Dose–Response Assay for Synthetic Mosquito (Diptera: Culicidae) Attractant Using a High-Throughput Screening System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Insecticide Susceptibility Assays
2.3. Chemical Attractant
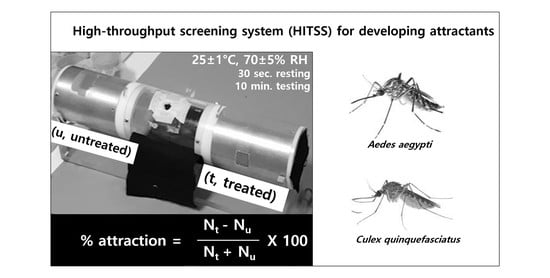
2.4. High-Throughput Screening System (HITSS)
2.5. Analysis
3. Results
3.1. WHO Bioassay
3.2. BG-Lure Effects
3.2.1. A Pack of BG-Lure (10 g)
3.2.2. Optimizing Dose of BG-Lure
3.2.3. Dry Ice (1 g)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.; Wint, G.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L. The current and future global distribution and population at risk of dengue. Nat. Micro. 2019, 4, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.A.; Walker, E.D. Mosquitoes (Culicidae). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Elsevier: London, UK, 2019; Chapter 15; pp. 261–325. [Google Scholar]
- Clements, A.N. Sensory reception and behavior. In The Biology of Mosquitoes; CABI: New York, NY, USA, 1999; Volume 2, pp. 433–479. [Google Scholar]
- Shen, H.H. Inner workings: How do mosquitoes smell us? The answers could help eradicate disease. Proc. Natl. Acad. Sci. USA 2017, 114, 2096–2098. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.J. Differentiation of host-seeking behavior from blood-feeding behavior in overwintering Culex pipiens (Diptera: Culicidae) and observations on gonotrophic dissociation. J. Med. Entomol. 1983, 20, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Clements, A.N. Transmission of viruses and interactions with bacteria. In The Biology of Mosquitoes; CABI: New York, NY, USA, 1992; Volume 3, pp. 1–88. [Google Scholar]
- Brown, A.; Sarkaria, D.; Thompson, R. Studies on the responses of the female Aëdes mosquito. Part I—The search for attractant vapours. Bull. Entomol. Res. 1951, 42, 105–114. [Google Scholar] [CrossRef]
- Takken, W. The role of olfaction in host-seeking of mosquitoes: A review. Int. J. Trop. Insect Sci. 1991, 12, 287–295. [Google Scholar] [CrossRef]
- Suh, E.; Bohbot, J.D.; Zwiebel, L.J. Peripheral olfactory signaling in insects. Curr. Opin. Insect Sci. 2014, 6, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keil, T.A. Sensory cilia in arthropods. Arthropod Struct. Dev. 2012, 41, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): A review. Bull. Entomol. Res. 1980, 70, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Grant, A.; Aghajanian, J.; O’Connell, R.; Wigton, B. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J. Comp. Physiol. 1995, 177, 389–396. [Google Scholar] [CrossRef]
- Syed, Z. Chemical ecology and olfaction in arthropod vectors of diseases. Curr. Opin. Insect Sci. 2015, 10, 83–89. [Google Scholar] [CrossRef]
- Acree, F.; Turner, R.; Gouck, H.; Beroza, M.; Smith, N. L-Lactic acid: A mosquito attractant isolated from humans. Science 1968, 161, 1346–1347. [Google Scholar] [CrossRef]
- Smith, C.N.; Smith, N.; Gouck, H.K.; Weidhaas, D.; Gilbert, I.; Mayer, M.; Smittle, B.; Hofbauer, A. L-lactic acid as a factor in the attraction of Aedes aegypti (Diptera: Culicidae) to human hosts. Ann. Entomol. Soc. Am. 1970, 63, 760–770. [Google Scholar] [CrossRef]
- Hall, D.; Beevor, P.; Cork, A.; Nesbitt, B.F.; Vale, G. 1-Octen-3-ol. Int. J. Trop. Insect Sci. 1984, 5, 335–339. [Google Scholar] [CrossRef]
- Smallegange, R.C.; Qiu, Y.T.; Loon, J.J.; Takken, W. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem. Senses 2005, 30, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sithiprasasna, R.; Jaichapor, B.; Chanaimongkol, S.; Khongtak, P.; Lealsirivattanakul, T.; Trong, S.; Burkett, D.A.; Perich, M.J.; Wirtz, R.A.; Coleman, R.E. Evaluation of candidate traps as tools for conducting surveillance for Anopheles mosquitoes in a malaria-endemic area in western Thailand. J. Med. Eentomol. 2004, 41, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Coutinho-Abreu, I.V.; Sharma, V.K.; Cui, L.; Yan, G.; Ray, A. Odorant ligands for the CO2 receptor in two Anopheles vectors of malaria. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Todd, J.L.; Baker, T.C. Function of peripheral olfactory organs. In Insect Olfaction; Hansson, B.S., Ed.; Springer: Heidelberg, Germany, 1999; Chapter 3; pp. 67–96. [Google Scholar]
- Bowen, M. The sensory physiology of host-seeking behavior in mosquitoes. Annu. Rev. Entomol. 1991, 36, 139–158. [Google Scholar] [CrossRef]
- Chuaycharoensuk, T.; Juntarajumnong, W.; Boonyuan, W.; Bangs, M.J.; Akratanakul, P.; Thammapalo, S.; Jirakanjanakit, N.; Tanasinchayakul, S.; Chareonviriyaphap, T. Frequency of pyrethroid resistance in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Thailand. J. Vector Ecol. 2011, 36, 204–212. [Google Scholar] [CrossRef] [PubMed]
- WHO. Test. Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes; World Health Organization: Geneva, Switzerland, 2016; p. 48. [Google Scholar]
- Grieco, J.P.; Achee, N.L.; Sardelis, M.R.; Chauhan, K.R.; Roberts, D.R. A novel high-throughput screening system to evaluate the behavioral response of adult mosquitoes to chemicals. J. Am. Mosq. Contr. Assoc. 2005, 21, 404–411. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Kline, D.L. Olfactory attractants for mosquito surveillance and control: 1-octen-3-ol. J. Am. Mosq. Contr. Assoc. 1994, 10, 280–287. [Google Scholar]
- Silver, J.B. Mosquito Ecology: Field Sampling Methods, 3rd ed.; Springer: New York, NY, USA, 2008; pp. 947–1025. [Google Scholar]
- Kline, D.L. Traps and trapping techniques for adult mosquito control. J. Am. Mosq. Contr. Assoc. 2006, 22, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Muirhead-Thompson, R. Trap Responses of Flying Insects: The Influence of Trap Design on Capture Efficiency; Academic Press: London, UK, 2012; pp. 180–258. [Google Scholar]
- Salazar, F.V.; Achee, N.L.; Grieco, J.P.; Prabaripai, A.; Ojo, T.A.; Eisen, L.; Dureza, S.; Polsomboon, S.; Chareonviriyaphap, T. Effect of Aedes aegypti exposure to spatial repellent chemicals on BG-Sentinel™ trap catches. Parasit. Vectors 2013, 6, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cribellier, A.; Erp, J.A.; Hiscox, A.; Lankheet, M.J.; Leeuwen, J.L.; Spitzen, J.; Muijres, F.T. Flight behaviour of malaria mosquitoes around odour-baited traps: Capture and escape dynamics. R. Soc. Open Sci. 2018, 5, 180246. [Google Scholar] [CrossRef] [Green Version]
- Van De Straat, B.; Hiscox, A.; Takken, W.; Burkot, T.R. Evaluating synthetic odours and trap designs for monitoring Anopheles farauti in Queensland, Australia. Malar. J. 2019, 18, 299. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; James, J. Attraction of Aedes aegypti (L.): Responses to human arms, carbon dioxide, and air currents in a new type of olfactometer. Bull. Entomol. Res. 1969, 58, 629–642. [Google Scholar] [CrossRef]
- Geier, M.; Sass, H.; Boeckh, J. A search for components in human body odour that attract females of Aedes aegypti. In Olfaction in Mosquitoes-Host Interactions; Bock, G.R., Cardew, G., Eds.; Ciba Fundation Symposium: London, UK, 1996; pp. 132–148. [Google Scholar]
- Geier, M.; Boeckh, J. A new Y-tube olfactometer for mosquitoes to measure the attractiveness of host odours. Entomol. Exp. Appl. 1999, 92, 9–19. [Google Scholar] [CrossRef]
- Kline, D.L.; Bernier, U.R.; Posey, K.H.; Barnard, D.R. Olfactometric evaluation of spatial repellents for Aedes aegypti. J. Med. Entomol. 2003, 40, 463–467. [Google Scholar] [CrossRef] [Green Version]
- WHO. Guidelines for Efficacy Testing of Spatial Repellents; World Health Oranization: Geneva, Switzerland, 2013; pp. 5–9. [Google Scholar]
- WHO. Efficacy-Testing of Traps for Control of Aedes spp. Mosquito Vectors; World Health Organization: Geneva, Switzerland, 2018; pp. 5–7. [Google Scholar]
- Knols, B.G.; Jong, R.D.; Takken, W. Trapping system for testing olfactory responses of the malaria mosquito Anopheles gambiae in a wind tunnel. Med. Vet. Entomol. 1994, 8, 386–388. [Google Scholar] [CrossRef]
- Feinsod, F.M.; Spielman, A. An olfactometer for measuring host-seeking behavior of female Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1979, 15, 282–285. [Google Scholar] [CrossRef]
- Bohbot, J.; Pitts, R.; Kwon, H.W.; Rütet, M.; Robertson, H.M.; Zwiebel, L. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol. Biol. 2007, 16, 525–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, G.H.; Riffell, J.A. Olfaction, experience and neural mechanisms underlying mosquito host preference. J. Exp. Biol. 2018, 221, 157131. [Google Scholar] [CrossRef] [Green Version]
- Sutcliffe, J.F. Sensory bases of attractancy: Morphology of mosquito olfactory sensilla-a review. J. Am. Mosq. Contr. 1994, 10, 309–315. [Google Scholar]
- Headlee, T.J. Further studies of the relative effects on insect metabolism of temperatures derived from constant and variable sources. J. Econ. Entomol. 1941, 34, 171–174. [Google Scholar] [CrossRef]
- Huffaker, C.B.; Back, R.C. A study of methods of sampling mosquito populations. J. Econ. Entomol. 1943, 36, 561–569. [Google Scholar] [CrossRef]
- Lacey, E.S.; Ray, A.; Carde, R.R. Close encounters: Contributions of carbon dioxide and human skin odour to finding and landing on a host in Aedes aegypti. Physiol. Entomol. 2014, 39, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanoudis, C.; Andreadis, S.; Bray, D.; Savopoulou-Soultani, M.; Ignell, R. Behavioural response of the house mosquitoes Culex quinquefasciatus and Culex pipiens molestus to avian odours and its reliance on carbon dioxide. Med. Vet. Entomol. 2020, 34, 129–137. [Google Scholar] [CrossRef]
- Willis, E.R. The olfactory responses of female mosquitoes. J. Econ. Entomol. 1947, 40, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.; Geier, M.; Cardé, R.T. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J. Exp. Biol. 2005, 208, 2963–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullens, B.A.; Gerry, A.C. Comparison of bait cattle and carbon dioxide-baited suction traps for collecting Culicoides variipennis sonorensis (Diptera: Ceratopogonidae) and Culex quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 1998, 35, 245–250. [Google Scholar] [CrossRef]
- Reeves, W. Quantitative field studies on a carbon dioxide chemotropism of mosquitoes. Am. J. Trop. Med. Hyg. 1953, 2, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pombi, M.; Jacobs, F.; Verhulst, N.O.; Caputo, B.; Torre, A.D.; Takken, W. Field evaluation of a novel synthetic odour blend and of the synergistic role of carbon dioxide for sampling host-seeking Aedes albopictus adults in Rome, Italy. Parasit. Vectors 2014, 7, 580. [Google Scholar] [CrossRef] [PubMed]
- Roiz, D.; Duperier, S.; Roussel, M.; Boussès, P.; Dontenille, D.; Simard, F.; Paupy, C. Trapping the Tiger: Efficacy of the novel BG-Sentinel 2 with several attractants and carbon dioxide for collecting Aedes albopictus (Diptera: Culicidae) in Southern France. J. Med. Entomol. 2015, 53, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Wright, R. Why mosquito repellents repel. Sci. Am. 1975, 233, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Ganguly, A.; Chakraborty, T.S.; Kumar, A.; Siddiqi, O. Synergism and combinatorial coding for binary odor mixture perception in Drosophila. Eneuro 2016, 3, 27588303. [Google Scholar] [CrossRef] [Green Version]
- Meza, F.C.; Roberts, J.M.; Sobhy, I.S.; Okumu, F.O.; Tripet, F.; Bruce, T.J. Behavioural and electrophysiological responses of female Anophels gambiae mosquitoes to volatiles from a mango bait. J. Chem. Ecol. 2020, 46, 387–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.; Sun, J.; Dai, J. Dose-dependent behavioral response of the mosquito Aedes albopictus to floral odorous compounds. J. Insect. Sci. 2013, 13, 127. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.T.; Ding, Y.M.; Mo, J.C. Behavioural response of female Culex pipiens pallens to common host plant volatiles and synthetic blends. Parasit. Vector. 2015, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nyasembe, V.O.; Teal, P.E.; Mukabana, W.R.; Tumlinson, J.H.; Torto, B. Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasit. Vector. 2012, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Henderson, J.P.; Westwood, R.; Galloway, T. An assessment of the effectiveness of the Mosquito Magnet pro model for suppression of nuisance mosquitoes. J. Am. Mosq. Contr. Assoc. 2006, 22, 401–407. [Google Scholar] [CrossRef]
- Smallegange, R.C.; Schmied, W.H.; Van Roey, K.J.; Verhulst, N.O.; Spitzen, J.; Mukabana, W.R.; Takken, W. Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae. Malar. J. 2010, 9, 292. [Google Scholar] [CrossRef] [Green Version]
- Jerry, D.C.; Mohammed, T.; Mohammed, A. Yeast-generated CO2: A convenient source of carbon dioxide for mosquito trapping using the BG-Sentinel® traps. Asian Pac. J. Trop. Biomed. 2017, 7, 896–900. [Google Scholar] [CrossRef]
- Kline, D.; Takken, W.; Wood, J.; Carlson, D. Field studies on the potential of butanone, carbon dioxide, honey extract, l-octen-3-ol, L-lactic acid and phenols as attractants for mosquitoes. Med. Vet. Entomol. 1990, 4, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.B.; Rossignol, P.A. An olfactometer for discriminating between attraction, inhibition, and repellency in mosquitoes (Diptera: Culicidae). J. Med. Entomol. 1999, 36, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.; Lacey, E.S.; Cardé, R.T. Waiting with bated breath: Opportunistic orientation to human odor in the malaria mosquito, Anopheles gambiae, is modulated by minute changes in carbon dioxide concentration. J. Chem. Ecol. 2015, 41, 59–66. [Google Scholar] [CrossRef]



| Species | Amount (g) | Mean ± SD No. of Females † in HITSS Chambers | p-Value † | Mean ± SD Percent Attraction †† | |
|---|---|---|---|---|---|
| Untreated | Treated | ||||
| Ae. aegypti (USDA) | 0.0 | 2.2 ± 1.7 | 1.3 ± 1.1 | 0.203 | −11.9 ± 72.2d |
| 0.005 | 0.6 ± 0.9 | 3.0 ± 2.1 | 0.011 * | 72.6 ± 42.0a | |
| 0.025 | 2.0 ± 1.6 | 2.2 ± 1.3 | 0.670 | 21.3 ± 57.4b | |
| 0.05 | 1.9 ± 1.5 | 6.4 ± 2.7 | 0.007 * | 53.5 ± 31.8ab | |
| 0.1 | 1.8 ± 1.1 | 1.1 ± 1.1 | 0.132 | −29.3 ± 52.9d | |
| 0.15 | 2.1 ± 0.9 | 2.6 ± 1.7 | 0.389 | 3.5 ± 47.4c | |
| 0.2 | 1.0 ± 0.9 | 0.2 ± 0.4 | 0.020 | −51.9 ± 47.5e | |
| 0.5 | 0.8 ± 1.4 | 0.2 ± 0.7 | 0.357 | −22.2 ± 66.7d | |
| 1.0 | 1.1 ± 1.5 | 0.2 ± 0.4 | 0.084 | −40.0 ± 70.0de | |
| 5.0 | 3.0 ± 1.0 | 0.2 ± 0.4 | 0.007 | −87.0 ± 26.1f | |
| 10.0 | 5.7 ± 3.5 | 0.1 ± 0.3 | 0.007 | −96.3 ± 11.1f | |
| 1.0 (dry ice) | 1.7 ± 0.9 | 2.6 ± 1.9 | 0.206 | 12.2 ± 39.6c | |
| Ae. aegypti (Pu Teuy) | 0.0 | 2.1 ± 1.2 | 2.0 ± 1.1 | 0.943 | 1.1 ± 56.9c |
| 0.005 | 1.0 ± 0.9 | 4.2 ± 1.8 | 0.007 * | 58.9 ± 27.9a | |
| 0.025 | 1.3 ± 1.3 | 0.7 ± 0.9 | 0.236 | −31.5 ± 56.8d | |
| 0.05 | 1.4 ± 0.7 | 3.2 ± 1.2 | 0.014 * | 37.4 ± 35.9b | |
| 0.1 | 1.6 ± 1.3 | 1.8 ± 1.0 | 0.480 | 17.0 ± 37.3c | |
| 0.15 | 2.7 ± 1.7 | 2.9 ± 2.0 | 0.621 | 3.3 ± 45.8c | |
| 0.2 | 1.9 ± 1.5 | 1.8 ± 1.2 | 0.994 | −2.2 ± 61.8d | |
| 0.5 | 3.7 ± 2.2 | 0.4 ± 0.7 | 0.011 | −82.0 ± 33.9e | |
| 1.0 | 1.3 ± 1.4 | 0.2 ± 0.4 | 0.039 | −48.1 ± 50.3d | |
| 5.0 | 1.7 ± 1.1 | 0.1 ± 0.3 | 0.016 | −77.8 ± 44.1e | |
| 10.0 | 5.4 ± 2.2 | 0.0 ± 0.0 | 0.008 | −100.0 ± 0.0f | |
| 1.0 (dry ice) | N/A | N/A | N/A | N/A | |
| Cx. quinquefasciatus (NIH) | 0.0 | 3.6 ± 2.7 | 3.7 ± 3.2 | 1.000 | −18.4 ± 47.4d |
| 0.005 | 0.4 ± 0.7 | 4.9 ± 3.3 | 0.011 * | 75.9 ± 45.0b | |
| 0.025 | 0.2 ± 0.7 | 6.0 ± 3.7 | 0.012 * | 88.9 ± 33.3a | |
| 0.05 | 1.1 ± 1.1 | 5.4 ± 2.2 | 0.007 * | 68.3 ± 25.4c | |
| 0.1 | 0.6 ± 0.9 | 4.8 ± 2.2 | 0.008 * | 83.7 ± 24.9b | |
| 0.15 | 0.4 ± 0.7 | 3.9 ± 3.4 | 0.028 * | 73.1 ± 65.6b | |
| 0.2 | 0.9 ± 1.2 | 3.2 ± 2.5 | 0.068 | 54.2 ± 63.5c | |
| 0.5 | 1.2 ± 1.1 | 1.3 ± 1.1 | 0.915 | 10.0 ± 63.6d | |
| 1.0 | 4.1 ± 2.1 | 1.9 ± 1.5 | 0.024 | −44.4 ± 46.1e | |
| 5.0 | 4.1 ± 2.4 | 1.0 ± 1.2 | 0.007 | −72.2 ± 26.8f | |
| 10.0 | 4.6 ± 3.1 | 1.2 ± 0.8 | 0.018 | −50.2 ± 34.7e | |
| 1.0 (dry ice) | 1.6 ± 1.8 | 10.1 ± 2.8 | 0.008 * | 76.0 ± 26.3b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-Y.; Leepasert, T.; Bangs, M.J.; Chareonviriyaphap, T. Dose–Response Assay for Synthetic Mosquito (Diptera: Culicidae) Attractant Using a High-Throughput Screening System. Insects 2021, 12, 355. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12040355
Kim D-Y, Leepasert T, Bangs MJ, Chareonviriyaphap T. Dose–Response Assay for Synthetic Mosquito (Diptera: Culicidae) Attractant Using a High-Throughput Screening System. Insects. 2021; 12(4):355. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12040355
Chicago/Turabian StyleKim, Dae-Yun, Theerachart Leepasert, Michael J. Bangs, and Theeraphap Chareonviriyaphap. 2021. "Dose–Response Assay for Synthetic Mosquito (Diptera: Culicidae) Attractant Using a High-Throughput Screening System" Insects 12, no. 4: 355. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12040355