Persistence of a Yeast-Based (Hanseniaspora uvarum) Attract-and-Kill Formulation against Drosophila suzukii on Grape Leaves
Abstract
:Simple Summary
Abstract
1. Introduction
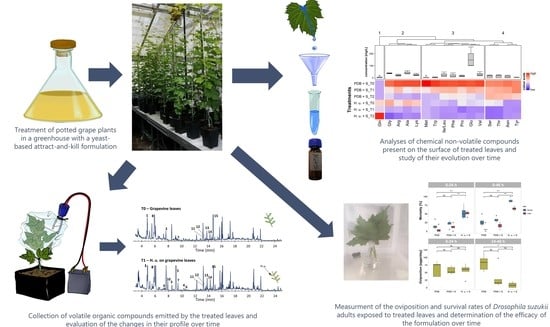
2. Materials and Methods
2.1. Insect Rearing
2.2. Yeast Cultivation
2.3. Grape Plants Cultivation
2.4. Treatments
2.5. Mortality and Oviposition Assays
2.6. Sample Preparation and Analysis of Chemical and Metabolic Compounds
2.7. Volatile Compounds Collection and Characterization by CLSA-GC-MS
2.8. Statistical Analysis
3. Results and Discussion
3.1. Mortality and Oviposition Assessment One Day after Treatment (T1)
3.2. Mortality and Oviposition Assessment One Week after Treatment (T2)
3.3. Carbohydrates and Sugar Alcohols
3.4. Amino Acids
3.5. Organic Acids
3.6. VOCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive Pest of Ripening Soft Fruit Expanding its Geographic Range and Damage Potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectol. 2012, 65, 149–160. [Google Scholar]
- Farnsworth, D.; Hamby, K.A.; Bolda, M.; Goodhue, R.E.; Williams, J.C.; Zalom, F.G. Economic analysis of revenue losses and control costs associated with the spotted wing drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. Pest Manag. Sci. 2017, 73, 1083–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haviland, D.R.; Beers, E.H. Chemical Control Programs for Drosophila suzukii that Comply with International Limitations on Pesticide Residues for Exported Sweet Cherries. J. Integr. Pest Manag. 2012, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mori, B.A.; Whitener, A.B.; Leinweber, Y.; Revadi, S.; Beers, E.H.; Witzgall, P.; Becher, P.G. Enhanced yeast feeding following mating facilitates control of the invasive fruit pest Drosophila suzukii. J. Appl. Ecol. 2017, 54, 170–177. [Google Scholar] [CrossRef]
- Noble, R.; Dobrovin-Pennington, A.; Phillips, A.; Cannon, M.F.L.; Shaw, B.; Fountain, M.T. Improved insecticidal control of spotted wing drosophila (Drosophila suzukii) using yeast and fermented strawberry juice baits. Crop Prot. 2019, 125, 104902. [Google Scholar] [CrossRef]
- Knight, A.L.; Basoalto, E.; Yee, W.; Hilton, R.; Kurtzman, C.P. Adding yeasts with sugar to increase the number of effective insecticide classes to manage Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in cherry. Pest Manag. Sci. 2016, 72, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.H.; Adams, T.; Werle, C.T.; Sampson, B.J.; Adamczyk, J.J.; Rogg, H.; Landolt, P.J. A four-component synthetic attractant for Drosophila suzukii (Diptera: Drosophilidae) isolated from fermented bait headspace. Pest. Manag. Sci. 2014, 70, 324–331. [Google Scholar] [CrossRef]
- Iglesias, L.E.; Nyoike, T.W.; Liburd, O.E. Effect of Trap Design, Bait Type, and Age on Captures of Drosophila suzukii (Diptera: Drosophilidae) in Berry Crops. J. Econ. Entomol. 2014, 107, 1508–1518. [Google Scholar] [CrossRef] [Green Version]
- Roubos, C.R.; Gautam, B.K.; Fanning, P.D.; Van Timmeren, S.; Spies, J.; Liburd, O.E.; Isaacs, R.; Curry, S.; Little, B.A.; Sial, A.A. Impact of phagostimulants on effectiveness of OMRI-listed insecticides used for control of spotted-wing drosophila (Drosophila suzukii Matsumura). J. Appl. Entomol. 2019, 143, 609–625. [Google Scholar] [CrossRef]
- Ljungdahl, P.O.; Daignan-Fornier, B. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics 2012, 190, 885–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitaler, U.; Bianchi, F.; Eisenstecken, D.; Castellan, I.; Angeli, S.; Dordevic, N.; Robatscher, P.; Vogel, R.F.; Koschier, E.H.; Schmidt, S. Yeast species affects feeding and fitness of Drosophila suzukii adults. J. Pest Sci. 2020, 93, 1295–1309. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Changes in the profle of volatile compounds and amino acids during cider fermentation using dessert variety of apples. Eur. Food Res. Technol. 2014, 239, 67–77. [Google Scholar] [CrossRef]
- Callejón, R.M.; Margulies, B.; Hirson, G.D.; Ebeler, S.E. Dynamic changes in volatile compounds during fermentation of Cabernet Sauvignon grapes with and without skins. Am. J. Enol. Vitic. 2012, 63, 301–312. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Rogg, H.; Landolt, P.J. Identification and Field Evaluation of Fermentation Volatiles from Wine and Vinegar that Mediate Attraction of Spotted Wing Drosophila, Drosophila suzukii. J. Chem. Ecol. 2012, 38, 1419–1431. [Google Scholar] [CrossRef]
- Fountain, M.T.; Bennett, J.; Cobo-Medina, M.; Conde Ruiz, R.; Deakin, G.; Delgado, A.; Harrison, R.; Harrison, N. Alimentary microbes of winter-form Drosophila suzukii. Insect Mol. Biol. 2018, 27, 383–392. [Google Scholar] [CrossRef]
- Hamby, K.A.; Hernández, A.; Boundy-Mills, K.; Zalom, F.G. Associations of yeasts with spotted-wing Drosophila (Drosophila suzukii; Diptera: Drosophilidae) in cherries and raspberries. Appl. Environ. Microbiol. 2012, 78, 4869–4873. [Google Scholar] [CrossRef] [Green Version]
- Bellutti, N.; Gallmetzer, A.; Innerebner, G.; Schmidt, S.; Zelger, R.; Koschier, E.H. Dietary yeast affects preference and performance in Drosophila suzukii. J. Pest Sci. 2018, 91, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.T.; Koivunen, E.E.; Swett, C.L.; Hamby, K.A. Associations between Drosophila suzukii (Diptera: Drosophilidae) and Fungi in Raspberries. Environ. Entomol. 2019, 48, 68–79. [Google Scholar] [CrossRef]
- Scheidler, N.H.; Liu, C.; Hamby, K.A.; Zalom, F.G.; Syed, Z. Volatile codes: Correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.T.; Hamby, K.A. Differential Impacts of Yeasts on Feeding Behavior and Development in Larval Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreazza, F.; Bernardi, D.; Baronio, C.A.; Pasinato, J.; Nava, D.E.; Botton, M. Toxicities and effects of insecticidal toxic baits to control Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae). Pest Manag. Sci. 2017, 73, 146–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H. ggplot2 Elegant Graphics for Data Analysis (Use R!), 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Cowles, R.S.; Rodriguez-Saona, C.; Holdcraft, R.; Loeb, G.M.; Elsensohn, J.E.; Hesler, S.P. Sucrose Improves Insecticide Activity Against Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2015, 108, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, S.L.; Mehlferber, E.; Moore, P.J. Life-history trade-offs under different larval diets in Drosophila suzukii (Diptera: Drosophilidae). Physiol. Entomol. 2015, 40, 2–9. [Google Scholar] [CrossRef]
- Clymans, R.; Van Kerckvoorde, V.; Bangels, E.; Akkermans, W.; Alhmedi, A.; De Clercq, P.; Beliën, T.; Bylemans, D. Olfactory preference of Drosophila suzukii shifts between fruit and fermentation cues over the season: Effects of physiological status. Insects 2019, 10, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swoboda-Bhattarai, K.A.; McPhie, D.R.; Burrack, H.J. Reproductive Status of Drosophila suzukii (Diptera: Drosophilidae) Females Influences Attraction to Fermentation-Based Baits and Ripe Fruits. J. Econ. Entomol. 2017, 110, 1648–1652. [Google Scholar] [CrossRef]
- Argüelles, J.C. Physiological roles of trehalose in bacteria and yeasts: A comparative analysis. Arch. Microbiol. 2000, 174, 217–224. [Google Scholar] [CrossRef]
- Zaunmüller, T.; Unden, G. Transport of sugars and sugar alcohols by lactic acid bacteria. In Biology of Microorganisms on Grapes, in Must and in Wine, 1st ed.; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Berlin, Germany, 2009; pp. 149–165. [Google Scholar]
- Wernke, M.J. Glycerol. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 754–756. [Google Scholar]
- Biolchini, M.; Murru, E.; Anfora, G.; Loy, F.; Banni, S.; Crnjar, R.; Sollai, G. Fat storage in Drosophila suzukii is influenced by different dietary sugars in relation to their palatability. PLoS ONE 2017, 12, 1–18. [Google Scholar] [CrossRef]
- Isono, K.; Morita, H.; Kohatsu, S.; Ueno, K.; Matsubayashi, H.; Yamamoto, M.T. Trehalose sensitivity of the gustatory receptor neurons expressing wild-type, mutant and ectopic Gr5a in Drosophila. Chem. Senses 2005, 30, 275–276. [Google Scholar] [CrossRef] [Green Version]
- Dahanukar, A.; Lei, Y.T.; Kwon, J.Y.; Carlson, J.R. Two Gr Genes Underlie Sugar Reception in Drosophila. Neuron 2007, 56, 503–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slone, J.; Daniels, J.; Amrein, H. Sugar Receptors in Drosophila. Curr. Biol. 2007, 17, 1809–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisotsky, Z.; Medina, A.; Freeman, E.; Dahanukar, A. Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nat. Neurosci. 2011, 14, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Kwon, J.Y.; Seo, J.T.; Shin, D.M.; Moon, S.J. Drosophila Gr64e mediates fatty acid sensing via the phospholipase C pathway. PLoS Genet. 2018, 14, e1007229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Huang, R.; Fu, X.; Wang, G.; Qi, W.; Mao, D.; Shi, Z.; Shen, W.L.; Wang, L. A post-ingestive amino acid sensor promotes food consumption in Drosophila. Cell Res. 2018, 28, 1013–1025. [Google Scholar] [CrossRef] [Green Version]
- Salthammer, T. Very volatile organic compounds: An understudied class of indoor air pollutants. Indoor Air 2016, 26, 25–38. [Google Scholar] [CrossRef]
- Grosjean, D. Atmospheric reactions of pyruvic acid. Atmos. Environ. 1983, 17, 2379–2382. [Google Scholar] [CrossRef]
- Charlu, S.; Wisotsky, Z.; Medina, A.; Dahanukar, A. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat. Commun. 2013, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Revadi, S.; Vitagliano, S.; Rossi Stacconi, M.V.; Ramasamy, S.; Mansourian, S.; Carlin, S.; Vrhovsek, U.; Becher, P.G.; Mazzoni, V.; Rota-Stabelli, O.; et al. Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol. Entomol. 2015, 40, 54–64. [Google Scholar] [CrossRef]
- Liman, E.R.; Zhang, Y.V.; Montell, C. Peripheral coding of taste. Neuron 2014, 81, 984–1000. [Google Scholar] [CrossRef] [Green Version]
- May, B.; Lange, B.M.; Wüst, M. Biosynthesis of sesquiterpenes in grape berry exocarp of Vitis vinifera L.: Evidence for a transport of farnesyl diphosphate precursors from plastids to the cytosol. Phytochemistry 2013, 95, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalal, M.; Winkler, J.B.; Gourrat, K.; Trouvelot, S.; Adrian, M.; Schnitzler, J.P.; Jamois, F.; Daire, X. Sesquiterpene volatile organic compounds (VOCs) are markers of elicitation by sulfated laminarine in grapevine. Front. Plant Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erland, L.A.E.; Rheault, M.R.; Mahmoud, S.S. Insecticidal and oviposition deterrent effects of essential oils and their constituents against the invasive pest Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Crop Prot. 2015, 78, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Batista, M.R.D.; Uno, F.; Chaves, R.D.; Tidon, R.; Rosa, C.A.; Klaczko, L.B. Differential attraction of drosophilids to banana baits inoculated with Saccharomyces cerevisiae and Hanseniaspora uvarum within a Neotropical forest remnant. PeerJ 2017, 5, e3063. [Google Scholar] [CrossRef] [Green Version]







| No | Compound | LRI ꓯ on HP-5MS | Reference LRI | Grapevine Leaves (T0) | Grapevine Leaves + H. uvarum (T1) | Grapevine Leaves + H. uvarum (T2) | Significance (ANOVA p Value, df = 2, 15) |
|---|---|---|---|---|---|---|---|
| ALDEHYDES 1 | Benzaldehyde α | 961 | 965 | 0.33 ± 0.28 a | 2.85 ± 1.35 b | 1.20 ± 1.07 ab | F = 8.039, 0.004 ** |
| ALCOHOLS 2 | 2-phenylethanol α | 1114 | 1116 | 0.18 ± 0.20 a | 1.09 ± 0.45 b | 0.48 ± 0.36 a | F = 8.648, 0.004 ** |
| ACIDS 3 | octanoic acid α | 1171 | 1175 | nd a | 0.70 ± 0.30 b | 0.53 ± 0.39 b | F = 8.431, 0.003 ** |
| ACETATES 4 | 2-phenylethyl acetate α | 1258 | 1265 | nd a | 1.48 ± 0.60 b | 0.96 ± 0.63 b | F = 11.27, 0.001 ** |
| AROMATICS | |||||||
| 5 | methyl salicylate α | 1193 | 1190 | nd a | 12.73 ± 9.00 b | 2.25 ± 2.17 a | F = 14.45, p < 0.001 *** |
| 6 | Indole α | 1291 | 1288 | nd a | 0.57 ± 0.24 ab | 1.82 ± 1.85 b | F = 3.75, 0.047 * |
| GLVs 7 | (Z)-3-hexenyl butyrate α | 1188 | 1180 | 0.06 ± 0.08 | 2.63 ± 2.39 | 3.87 ± 4.99 | F = 1.846, ns |
| TERPENES | |||||||
| 8 | 1,8-cineole | 1030 | 1030 | 7.80 ± 4.37 | 24.95 ± 14.86 | 15.98 ± 12.13 | F = 2.851, ns |
| 9 | Linalool α | 1103 | 1101 | nd a | 1.53 ± 0.88 ab | 2.79 ± 1.54 b | F = 9.299, 0.002 ** |
| 10 | DMNT α | 1117 | 1105 | nd a | 9.07 ± 4.70 b | 7.51 ± 5.32 b | F = 6.996, 0.007 ** |
| 11 | beta-caryophyllene α | 1418 | 1418 | 0.20 ± 0.15 a | 8.90 ± 3.83 b | 5.80 ± 5.39 ab | F = 6.659, 0.008 ** |
| 12 | Humulene α | 1452 | 1440 | 0.29 ± 0.11 a | 5.11 ± 1.94 b | 3.49 ± 2.66 b | F = 8.334, 0.004 ** |
| 13 | germacrene D α | 1480 | 1480 | 0.80 ± 0.46 a | 4.61 ± 2.08 b | 3.35 ± 2.41 b | F = 5.478, 0.016 * |
| 14 | trans-alpha-bergamotene | 1495 | 1496 | 0.15 ± 0.22 | 1.99 ± 0.62 | 3.63 ± 3.66 | F = 3.284, ns |
| 15 | (E,E)-alpha-farnesene α | 1508 | 1500 | 0.13 ± 0.11 a | 25.02 ± 13.40 a | 136.25 ± 115.62 b | F = 5.816, 0.013 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, F.; Spitaler, U.; Castellan, I.; Cossu, C.S.; Brigadoi, T.; Duménil, C.; Angeli, S.; Robatscher, P.; Vogel, R.F.; Schmidt, S.; et al. Persistence of a Yeast-Based (Hanseniaspora uvarum) Attract-and-Kill Formulation against Drosophila suzukii on Grape Leaves. Insects 2020, 11, 810. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110810
Bianchi F, Spitaler U, Castellan I, Cossu CS, Brigadoi T, Duménil C, Angeli S, Robatscher P, Vogel RF, Schmidt S, et al. Persistence of a Yeast-Based (Hanseniaspora uvarum) Attract-and-Kill Formulation against Drosophila suzukii on Grape Leaves. Insects. 2020; 11(11):810. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110810
Chicago/Turabian StyleBianchi, Flavia, Urban Spitaler, Irene Castellan, Carlo S. Cossu, Timothy Brigadoi, Claire Duménil, Sergio Angeli, Peter Robatscher, Rudi F. Vogel, Silvia Schmidt, and et al. 2020. "Persistence of a Yeast-Based (Hanseniaspora uvarum) Attract-and-Kill Formulation against Drosophila suzukii on Grape Leaves" Insects 11, no. 11: 810. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110810