Ultrastructural post-oviposition changes in the egg chorion of the dragon-fly, Zyxomma petiolatum Rambur (Odonata: Libellulidae). Int. J. Insect Morphol. Embryol. 1995, 24, 235–238. [Google Scholar] [CrossRef]
- Andrew, R.J.; Tembhare, D.B. Surface ultrastructure of the egg chorion of Bradinopyga geminata (Rambur) and Rhyothemis variegata variegata (Linn.). Fraseria 1996, 3, 1–5. [Google Scholar]
- Andrew, R.J. Egg chorionic ultrastructure of the dragonfly Tramea virginia (Rambur) (Anisoptera: Libellulidae). Odonatologica 2002, 31, 171–175. [Google Scholar]
- Gaino, E.; Piersanti, S.; Rebora, M. Egg envelope synthesis and chorion modification after oviposition in the dragonfly Libellula depressa (Odonata, Libellulidae). Tissue Cell 2008, 40, 317–324. [Google Scholar] [CrossRef]
- Gaino, E.; Rebora, M. Synthesis and function of the fibrous layers covering the eggs of Siphlonurus lacustris (Ephemeroptera, Siphlonuridae). Acta Zool. 2001, 82, 41–48. [Google Scholar] [CrossRef]
- Brinck, P. Reproductive system and mating in Ephemeroptera. Opusc. Entomol. 1957, 22, 1–37. [Google Scholar]
- Koss, R.W. Ephemeroptera Eggs: Sperm Guide Morphology and Adhesive Layer Formation. Trans. Am. Micros. Soc. 1970, 89, 295–299. [Google Scholar] [CrossRef]
- Gaino, E.; Mazzini, M. Scanning electron microscopy of the egg attachment structures of Electrogena zebrata (Ephemeroptera: Heptageniidae). Trans. Amer. Micros. Soc. 1987, 106, 114–119. [Google Scholar] [CrossRef]
- Gaino, E.; Mazzini, M. Fine structure of the chorionic projections of the egg of Rhithrogena kimminsi Thomas (Ephemeroptera: Heptageniidae) and their role in egg adhesion. Int. J. Insect Morphol. Embryol. 1988, 17, 113–120. [Google Scholar] [CrossRef]
- Gaino, E.; Mazzini, M. Chorionic adhesive material of the egg of the mayfly Habrophlebia eldae (Ephemeroptera, Leptophlebiidae): Morphology and synthesis. Boll. Zool. 1989, 56, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Wohlfart, E.; Wolff, J.O.; Arzt, E.; Gorb, S.N. The whole is more than the sum of all its parts: Collective effect of spider attachment organs. J. Exp. Biol. 2014, 217, 222–224. [Google Scholar] [CrossRef] [Green Version]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Gorb, E.V.; Kovalev, A.E.; Gorb, S.N. Attachment ability of the southern green stink bug Nezara viridula (Heteroptera: Pentatomidae). J. Comp. Physiol. A 2017, 203, 601–611. [Google Scholar] [CrossRef]
- Schaber, C.F.; Kreitschitz, A.; Gorb, S.N. Friction-active surfaces based on free-standing anchored cellulose nanofibrils. ACS Appl. Mater. Interfaces 2018, 10, 37566–37574. [Google Scholar] [CrossRef]
- Santos, R.; Gorb, S.N.; Jamar, V.; Flammang, P. Adhesion of echinoderm tube feet to rough surfaces. J. Exp. Biol. 2005, 208, 2555–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorb, S.N. Biological attachment devices: Exploring nature’s diversity for biomimetics. Phil. Trans. Math. Phys. Eng. Sci. 2008, 366, 1557–1574. [Google Scholar] [CrossRef]
- Scherge, M.; Gorb, S.N. Biological Micro- and Nanotribology; Springer: Heidelberg/Berlin, Germany, 2001. [Google Scholar]
- Filippov, A.; Popov, V.L.; Gorb, S.N. Shear induced adhesion: Contact mechanics of biological spatula-like attachment devices. J. Theor. Biol. 2011, 276, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Persson, B.N.J. On the mechanism of adhesion in biological systems. J. Chem. Phys. 2003, 118, 7614–7621. [Google Scholar] [CrossRef] [Green Version]
- Persson, B.N.J.; Gorb, S.N. The effect of surface roughness on the adhesion of elastic plates with application to biological systems. J. Chem. Phys. 2003, 119, 11437–11444. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.L.; Kendall, K.; Roberts, A.D.; Tabor, D. Surface energy and the contact of elastic solids. Proc. R. Soc. A 1971, 324, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Arzt, E.V.; Gorb, S.N.; Spolenak, R. From micro to nano contacts in biological attachment devices. Proc. Natl. Acad. Sci. USA 2003, 100, 10603–10606. [Google Scholar] [CrossRef] [Green Version]
- Gorb, S.N.; Heepe, L. Biological fibrillar adhesives: Functional principles and biomimetic applications. In Handbook of Adhesion Technology; da Silva, L., Oċhsner, A., Adams, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–37. [Google Scholar]
- Gorb, S.N. Attachment Devices of Insect Cuticle; Kluwer Academic: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Peressadko, A.; Gorb, S.N. Surface profile and friction force generated by insects. In Proceedings of the First International Industrial Conference Bionik 2004, Hannover, Germany, 22–23 April 2004; Boblan, I., Bannasch, R., Eds.; VDI Verlag: Düsseldorf, Germany, 2004; pp. 257–263. [Google Scholar]
- Voigt, D.; Schuppert, J.M.; Dattinger, S.; Gorb, S.N. Sexual dimorphism in the attachment ability of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) to rough substrates. J. Insect Physiol. 2008, 54, 765–776. [Google Scholar] [CrossRef]
- Bullock, J.M.R.; Federle, W. Division of labour and sex differences between fibrillar, tarsal adhesive pads in beetles: Effective elastic modulus and attachment performance. J. Exp. Biol. 2009, 212, 1876–1888. [Google Scholar] [CrossRef] [Green Version]
- Gorb, E.V.; Gorb, S.N. Effects of surface topography and chemistry of Rumex obtusifolius leaves on the attachment of the beetle Gastrophysa viridula. Entomol. Exp. Appl. 2009, 130, 222–228. [Google Scholar] [CrossRef]
- Gorb, E.V.; Hosoda, N.; Miksch, C.; Gorb, S.N. Slippery pores: Anti-adhesive effect of nanoporous substrates on the beetle attachment system. J. R. Soc. Interface 2010, 7, 1571–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prüm, B.; Seidel, R.; Bohn, H.F.; Speck, T. Plant surfaces with cuticular folds are slippery for beetles. J. R. Soc. Interface 2012, 9, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramaswamy, S.B.; Ma, W.K.; Baker, G.T. Sensory cues and receptors for oviposition by Heliothis virescens. Entomol. Exp. Appl. 1987, 43, 159–168. [Google Scholar] [CrossRef]
- Ramaswamy, S.B. Host finding by moths: Sensory modalities and behaviours. J. Insect Physiol. 1988, 34, 235–249. [Google Scholar] [CrossRef]
- Hilker, M.; Meiners, T. Early herbivore alert: Insect eggs induce plant defense. J. Chem. Ecol. 2006, 32, 1379–1397. [Google Scholar] [CrossRef]
- Grohmann, C.; Blankenstein, A.; Koops, S.; Gorb, S.N. Attachment of Galerucella nymphaeae (Coleoptera, Chrysomelidae) to surfaces with different surface energy. J. Exp. Biol. 2014, 217, 4213–4220. [Google Scholar] [CrossRef] [Green Version]
- Israelachvili, J. Intermolecular and Surface Forces; Academic Press: London, UK, 1992; pp. 415–467. [Google Scholar]
- Kendall, K. The adhesion and surface energy of elastic solids. J. Phys. D 1971, 4, 1186. [Google Scholar] [CrossRef]
- Lüken, D.; Voigt, D.; Gorb, S.N.; Zebitz, C.P.W. Die Tarsenmorphologie und die Haftfähigkeit des Schwarzen Batatenkäfers Cylas puncticollis (Boheman) auf glatten Oberflächen mit unterschiedlichen physiko-chemischen Eigenschaften. Mitt. Dtsch. Ges. Allg. Angew. Ent. 2009, 17, 109–113. [Google Scholar]
- Voigt, D.; Gorb, S.N. Attachment ability of sawfly larvae to smooth surfaces. Arthropod Struct. Dev. 2012, 41, 145–153. [Google Scholar] [CrossRef]
- Thomas, J.; Peppas, N. Adhesives. In Encyclopedia of Biomaterials and Biomedical Engineering; Wnek, G.E., Bowlin, G.L., Eds.; Taylor & Francis: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2008; pp. 1–7. [Google Scholar] [CrossRef]
- Woolman, C.; Dhannasiri, B. Food plants for Phyllium bioculatum Gray in Sri Lanka. Phasmid Stud. 1995, 4, 33. [Google Scholar]
- Wang, H.; Shi, H.; Li, Y.; Yu, Y.; Zhang, J. Seasonal variations in leaf capturing of particulate matter, surface wettability and micromorphology in urban tree species. Front. Env. Sci. Eng. 2013, 7, 579–588. [Google Scholar] [CrossRef]
- Mohammed-Ziegler, I.; Oszlánczi, Á.; Somfai, B.; Hórvölgyi, Z.; Pászli, I.; Holmgren, A.; Forsling, W. Surface free energy of natural and surface-modified tropical and European wood species. J. Adhes. Sci. Technol. 2004, 18, 687–713. [Google Scholar] [CrossRef]
- Prüm, B.; Bohn, H.F.; Seidel, R.; Rubach, S.; Speck, T. Plant surfaces with cuticular folds and their replicas: Influence of microstructuring and surface chemistry on the attachment of a leaf beetle. Acta Biomater. 2013, 9, 6360–6368. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Wright, J. Spatial patterns and recent trends in the climate of tropical rainforest regions. Philos. Trans. R. Soc. B 2004, 359, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Yago, M. Curious oviposition behavior in Phyllium westwoodii (Phasmatodea: Phylliidae): Preliminary observations. J. Insect Sci. 2015, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Subramani, K.; Ahmed, W. Self-assembly of proteins and peptides and their applications in bionanotechnology and dentistry. In Emerging Nanotechnologies in Dentistry 2; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–249. [Google Scholar] [CrossRef]
- Beament, J.W.L.; Lal, R. Penetration through the Egg-shell of Pieris brassicae (L.). Bull. Entomol. Res. 1957, 48, 109–125. [Google Scholar] [CrossRef]
- Riley, R.C.; Forgash, A.J. Drosophila melanogaster eggshell adhesive. J. Insect Physiol. 1967, 13, 509–517. [Google Scholar] [CrossRef]
- Amornsak, W.; Noda, T.; Yamashita, O. Accumulation of glue proteins in the developing colleterial glands of the silkworm, Bombyx mori. J. Seric. Sci. Jpn. 1992, 61, 123–130. [Google Scholar] [CrossRef]
- Burkhart, C.N.; Stankiewicz, B.A.; Pchalek, I.; Kruge, M.A.; Burkhart, C.G. Molecular composition of the louse sheath. J. Parasitol. 1999, 85, 559–561. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Y.; Jiang, Y.; Xu, M. Proteome analysis of the silkworm (Bombyx mori. L) colleterial gland during different development stages. Arch. Insect Biochem. Physiol. 2006, 61, 42–50. [Google Scholar] [CrossRef]
- Betz, O. Adhesive Exocrine Glands in Insects: Morphology, Ultrastructure; Adhesive Secretion. In Biological Adhesive, Systems; von Byern, J., Grunwald., I., Eds.; Springer: Vienna, Austria, 2010; pp. 111–152. [Google Scholar] [CrossRef]
- Burgess, I.F. Do nit removal formulations and other treatments loosen head louse eggs and nits from hair? Med. Vet. Entomol. 2010, 24, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.D. Biological adhesives from nature. In Encyclopedia of Biomaterials and Biomedical Engineering; Wnek, G.E., Bowlin, G.L., Eds.; Taylor & Francis: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2008; pp. 236–253. [Google Scholar]
- Dalziel, M.; Crispin, M.; Scanlan, C.N.; Zitzmann, N.; Dwek, R.A. Emerging principles for the therapeutic exploitation of glycosylation. Science 2014, 343, 1235681. [Google Scholar] [CrossRef] [PubMed]
- Xi, E.; Venkateshwaran, V.; Li, L.; Rego, N.; Patel, A.J.; Garde, S. Hydrophobicity of proteins and nanostructured solutes is governed by topographical and chemical context. Proc. Natl. Acad. Sci. USA 2017, 114, 13345–13350. [Google Scholar] [CrossRef] [Green Version]
- Beňová-Liszeková, D.; Beňo, M.; Farkaš, R. Fine infrastructure of released and solidified Drosophila larval salivary secretory glue using SEM. Bioinspir. Biomim. 2019, 14, 055002. [Google Scholar] [CrossRef]
- Borne, F.; Kovalev, A.E.; Gorb, S.N.; Courtier-Orgogozo. The glue produced by Drosophila melanogaster for pupa adhesion is universal. J. Exp. Biol. 2020, 223, jeb220608. [Google Scholar] [CrossRef] [PubMed]
- Vandermeer, J.H.; Goldberg, D.E. Population Ecology: First Principles, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Rentz, D.C. Grasshopper Country: The Abundant Orthopteroid Insects of Australia; UNSW Press: Sydney, Australia, 1996. [Google Scholar]
- Patel, A.K.; Mathias, J.-D.; Michaud, P. Polysaccharides as adhesives. Rev. Adhes. Adhes. 2013, 1, 312–345. [Google Scholar] [CrossRef]
- Karak, N. Biopolymers for paints and surface coatings. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 333–368. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büscher, T.H.; Quigley, E.; Gorb, S.N. Adhesion Performance in the Eggs of the Philippine Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae). Insects 2020, 11, 400. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11070400
Büscher TH, Quigley E, Gorb SN. Adhesion Performance in the Eggs of the Philippine Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae). Insects. 2020; 11(7):400. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11070400
Chicago/Turabian StyleBüscher, Thies H., Elise Quigley, and Stanislav N. Gorb. 2020. "Adhesion Performance in the Eggs of the Philippine Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae)" Insects 11, no. 7: 400. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11070400