Toxicity of Insecticides on Various Life Stages of Two Tortricid Pests of Cranberries and on a Non-Target Predator
Abstract
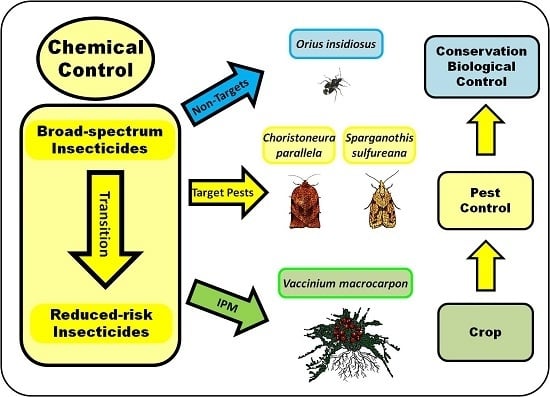
:1. Introduction
2. Experimental Section
2.1. Insects
2.2. Treatments
2.3. Laboratory Assays
2.3.1. Egg Toxicity
2.3.2. Larval Toxicity
2.3.3. Adult Toxicity
2.4. Extended Laboratory Experiments
2.5. Non-Target Effects
2.6. Statistical Analysis
3. Results
3.1. Laboratory Assays
3.1.1. Egg Toxicity
3.1.2. Larval Toxicity
3.1.3. Adult Toxicity
3.2. Extended Laboratory Experiments
3.3. Non-Target Effects
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| USA | United States of America |
| IPM | Integrated Pest Management |
| IRAC | Insecticide Resistance Action Committee |
| DAT | Days After Treatment |
| EPA | Environmental Protection Agency |
| ANOVA | Analysis of Variance |
References
- Wheeler, W.B. Role of research and regulation in 50 years of pest management in agriculture. J. Agric. Food Chem. 2002, 50, 4151–4155. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- EPA. Environmental Protection Agent. Consideration of the FQPA Safety Factor and Other Uncertainty Factors in Cumulative Risk Assessment of Chemicals Sharing a Common Mechanism of Toxicity. Available online: http://www2.epa.gov/pesticide-science-and-assessing-pesticide-risks/consideration-fqpa-safety-factor-and-other (accessed on 28 October 2015).
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2014, 70, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Grafton-Cardwell, E.; Godfrey, L.D.; Chaney, W.E.; Bentley, W.J. Various novel insecticides are less toxic to humans, more specific to key pests. Calif. Agric. 2005, 59, 29–34. [Google Scholar] [CrossRef]
- Boiteau, G.; Noronha, C. Topical, residual and ovicidal contact toxicity of three reduced-risk insecticides against the European corn borer, Ostrinia nubilalis (Lepidoptera: Crambidae), on potato. Pest Manag. Sci. 2007, 63, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Shearer, P.W.; Hamilton, G.C. Toxicity of insecticides to Halyomorpha halys (Hemiptera: Pentatomidae) using glass-vial bioassays. J. Econ. Entomol. 2008, 101, 1439–1442. [Google Scholar] [CrossRef]
- Eck, P. The American Cranberry; Rutgers University Press: New Brunswick, NJ, USA, 1990; p. 420. [Google Scholar]
- USDA NASS. United States Department of Agriculture. National Agricultural Statistics Service. Available online: http://www.nass.usda.gov/ (accessed on 2 February 2016).
- Dao, C.A.; Patel, K.; Neto, C.C. Phytochemicals from the fruit and foliage of cranberry (Vaccinium macrocarpon)-Potential benefits for human health. In Emerging Trends in Dietary Components for Preventing and Combating Disease; Patil, B.S., Jayaprakasha, G.K., Murthy, K.N.C., Seeram, N.P., Eds.; American Chemical Society: Washington, DC, USA, 2012; pp. 79–94. [Google Scholar]
- Neto, C.C.; Dao, C.A.; Salvas, M.R.; Autio, W.R.; Heuvel, J.E.V. Derivatives and quercetin glycosides in foliage of cranberry that may play a role in pest deterrence. J. Am. Soc. Hort. Sci. 2010, 135, 494–500. [Google Scholar]
- Rodriguez-Saona, C.; Vorsa, N.; Singh, A.P.; Johnson-Cicalese, J.; Szendrei, Z.; Mescher, M.C.; Frost, C.J. Tracing the history of plant traits under domestication in cranberries: Potential consequences on anti-herbivore defences. J. Exp. Bot. 2011, 62, 2633–2644. [Google Scholar] [CrossRef] [PubMed]
- Risch, S.J.; Andow, D.; Altieri, M.A. Agroecosystem diversity and pest control: Data, tentative conclusions, and new research directions. Environ. Entomol. 1983, 12, 625–629. [Google Scholar] [CrossRef]
- Winnett, G.; Marucci, P.; Reduker, S.; Uchrin, C.G. Fate of parathion in ground water in commercial cranberry culture in the New Jersey pinelands. Bull. Environ. Contam. Toxicol. 1990, 45, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.J.; Lienk, S.E. Tortricid Fauna of Apple in New York (Lepidoptera: Tortricidae)—Including an Account of Apples’ Occurence in the State, Especially as a Naturalized Plant; New York State Agricultural Experiment Station, Cornell University: Geneva, NY, USA, 1971. [Google Scholar]
- Averill, A.E.; Sylvia, M.M. Cranberry Insects of the Northeast; University of Massachusetts: Amherst, MA, USA, 1998; p. 112. [Google Scholar]
- Sandberg, S.; Passoa, S. New host records and morphological notes on four tortricines (Tortricidae). J. Res. Lep. 1988, 27, 104–108. [Google Scholar]
- Beckwith, C.S. Sparganothis sulfureana Clem., a cranberry pest in New Jersey. J. Econ. Entomol. 1938, 31, 253–256. [Google Scholar] [CrossRef]
- Blake, G.; Sandler, H.A.; Coli, W.; Pober, D.M.; Coggins, C. An assessment of grower perceptions and factors influencing adoption of IPM in commercial cranberry production. Renew. Agric. Food. Syst. 2007, 22, 134–144. [Google Scholar] [CrossRef]
- Polavarapu, S.; Lonergan, G.C.; Peng, H.; Neilsen, K. Potential for mating disruption of Sparganothis sulfureana Clemens (Lepidoptera: Tortricidae) in cranberries. J. Econ. Entomol. 2001, 94, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Stuart, R.J.; Polavarapu, S. Egg-mass variability and differential parasitism of Choristoneura parallela (Lepidoptera: Tortricidae) by endemic Trichogramma minutum (Hymenoptera: Trichogrammatidae). Ann. Entomol. Soc. Am. 2000, 93, 1076–1084. [Google Scholar] [CrossRef]
- Liburd, O.E.; Finn, E.M. Small fruit pests and their management. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer Netherlands: Dordrecht, Netherlands, 2004; Volume 4, pp. 2014–2029. [Google Scholar]
- Marucci, P.E. The effect of cranberry tipworm attack on the fruit bud production of the cranberry plant. In Proceedings of the 84th Annual Meeting of the American Cranberry Growers’ Association, Pemberton, NJ, USA, 4 February 1954.
- Rodriguez-Saona, C.R.; Byers, J.A.; Schiffhauer, D. Effect of trap color and height on captures of blunt-nosed and sharp-nosed leafhoppers (Hemiptera: Cicadellidae) and non-target arthropods in cranberry bogs. Crop Prot. 2012, 40, 132–144. [Google Scholar] [CrossRef]
- Mendes, S.M.; Bueno, V.H.P.; Argolo, V.M.; Silveira, L.C.P. Type of prey influences Biology and consumption rate of Orius insidiosus (Say) (Hemiptera: Anthocoridae). Rev. Bras. Entomol. 2002, 46, 99–103. [Google Scholar] [CrossRef]
- Isenhour, D.J.; Layton, R.C.; Wiseman, B.R. Potential of adult Orius insidiosus [Hemiptera: Anthocoridae] as a predator of the fall armyworm, Spodoptera frugiperda [Lepidoptera: Noctuidae]. Entomophaga 1990, 35, 269–275. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Reduced Risk and Organophosphate Alternative Decisions for Conventional Pesticides. Available online: http://www.epa.gov/pesticide-registration/reduced-risk-and-organophosphate-alternative-decisions-conventional (accessed on 12 April 2016).
- Oudemans, P.; Majek, B.; Pavlis, G.; Polk, D.; Rodriguez-Saona, C.; Ward, D. Commercial Blueberry Pest Control Recommendations for New Jersey; Factsheet No. E265; Rutgers NJAES Cooperative Extension: Rutgers, NJ, USA, 2015; p. 53. [Google Scholar]
- De Lange, E.S.; Rodriguez-Saona, C. Sparganothis Fruitworm: A Pest of Cranberry in New Jersey; Factsheet No. 1249; The State University of New Jersey, Cooperative Extension NJ: Rutgers, NJ, USA, 2015; pp. 1–3. [Google Scholar]
- De Lange, E.S.; Rodriguez-Saona, C. Spotted Fireworm: A Pest of Cranberry in New Jersey; Factsheet No. 1247; The State University of New Jersey, Cooperative Extension NJ: Rutgers, NJ, USA, 2015; pp. 1–2. [Google Scholar]
- Studebaker, G.E.; Kring, T.J. Effects of insecticides on Orius insidiosus (Hemiptera: Anthocoridae), measured by field, greenhouse and Petri dish bioassays. Fla. Entomol. 2003, 86, 178–185. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Rodriguez-Saona, C.; Holdcraft, R.; Vera Kyryczenko-Roth, P.E.; Marucci Center for Blueberry & Cranberry Research & Extension. Rutgers University of NJ, Chatsworth, NJ, USA. Unpublished data. 2016.
- Charlet, L.D.; Busacca, J.D. Insecticidal control of banded sunflower moth, Cochylis hospes (Lepidoptera: Cochylidae), larvae at different sunflower growth stages and dates of planting in North Dakota. J. Econ. Entomol. 1986, 79, 648–650. [Google Scholar] [CrossRef]
- Wise, J.C.; Jenkins, P.E.; Vander Poppen, R.; Isaacs, R. Activity of broad-spectrum and reduced-risk insecticides on various life stages of cranberry fruitworm (Lepidoptera: Pyralidae) in highbush blueberry. J. Econ. Entomol. 2010, 103, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Hardke, J.T.; Temple, J.H.; Leonard, B.R.; Jackson, R.E. Laboratory toxicity and field efficacy of selected insecticides against fall armyworm (Lepidoptera: Noctuidae). Fla. Entomol. 2011, 94, 272–278. [Google Scholar] [CrossRef]
- Maienfisch, P.; Angst, M.; Brandl, F.; Fischer, W.; Hofer, D.; Kayser, H.; Kobel, W.; Rindlisbacher, A.; Senn, R.; Steinemann, A.; Widmer, H. Chemistry and biology of thiamethoxam: A second generation neonicotinoid. Pest Manag. Sci. 2001, 57, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Ebbinghaus-Kintscher, U.; Salgado, V.L.; Kaussmann, M. Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants. Pest. Biochem. Physiol. 2003, 76, 55–69. [Google Scholar] [CrossRef]
- Yue, B.; Wilde, G.E.; Arthur, F. Evaluation of thiamethoxam and imidacloprid as seed treatments to control European corn borer and Indian meal moth (Lepidoptera: Pyralidae) larvae. J. Econ. Entomol. 2003, 96, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R. Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.R.; Dhadialla, T.S.; Hunter, R.; Jansson, R.K.; Jany, C.S.; Lidert, Z.; Slawecki, R.A. The chemical and biological properties of methoxyfenozide, a new insecticidal ecdysteroid agonist. Pest Manag. Sci. 2001, 57, 115–119. [Google Scholar] [CrossRef]
- Post, L.C.; De Jong, B.J.; Vincent, W.R. 1-(2,6-disubstituted benzoyl)-3-phenylurea insecticides: Inhibitors of chitin synthesis. Pest. Biochem. Physiol. 1974, 4, 473–483. [Google Scholar] [CrossRef]
- Isaacs, R.; Mason, K.S.; Maxwell, E. Stage-specific control of grape berry moth, Endopiza viteana (Clemens) (Lepidoptera: Tortricidae), by selective and broad-spectrum insecticides. J. Econ. Entomol. 2005, 98, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, L.C.; Walgenbach, J.F. Life stage toxicity and residual activity of insecticides to codling moth and oriental fruit moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2011, 104, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.; Scott-Dupree, C.D.; Tolman, J.H.; Ronald Harris, C. Acute and sublethal toxicity of novaluron, a novel chitin synthesis inhibitor, to Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2005, 61, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.R.; Wise, J.C.; Polk, D.; Leskey, T.C.; Vandervoort, C. Lethality of reduced-risk insecticides against plum curculio (Coleoptera: Curculionidae) in blueberries, with emphasis on their curative activity. Pest Manag. Sci. 2013, 69, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Brittain, C.; Potts, S.G. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol. 2011, 12, 321–331. [Google Scholar] [CrossRef]
- Hodgson, E.W.; Pitts-Singer, T.L.; Barbour, J.D. Effects of the insect growth regulator, novaluron on immature alfalfa leafcutting bees, Megachile rotundata. J. Insect Sci. 2011, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluijs, J.P.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.M.; Belzunces, L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013, 5, 293–305. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pest. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Insecticidal anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.H.; Bommireddy, P.L.; Cook, D.R.; Maeçon, P.; Leonard, B.R. Susceptibility of selected Lepidopteran pests to rynaxypyr®, a novel insecticide. J. Cotton Sci. 2009, 13, 23–31. [Google Scholar]
- Liu, T.X.; Sparks, A.N.; Chen, W. Toxicity, persistence and efficacy of indoxacarb and two other insecticides on Plutella xylostella (Lepidoptera: Plutellidae) immatures in cabbage. Int. J. Pest Manag. 2003, 49, 235–241. [Google Scholar] [CrossRef]
- Pozzebon, A.; Tirello, P.; Moret, R.; Pederiva, M.; Duso, C. A fundamental step in IPM on grapevine: Evaluating the side effects of pesticides on predatory mites. Insects 2015, 6, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Gentz, M.C.; Murdoch, G.; King, G.F. Tandem use of selective insecticides and natural enemies for effective, reduced-risk pest management. Biol. Control 2010, 52, 208–215. [Google Scholar] [CrossRef]
- Roubos, C.R.; Rodriguez-Saona, C.; Holdcraft, R.; Mason, K.S.; Isaacs, R. Relative toxicity and residual activity of insecticides used in blueberry pest management: Mortality of natural enemies. J. Econ. Entomol. 2014, 107, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, D.G.; MacCarthy, H.R. The movement and persistence of insecticides in plant tissue. Residue Rev. 1965, 9, 114–152. [Google Scholar] [PubMed]
- Zhu, J.W.; Polavarapu, S.; Park, K.C.; Garvey, C.; Mahr, D.; Nojima, S.; Roelofs, W.; Tom Baker, T. Reidentification of pheromone composition of Sparganothis sulfureana (Clemens) and evidence of geographic variation in male responses from two US states. J. Asia Pacific Entomol. 2009, 12, 247–252. [Google Scholar] [CrossRef]
- Polavarapu, S.; Lonergan, G. Sex pheromone of Choristoneura parallela (Lepidoptera: Tortricidae): Components and development of a pheromone lure for population monitoring. Environ. Entomol. 1998, 27, 1242–1249. [Google Scholar] [CrossRef]
- Neal, J.W.; Klun, J.A.; Bierl-Leonhardt, B.A.; Schwarz, M. Female sex pheromone of Choristoneura parallela (Lepidoptera: Tortricidae). Environ. Entomol. 1982, 11, 893–896. [Google Scholar] [CrossRef]
- Deutsch, A.E.; Rodriguez-Saona, C.; Kyryczenko-Roth, V.; Sojka, J.; Zalapa, J.E.; Steffan, S.A. Degree-day benchmarks for Sparganothis sulfureana (Lepidoptera: Tortricidae) development in cranberries. J. Econ. Entomol. 2014, 107, 2130–2136. [Google Scholar] [CrossRef] [PubMed]









| Active Ingredient | Trade Name | Class | IRAC Group a | Manufacturer | Rate (per Hectare) b | Year(s) c |
|---|---|---|---|---|---|---|
| Thiamethoxam d | Actara 25WG | Nicotinoid | 4A | Syngenta | 0.28 kg | 2015 |
| Acetamiprid d,e | Assail 30SG | Nicotinoid | 4A | UPI | 0.48 kg | 2012 |
| Spinetoram e | Delegate 25WG | Spinosyn | 5 | Dow AgroSciences | 0.42 kg | 2012, 2013, 2014 |
| Chlorantraniliprole e | Altacor 35WDG | Diamide | 28 | DuPont | 0.28 kg | 2012, 2013, 2014 |
| Cyantraniliprole e | Exirel 10SE | Diamide | 28 | DuPont | 0.95 kg | 2012, 2013, 2014 |
| Methoxyfenozide d,e | Intrepid 2F | Insect Growth Regulator | 18 | Dow AgroSciences | 1.17 L | 2012, 2013, 2014 |
| Novaluron d,e | Rimon 0.8EC | Benzoylurea | 15 | Chemtura | 0.88 L | 2012 |
| Indoxacarb d,e | Avaunt 30WDG | Oxadiazine | 22A | DuPont | 0.42 kg | 2012, 2015 |
| Chlorpyrifos | Lorsban 4E | Organophosphate | 1B | Dow AgroSciences | 3.51 L | 2012, 2013, 2014, 2015 |
| Life Stage | Variables | S. sulfureana | Ch. Parallela | ||||
|---|---|---|---|---|---|---|---|
| Factor | df | F | p 1 | df | F | p 1 | |
| Eggs | |||||||
| Viability | Treatment | 9, 90 | 1.70 | 0.09 | 9, 60 | 1.37 | 0.221 |
| Hatch | Treatment | 9, 90 | 15.79 | ≤0.001 | 9, 60 | 8.26 | ≤0.001 |
| Larvae | |||||||
| Mortality | Treatment | 9, 360 | 33.71 | ≤0.001 | 9, 360 | 76.7 | ≤0.001 |
| Instar | 1, 360 | 56.77 | ≤0.001 | 1, 360 | 19.4 | ≤0.001 | |
| Date | 1, 360 | 13.29 | ≤0.001 | 1, 360 | 4.29 | ≤0.05 | |
| Treatment × Instar | 9, 360 | 6.33 | ≤0.001 | 9, 360 | 5 | ≤0.001 | |
| Treatment × Date | 9, 360 | 5.18 | ≤0.001 | 9, 360 | 1.26 | 0.251 | |
| Treatment × Instar × Date | 9, 360 | 2.13 | ≤0.05 | 9, 360 | 0.54 | 0.842 | |
| Adults | |||||||
| Moribund | Treatment | 9, 240 | 11.45 | ≤0.001 | 9, 240 | 38.83 | ≤0.001 |
| Sex | 1, 240 | 0.71 | 0.39 | 1, 240 | 0.12 | 0.723 | |
| Date | 2, 240 | 1.22 | 0.29 | 2, 240 | 1.9 | 0.15 | |
| Treatment × Sex | 9, 240 | 1.98 | ≤0.05 | 9, 240 | 0.4 | 0.932 | |
| Treatment × Date | 18, 240 | 3.60 | ≤0.001 | 18, 240 | 1.59 | 0.06 | |
| Treatment × Sex × Date | 18, 240 | 1.86 | ≤0.05 | 18, 240 | 1.87 | ≤0.05 | |
| Mortality | Treatment | 9, 240 | 50.18 | ≤0.001 | 9, 240 | 40.46 | ≤0.001 |
| Sex | 1, 240 | 11.20 | ≤0.001 | 1, 240 | 0.22 | 0.637 | |
| Date | 2, 240 | 11.20 | ≤0.001 | 2, 240 | 9.72 | ≤0.001 | |
| Treatment × Sex | 9, 240 | 3.58 | ≤0.001 | 9, 240 | 0.34 | 0.958 | |
| Treatment × Date | 18, 240 | 5.42 | ≤0.001 | 18, 240 | 2.19 | ≤0.01 | |
| Treatment × Sex × Date | 18, 240 | 0.41 | 0.98 | 18, 240 | 1.99 | ≤0.05 | |
| Species | Variables | df | F | p 1 |
|---|---|---|---|---|
| Factor | ||||
| S. sulfureana | ||||
| Mortality | Treatment | 9, 416 | 18.47 | ≤0.001 |
| Instar | 1, 416 | 115.57 | ≤0.001 | |
| Date | 2, 416 | 35.09 | ≤0.001 | |
| Treatment × Instar | 9, 416 | 9.97 | ≤0.001 | |
| Insecticide × Date | 14, 416 | 1.74 | ≤0.05 | |
| Insecticide × Instar × Date | 14, 416 | 3.15 | ≤0.001 | |
| Mass | Treatment | 9, 78 | 4.35 | ≤0.001 |
| Date | 1, 78 | 0.60 | 0.44 | |
| Treatment × Date | 7, 78 | 1.36 | 0.23 | |
| Ch. parallela | ||||
| Mortality | Treatment | 9, 459 | 36.94 | ≤0.001 |
| Instar | 1, 459 | 166.90 | ≤0.001 | |
| Date | 2, 459 | 9.51 | ≤0.001 | |
| Treatment × Instar | 9, 459 | 6.12 | ≤0.001 | |
| Insecticide × Date | 15, 459 | 6.93 | ≤0.001 | |
| Insecticide × Instar × Date | 14, 459 | 2.64 | ≤0.01 | |
| Mass | Treatment | 7, 59 | 10.01 | ≤0.001 |
| Date | 1, 59 | 31.32 | ≤0.001 | |
| Treatment × Date | 4, 59 | 3.57 | ≤0.05 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Saona, C.; Wanumen, A.C.; Salamanca, J.; Holdcraft, R.; Kyryczenko-Roth, V. Toxicity of Insecticides on Various Life Stages of Two Tortricid Pests of Cranberries and on a Non-Target Predator. Insects 2016, 7, 15. https://0-doi-org.brum.beds.ac.uk/10.3390/insects7020015
Rodriguez-Saona C, Wanumen AC, Salamanca J, Holdcraft R, Kyryczenko-Roth V. Toxicity of Insecticides on Various Life Stages of Two Tortricid Pests of Cranberries and on a Non-Target Predator. Insects. 2016; 7(2):15. https://0-doi-org.brum.beds.ac.uk/10.3390/insects7020015
Chicago/Turabian StyleRodriguez-Saona, Cesar, Andrea Carolina Wanumen, Jordano Salamanca, Robert Holdcraft, and Vera Kyryczenko-Roth. 2016. "Toxicity of Insecticides on Various Life Stages of Two Tortricid Pests of Cranberries and on a Non-Target Predator" Insects 7, no. 2: 15. https://0-doi-org.brum.beds.ac.uk/10.3390/insects7020015