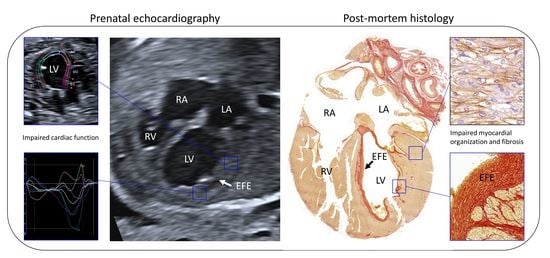
Deficient Myocardial Organization and Pathological Fibrosis in Fetal Aortic Stenosis—Association of Prenatal Ultrasound with Postmortem Histology
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Case Description
3.2. Ultrasonographic Myocardial Deformation
3.3. Histological Data
3.3.1. Myocardial Organization and Differentiation
3.3.2. Endocardial and Myocardial Fibrosis Patterns
3.3.3. Epicardium
4. Discussion
5. Perspectives, Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [Green Version]
- Freud, L.R.; Moon-Grady, A.; Escobar-Diaz, M.C.; Gotteiner, N.L.; Young, L.T.; McElhinney, D.B. Low rate of prenatal diagnosis among neonates with critical aortic stenosis: Insight into the natural history in utero. Ultrasound Obstet. Gynecol. 2015, 45, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Hornberger, L.K.; Sanders, S.P.; Rein, A.J.; Spevak, P.J.; Parness, I.A.; Colan, S.D. Left heart obstructive lesions and left ventricular growth in the midtrimester fetus. A longitudinal study. Circulation 1995, 92, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.W.; Ren, M.; Wiputra, H.; Mojumder, J.; Chan, W.X.; Tulzer, A.; Tulzer, G.; Buist, M.L.; Matter, C.N.Z.; Lee, L.C.; et al. Biomechanics of Human Fetal Hearts with Critical Aortic Stenosis. Ann. Biomed. Eng. 2020, 49, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- Harh, J.Y.; Paul, M.H.; Gallen, W.J.; Friedberg, D.Z.; Kaplan, S. Experimental production of hypoplastic left heart syndrome in the chick embryo. Am. J. Cardiol. 1973, 31, 51–56. [Google Scholar] [CrossRef]
- Simpson, J.M.; Sharland, G.K. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart 1997, 77, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, D.; Allan, L.; Tynan, M.J. Balloon dilatation of the aortic valve in the fetus: A report of two cases. Br. Heart J. 1991, 65, 256–258. [Google Scholar] [CrossRef]
- Tworetzky, W.; Wilkins-Haug, L.; Jennings, R.W.; van der Velde, M.E.; Marshall, A.C.; Marx, G.R.; Colan, S.D.; Benson, C.B.; Lock, J.E.; Perry, S.B. Balloon dilation of severe aortic stenosis in the fetus: Potential for prevention of hypoplastic left heart syndrome: Candidate selection, technique, and results of successful intervention. Circulation 2004, 110, 2125–2131. [Google Scholar] [CrossRef] [Green Version]
- Freud, L.R.; McElhinney, D.B.; Marshall, A.C.; Marx, G.R.; Friedman, K.G.; del Nido, P.J.; Emani, S.M.; Lafranchi, T.; Silva, V.; Wilkens-Haug, L.E.; et al. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: Postnatal outcomes of the first 100 patients. Circulation 2014, 130, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Pickard, S.S.; Wong, J.B.; Bucholz, E.M.; Newburger, J.W.; Tworetzky, W.; Lafranchi, T.; Benson, C.B.; Wilkins-Haug, L.E.; Porras, D.; Callahan, R.; et al. Fetal Aortic Valvuloplasty for Evolving Hypoplastic Left Heart Syndrome: A Decision Analysis. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006127. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Marshall, A.C.; Wilkins-Haug, L.E.; Brown, D.W.; Benson, C.B.; Silva, V.; Marx, G.R.; Mizrahi-Arnaud, A.; Lock, J.E.; Tworetzky, W. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation 2009, 120, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, C.; Wertaschnigg, D.; Wieser, I.; Arzt, W.; Tulzer, G. Tissue Doppler imaging in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome before and after fetal aortic valvuloplasty. Ultrasound Obstet. Gynecol. 2016, 47, 608–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jantzen, D.W.; Gelehrter, S.K.; Yu, S.; Donohue, J.E.; Fifer, C.G. Echocardiographic factors discriminating biventricular versus univentricular approach in the foetus with borderline left ventricle. Cardiol. Young 2015, 25, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, E.A.; Gittenberger-de Groot, A.C.; Vicente-Steijn, R.; Lie-Venema, H.; Rijlaarsdam, M.E.; Hazekamp, M.G.; Bartelings, M.M. Disturbed myocardial connexin 43 and N-cadherin expressions in hypoplastic left heart syndrome and borderline left ventricle. J. Thorac. Cardiovasc. Surg. 2012, 144, 1315–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElhinney, D.B.; Vogel, M.; Benson, C.B.; Marshall, A.C.; Wilkins-Haug, L.E.; Silva, V.; Tworetzky, W. Assessment of left ventricular endocardial fibroelastosis in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome. Am. J. Cardiol. 2010, 106, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Bijnens, B.; Cikes, M.; Butakoff, C.; Sitges, M.; Crispi, F. Myocardial motion and deformation: What does it tell us and how does it relate to function? Fetal Diagn. Ther. 2012, 32, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Enzensberger, C.; Achterberg, F.; Degenhardt, J.; Wolter, A.; Graupner, O.; Herrmann, J.; Axt-Fliedner, R. Feasibility and Reproducibility of Two-Dimensional Wall Motion Tracking (WMT) in Fetal Echocardiography. Ultrasound Int. Open. 2017, 3, E26–E33. [Google Scholar] [CrossRef] [Green Version]
- Vigneswaran, T.; Akolekar, R.; Syngelaki, A.; Charakida, M.; Allan, L.; Nicolaides, K.; Zidere, V.; Simpson, J.M. Reference Ranges for the Size of the Fetal Cardiac Outflow Tracts From 13 to 36 Weeks Gestation: A Single-Center Study of Over 7000 Cases. Circ. Cardiovasc. Imaging. 2018, 11, e007575. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; McCrindle, B.; Carvalho, J.; Hornberger, L.; McCarthy, K.; Daubeney, P. Development of Z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet. Gynecol. 2005, 26, 599–605. [Google Scholar] [CrossRef]
- Tan, J.; Silverman, N.H.; Hoffman, J.I.E.; Villegas, M.; Schmidt, K.G. Cardiac dimensions determined by cross-sectional echocardiography in the normal human fetus from 18 weeks to term. Am. J. Cardiol. 1992, 70, 1459–1467. [Google Scholar] [CrossRef]
- van Oostrum, N.H.M.; de Vet, C.M.; van der Woude, D.A.A.; Kemps, H.M.C.; Oei, S.G.; van Laar, J. Fetal strain and strain rate during pregnancy measured with speckle tracking echocardiography: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 250, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Weixler, V.; Marx, G.R.; Hammer, P.E.; Emani, S.M.; Del Nido, P.J.; Friehs, I. Flow disturbances and the development of endocardial fibroelastosis. J. Thorac. Cardiovasc. Surg. 2020, 159, 637–646. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Watabe, T. Roles of TGF-beta signals in endothelial-mesenchymal transition during cardiac fibrosis. Int. J. Inflam. 2011, 2011, 724080. [Google Scholar] [PubMed] [Green Version]
- Zhang, H.; Huang, X.; Liu, K.; Tang, J.; He, L.; Pu, W.; Liu, Q.; Li, Y.; Tian, X.; Wang, Y. Fibroblasts in an endocardial fibroelastosis disease model mainly originate from mesenchymal derivatives of epicardium. Cell Res. 2017, 27, 1157–1177. [Google Scholar] [CrossRef] [Green Version]
- Dronkers, E.; Wauters, M.M.M.; Goumans, M.J.; Smits, A.M. Epicardial TGF beta and BMP Signaling in Cardiac Regeneration: What Lesson Can We Learn from the Developing Heart? Biomolecules 2020, 10, 404. [Google Scholar] [CrossRef] [Green Version]
- Pervolaraki, E.; Dachtler, J.; Anderson, R.A.; Holden, A.V. Ventricular myocardium development and the role of connexins in the human fetal heart. Sci. Rep. 2017, 7, 12272. [Google Scholar] [CrossRef]
- Buckberg, G.; Hoffman, J.I.; Mahajan, A.; Saleh, S.; Coghlan, C. Cardiac mechanics revisited: The relationship of cardiac architecture to ventricular function. Circulation 2008, 118, 2571–2587. [Google Scholar] [CrossRef] [Green Version]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [Green Version]
- Villari, B.; Campbell, S.E.; Hess, O.M.; Mall, G.; Vassalli, G.; Weber, K.T.; Krayenbuehl, H.P. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J. Am. Coll. Cardiol. 1993, 22, 1477–1484. [Google Scholar] [CrossRef] [Green Version]
- Fabiani, I.; Scatena, C.; Mazzanti, C.M.; Conte, L.; Pugliese, N.R.; Franceschi, S.; Lessi, F.; Menicagli, M.; De Martino, A.; Pratali, S. Micro-RNA-21 (biomarker) and global longitudinal strain (functional marker) in detection of myocardial fibrotic burden in severe aortic valve stenosis: A pilot study. J. Transl. Med. 2016, 14, 248. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Cho, S.W.; Kim, S.M.; Ahn, J.; Carriere, K.; Jeong, D.S.; Lee, S.C.; Park, S.W.; Choe, Y.H.; Park, P.W. Assessment of Myocardial Fibrosis Using Multimodality Imaging in Severe Aortic Stenosis: Comparison with Histologic Fibrosis. JACC Cardiovasc Imaging. 2019, 12, 109–119. [Google Scholar] [CrossRef]
- Beyhoff, N.; Brix, S.; Betz, I.R.; Klopfleisch, R.; Foryst-Ludwig, A.; Krannich, A.; Stawowy, P.; Knebel, F.; Grune, J.; Kintscher, U. Application of Speckle-Tracking Echocardiography in an Experimental Model of Isolated Subendocardial Damage. J. Am. Soc. Echocardiogr. 2017, 30, 1239–1250.e2. [Google Scholar] [CrossRef]
- Peng, Y.; Popovic, Z.B.; Sopko, N.; Drinko, J.; Zhang, Z.; Thomas, J.D.; Penn, M.S. Speckle tracking echocardiography in the assessment of mouse models of cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H811–H820. [Google Scholar] [CrossRef] [PubMed]
- Day, T.G.; Charakida, M.; Simpson, J.M. Using speckle-tracking echocardiography to assess fetal myocardial deformation: Are we there yet? Ultrasound Obstet. Gynecol. 2019, 54, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; McElhinney, D.B.; Harrild, D.M.; Marcus, E.N.; Sahn, D.J.; Truong, U.; Tworetzky, W. Ventricular strain in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome before and after prenatal aortic valvuloplasty. Fetal Diagn. Ther. 2014, 35, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Vreeker, A.; van Stuijvenberg, L.; Hund, T.J.; Mohler, P.J.; Nikkels, P.G.; van Veen, T.A. Assembly of the cardiac intercalated disk during pre- and postnatal development of the human heart. PLoS ONE 2014, 9, e94722. [Google Scholar]
- Vetter, C.; Zweifel, M.; Zuppinger, C.; Carrel, T.; Martin, D.; Haefliger, J.A.; Delacrétaz, E. Connexin 43 expression in human hypertrophied heart due to pressure and volume overload. Physiol. Res. 2010, 59, 35–42. [Google Scholar] [CrossRef]
- Formigli, L.; Ibba-Manneschi, L.; Perna, A.M.; Pacini, A.; Polidori, L.; Nediani, C.; Modesti, P.A.; Nosi, D.; Tani, A.; Celli, A.; et al. Altered Cx43 expression during myocardial adaptation to acute and chronic volume overloading. Histol. Histopathol. 2003, 18, 359–369. [Google Scholar]
- Kostin, S.; Dammer, S.; Hein, S.; Klovekorn, W.P.; Bauer, E.P.; Schaper, J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc. Res. 2004, 62, 426–436. [Google Scholar] [CrossRef]
- Weeke-Klimp, A.; Bax, N.A.; Bellu, A.R.; Winter, E.M.; Vrolijk, J.; Plantinga, J.; Maas, S.; Brinker, M.; Mahtab, E.A.F.; Gittenberger-de Groot, A.C.; et al. Epicardium-derived cells enhance proliferation, cellular maturation and alignment of cardiomyocytes. J. Mol. Cell Cardiol. 2010, 49, 606–616. [Google Scholar] [CrossRef]
- Pesevski, Z.; Kvasilova, A.; Stopkova, T.; Nanka, O.; Drobna, K.E.; Buffinton, C.; Kockova, R.; Eckhardt, A.; Sedmera, D. Endocardial Fibroelastosis is Secondary to Hemodynamic Alterations in the Chick Embryonic Model of Hypoplastic Left Heart Syndrome. Dev. Dyn. 2018, 247, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Shimada, S.; Robles, C.; Illigens, B.M.; Casar Berazaluce, A.M.; del Nido, P.J.; Friehs, I. Distention of the Immature Left Ventricle Triggers Development of Endocardial Fibroelastosis: An Animal Model of Endocardial Fibroelastosis Introducing Morphopathological Features of Evolving Fetal Hypoplastic Left Heart Syndrome. Biomed. Res. Int. 2015, 2015, 462469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Singh, S.; Dawson, D.; Frenneaux, M.P. Right ventricular function in left ventricular disease: Pathophysiology and implications. Heart Lung Circ. 2013, 22, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Mantri, M.; Scuderi, G.J.; Abedini-Nassab, R.; Wang, M.F.Z.; McKellar, D.; Shi, H.; Grodner, B.; Butcher, J.T.; De Vlaminck, I. Spatiotemporal single-cell RNA sequencing of developing chicken hearts identifies interplay between cellular differentiation and morphogenesis. Nat. Commun. 2021, 12, 1771. [Google Scholar] [CrossRef] [PubMed]






| Primary Antibodies | Indication | Species | Clonality | Dilution | Source |
|---|---|---|---|---|---|
| Cardiac troponin-I | Myocardial structure | Rabbit | Polyclonal | 1:1000 | Abcam; AB47003 |
| WT-1 | Epicardial cells and EEMT | Rabbit | Monoclonal | 1:200 | Abcam; AB89901 |
| TUBB3 | Cardiac innervation | Rabbit | Polyclonal | 1:8000 | Sigma-Aldrich; T3952 |
| N-cadherin * | Adherence junctions | Mouse | Monoclonal | 1:200 | Sigma-Aldrich; C3865 |
| Cx43 * | Gap junctions | Rabbit | Polyclonal | 1:600 | Abcam; AB11370 |
| PECAM1 * | Endothelial cells | Rabbit | Polyclonal | 1:1000 | Santa Cruz; sc1506R |
| α-SMA * | Activated cardiac fibroblasts | Mouse | Monoclonal | 1:10,000 | Sigma-Aldrich; A2547 |
| pSmad2 | Activated TGF-β signaling, specific for EndMT or EMT | Rabbit | Monoclonal | 1:100 | Cell Signaling; 138D4 |
| Secondary Antibodies | |||||
| Antirabbit-Biotin Alexa Fluor 594 anti-mouse IgG Alexa Fluor 488 anti-rabbit IgG Alexa Fluor 488 anti-mouse IgG Alexa Fluor 555 anti-rabbit IgG | Goat Donkey Donkey Donkey Donkey | Polyclonal Polyclonal Polyclonal Polyclonal Polyclonal | 1:200 1:200 1:200 1:200 1:200 | Vector Laboratories; BA1000 Thermofisher Scientific; A21203 Thermofisher Scientific; A21206 Thermofisher Scientific; A21202 Thermofisher Scientific; A31572 | |
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| GA (wks) first presentation | 20 + 3 | 19 + 5 | 20 + 1 | 21 + 1 |
| Diagnosis | Critical AS | Critical AS | Severe AS | Severe AS |
| Valvuloplasty | Yes, prenatal at 21 + 1 wks | Offered but declined by parents | No | Yes, postnatal |
| AoV diam (Percentile 1) | 2.5 (P0) | 2.4 (−1.940/−2.326) | 2.5 (P1) | 2.9 (P4) |
| AoV velocity cm/s | 210 | 250 | 313 | 301 |
| MV diam (Percentile 2) | 4.6 (P4) | 4.6 (P7) | 4.8 (P10) | 5.5 (P24) |
| TV diam (Percentile 2) | 5 (P17) | 4.8 (P18) | 5.1 (P22) | 5.6 (P31) |
| LV length (Percentile 3) | 12.8 (P44) | 15.4 (P66) | 13 (P47) | 15.2 (P53) |
| RV length (Percentile 3) | 11.8 (P49) | 12.3 (P57) | 11.4 (P48) | 14.5 (P62) |
| LV shape | Spherical | Spherical | Normal | Normal |
| Aortic arch flow | Retrograde | Retrograde | Forward | Forward |
| EFE | Moderate | Mild | None | None |
| MV flow | Monophasic, regurgitation | Minimal forward, monophasic, regurgitation | Biphasic, mild regurgitation | Biphasic, mild regurgitation |
| FO flow | Left–right | Left–right | Right–left | Right–left |
| PV flow | Normal | Normal | Normal | Normal |
| GA of TOP (wks) | 23 + 1 | 21 + 2 | 21 + 2 | N/A |
| Normal Hearts | Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|---|
| FPS | 68 | 115 | 65 | 60 | 63 |
| Left Ventricle | |||||
| GLS | −19.6% | −2.0% | −0.9% | −11.8% | −10.5% |
| SLS—basal septal | −16.4% | −1.0% | −2.7% | −14.3% | −16.0% |
| SLS—mid septal | −18.8% | −0.6% | −1.0% | −13.7% | −13.3% |
| SLS—apical septal | −22.0% | −3.0% | −1.0% | −13.0% | −7.0% |
| SLS—basal lateral | −20.7% | −1.6% | −1.3% | −8.7% | −5.3% |
| SLS—mid lateral | −20.2% | −2.6% | −0.3% | −10.7% | −12.3% |
| SLS—apical lateral | −20.5% | −4.6% | −0.3% | −11.7 % | −11.7% |
| Right Ventricle | |||||
| GLS | −19.2% | −19.0% | −16.0% | −17.6% | −21.2% |
| SLS—basal septal | −15.5% | −4.0% | −4.0% | −9.7% | −6.0% |
| SLS—mid septal | −17.2% | −5.7% | −7.0% | −10.7% | −15.0% |
| SLS—apical septal | −18.5% | −15.3% | −14.7% | −12.7% | −29.3% |
| SLS—basal lateral | −20.9% | −28.7% | −20.0% | −26.3% | −25.0% |
| SLS—mid lateral | −22.9% | −26.0% | −24.0% | −26.3% | −23.7% |
| SLS—apical lateral | −18.9% | −27.3% | −24.3% | −15.3% | −30.0% |
| Modality | Indication | Normal Hearts | Severe AS | Critical AS |
|---|---|---|---|---|
| Fetal Echocardiography | Myocardial deformation | Normal strain between −15.5% and −22.9% | Mildly decreased strain between −7.0% and −16.0% | Extremely decreased strain between −0.3% and −4.6% |
| Histology | Myocardial organization | Well-organized cardiomyocyte alignment | Moderate disturbed cardiomyocyte organization | Severely disturbed cardiomyocyte network |
| Histology | Myocardial differentiation | Proper Cx43 and N-cadh expression at lateral borders and intercalated disks | Reduced Cx43 and N-cadherin expression, mainly as diffuse intracellular pattern | Reduced Cx43 and N-cadh expression, mainly as diffuse intracellular pattern |
| Histology | Myocardial fibrosis | No EFE | Mild EFE | Overt EFE with patchy fibrosis patterns in myocardium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwanenburg, F.; DeRuiter, M.C.; Wisse, L.J.; van Munsteren, C.J.; Bartelings, M.M.; Goumans, M.-J.; Ten Harkel, A.D.J.; Jongbloed, M.R.M.; Haak, M.C. Deficient Myocardial Organization and Pathological Fibrosis in Fetal Aortic Stenosis—Association of Prenatal Ultrasound with Postmortem Histology. J. Cardiovasc. Dev. Dis. 2021, 8, 121. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd8100121
Zwanenburg F, DeRuiter MC, Wisse LJ, van Munsteren CJ, Bartelings MM, Goumans M-J, Ten Harkel ADJ, Jongbloed MRM, Haak MC. Deficient Myocardial Organization and Pathological Fibrosis in Fetal Aortic Stenosis—Association of Prenatal Ultrasound with Postmortem Histology. Journal of Cardiovascular Development and Disease. 2021; 8(10):121. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd8100121
Chicago/Turabian StyleZwanenburg, Fleur, Marco C. DeRuiter, Lambertus J. Wisse, Conny J. van Munsteren, Margot M. Bartelings, Marie-Jose Goumans, Arend D. J. Ten Harkel, Monique R. M. Jongbloed, and Monique C. Haak. 2021. "Deficient Myocardial Organization and Pathological Fibrosis in Fetal Aortic Stenosis—Association of Prenatal Ultrasound with Postmortem Histology" Journal of Cardiovascular Development and Disease 8, no. 10: 121. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd8100121