Cardiac Resynchronization Therapy beyond Nominal Settings: An IEGM-Based Approach for Paced and Sensed Atrioventricular Delay Offset Optimization in Daily Clinical Practice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Device Implantation
2.3. Echocardiographic Optimization Protocol
2.4. IEGM Method
2.5. Follow Up
2.6. Statistical Analyses
3. Results
3.1. Echocardiographic AV Optimization
3.2. IEGM-Based AV Delays
3.3. CRT Response
4. Discussion
4.1. AV Optimization Methods
4.2. AV Delay: Selected Groups and Outcome Markers
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auricchio, A.; Stellbrink, C.; Sack, S.; Block, M.; Vogt, J.; Bakker, P.; Huth, C.; Schöndube, F.; Wolfhard, U.; Böcker, D.; et al. long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J. Am. Coll. Cardiol. 2002, 39, 2026–2033. [Google Scholar] [CrossRef]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef]
- Cazeau, S.; Leclercq, C.; Lavergne, T.; Walker, S.; Varma, C.; Linde, C.; Garrigue, S.; Kappenberger, L.; Haywood, G.A.; Santini, M.; et al. Effects of Multisite Biventricular Pacing in Patients with Heart Failure and Intraventricular Conduction Delay. N. Engl. J. Med. 2001, 344, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L.; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [Green Version]
- Mullens, W.; Grimm, R.A.; Verga, T.; Dresing, T.; Starling, R.C.; Wilkoff, B.L.; Tang, W.W. Insights from a Cardiac Resynchronization Optimization Clinic as Part of a Heart Failure Disease Management Program. J. Am. Coll. Cardiol. 2009, 53, 765–773. [Google Scholar] [CrossRef]
- Daubert, C.; Behar, N.; Martins, R.P.; Mabo, P.; Leclercq, C. Avoiding non-responders to cardiac resynchronization therapy: A practical guide. Eur. Heart J. 2017, 38, 1463–1472. [Google Scholar] [CrossRef]
- Gras, D.; Gupta, M.S.; Boulogne, E.; Guzzo, L.; Abraham, W.T. Optimization of AV and VV Delays in the Real-World CRT Patient Population: An International Survey on Current Clinical Practice. Pacing Clin. Electrophysiol. 2009, 32, S236–S239. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Ding, J.; Spinelli, J.C.; Kramer, A.P.; Salo, R.W.; Hoersch, W.; KenKnight, B.H.; Klein, H.U. Cardiac resynchronization therapy restores optimal atrioventricular mechanical timing in heart failure patients with ventricular conduction delay. J. Am. Coll. Cardiol. 2002, 39, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- Sawhney, N.S.; Waggoner, A.D.; Garhwal, S.; Chawla, M.K.; Osborn, J.; Faddis, M.N. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm. 2004, 1, 562–567. [Google Scholar] [CrossRef]
- Morales, M.-A.; Startari, U.; Panchetti, L.; Rossi, A.; Piacenti, M. Atrioventricular Delay Optimization by Doppler-Derived Left Ventricular dP/dt Improves 6-Month Outcome of Resynchronized Patients. Pacing Clin. Electrophysiol. 2006, 29, 564–568. [Google Scholar] [CrossRef]
- Hardt, S.E.; Yazdi, S.H.F.; Bauer, A.; Filusch, A.; Korosoglou, G.; Hansen, A.; Bekeredjian, R.; Ehlermann, P.; Remppis, A.; Katus, H.A.; et al. Immediate and chronic effects of AV-delay optimization in patients with cardiac resynchronization therapy. Int. J. Cardiol. 2007, 115, 318–325. [Google Scholar] [CrossRef]
- Ellenbogen, K.A.; Gold, M.R.; Meyer, T.E.; Lozano, I.F.; Mittal, S.; Waggoner, A.D.; Lemke, B.; Singh, J.P.; Spinale, F.G.; Van Eyk, J.E.; et al. Primary Results from the SmartDelay Determined AV Optimization: A Comparison to Other AV Delay Methods Used in Cardiac Resynchronization Therapy (SMART-AV) Trial. Circulation 2010, 122, 2660–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.O.; Lemke, B.; Birnie, D.; Krum, H.; Lee, K.L.-F.; Aonuma, K.; Gasparini, M.; Starling, R.C.; Milasinovic, G.; Rogers, T.; et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: Results of the adaptive CRT trial. Heart Rhythm. 2012, 9, 1807–1814.e1. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, E.; Saba, S. Right atrial pacing and the risk of postimplant atrial fibrillation in cardiac resynchronization therapy recipients. Am. Heart J. 2008, 155, 94–99. [Google Scholar] [CrossRef]
- Bernheim, A.; Ammann, P.; Sticherling, C.; Burger, P.; Schaer, B.; Rocca, H.P.B.-L.; Eckstein, J.; Kiencke, S.; Kaiser, C.; Linka, A.; et al. Right Atrial Pacing Impairs Cardiac Function During Resynchronization Therapy: Acute Effects of DDD Pacing Compared to VDD Pacing. J. Am. Coll. Cardiol. 2005, 45, 1482–1487. [Google Scholar] [CrossRef]
- Gold, M.R.; Niazi, I.; Giudici, M.; Leman, R.B.; Sturdivant, J.L.; Kim, M.H.; Waggoner, A.D.; Ding, J.; Arcot-Krishnamurthy, S.; Daum, D.; et al. Acute Hemodynamic Effects of Atrial Pacing with Cardiac Resynchronization Therapy. J. Cardiovasc. Electrophysiol. 2009, 20, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.-F.; Siu, C.-W.; Lee, K.L.; Fan, K.; Chan, H.-W.; Tang, M.-O.; Tsang, V.; Lee, S.W.; Lau, C.-P. The Incremental Benefit of Rate-Adaptive Pacing on Exercise Performance During Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2005, 46, 2292–2297. [Google Scholar] [CrossRef] [Green Version]
- Maass, A.H.; Buck, S.; Nieuwland, W.; Brügemann, J.; VAN Veldhuisen, D.J.; VAN Gelder, I.C. Importance of Heart Rate During Exercise for Response to Cardiac Resynchronization Therapy. J. Cardiovasc. Electrophysiol. 2009, 20, 773–780. [Google Scholar] [CrossRef]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.-A.; Cleland, J.G.F.; Deharo, J.-C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2013, 34, 2281–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schouwenburg, J.J.; Klinkenberg, T.J.; Maass, A.H.; Mariani, M.A. Video-Assisted Thoracic Placement of Epicardial Leads. J. Card. Surg. 2014, 29, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Cobb, D.B.; Gold, M.R. The Role of Atrioventricular and Interventricular Optimization for Cardiac Resynchronization Therapy. Heart Fail. Clin. 2017, 13, 209–223. [Google Scholar] [CrossRef]
- Luo, H.; Westphal, P.; Shahmohammadi, M.; Heckman, L.I.; Kuiper, M.; Cornelussen, R.N.; Delhaas, T.; Prinzen, F.W. Heart sound–derived systolic time intervals for atrioventricular delay optimization in cardiac resynchronization therapy. Heart Rhythm. 2023, 20, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Whinnett, Z.I.; Sohaib, S.A.; Mason, M.; Duncan, E.; Tanner, M.; Lefroy, D.; Al-Obaidi, M.; Ellery, S.; Leyva-Leon, F.; Betts, T.; et al. Multicenter Randomized Controlled Crossover Trial Comparing Hemodynamic Optimization Against Echocardiographic Optimization of AV and VV Delay of Cardiac Resynchronization Therapy. JACC Cardiovasc. Imaging 2019, 12, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.R.; Mur, J.L.M.; Moreno, J.; Fernández-Golfín, C.; Franco, E.; Berlot, B.; Monteagudo, J.M.; Francés, R.M.; Madrid, A.H.; Zamorano, J.L. Mitral-Aortic Flow Reversal in Cardiac Resynchronization Therapy. Circ. Arrhythmia Electrophysiol. 2017, 10, e004927. [Google Scholar] [CrossRef]
- Brugada, J.; Delnoy, P.P.; Brachmann, J.; Reynolds, D.; Padeletti, L.; Noelker, G.; Kantipudi, C.; Lopez, J.M.R.; Dichtl, W.; Borri-Brunetto, A.; et al. Contractility sensor-guided optimization of cardiac resynchronization therapy: Results from the RESPOND-CRT trial. Eur. Heart J. 2016, 38, 730–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maass, A.H.; Vernooy, K.; Wijers, S.C.; van’t Sant, J.; Cramer, M.J.; Meine, M.; Allaart, C.P.; De Lange, F.J.; Prinzen, F.W.; Gerritse, B.; et al. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: Results from the Markers and Response to CRT (MARC) study. EP Eur. 2018, 20, e1–e10. [Google Scholar] [CrossRef]
- Kloosterman, M.; Maass, A.H. Sex differences in optimal atrioventricular delay in patients receiving cardiac resynchronization therapy. Clin. Res. Cardiol. 2020, 109, 124–127. [Google Scholar] [CrossRef]
- Gold, M.R.; Yu, Y.; Singh, J.P.; Birgersdotter-Green, U.; Stein, K.M.; Wold, N.; Meyer, T.E.; Ellenbogen, K.A. Effect of Interventricular Electrical Delay on Atrioventricular Optimization for Cardiac Resynchronization Therapy. Circ. Arrhythmia Electrophysiol. 2018, 11, e006055. [Google Scholar] [CrossRef]
- Korach, R.; Kahr, P.C.; Ruschitzka, F.; Steffel, J.; Flammer, A.J.; Winnik, S. Long-term follow-up after cardiac resynchronization therapy-optimization in a real-world setting: A single-center cohort study. Cardiol. J. 2021, 28, 728–737. [Google Scholar] [CrossRef] [Green Version]
- Martens, P.; Deferm, S.; Bertrand, P.B.; Verbrugge, F.H.; Ramaekers, J.; Verhaert, D.; Dupont, M.; Vandervoort, P.M.; Mullens, W. The Detrimental Effect of RA Pacing on LA Function and Clinical Outcome in Cardiac Resynchronization Therapy. JACC Cardiovasc. Imaging 2020, 13, 895–906. [Google Scholar] [CrossRef]
- O’Donnell, D.; Nadurata, V.; Hamer, A.; Kertes, P.; Mohammed, U. Long-Term Variations in Optimal Programming of Cardiac Resynchronization Therapy Devices. Pacing Clin. Electrophysiol. 2005, 28, S24–S26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fung, J.W.-H.; Chan, Y.-S.; Chan, H.C.-K.; Lin, H.; Chan, S.; Yu, C.-M. The role of repeating optimization of atrioventricular interval during interim and long-term follow-up after cardiac resynchronization therapy. Int. J. Cardiol. 2008, 124, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, M.; Rienstra, M.; Mulder, B.A.; Van Gelder, I.C.; Maass, A.H. Atrial reverse remodelling is associated with outcome of cardiac resynchronization therapy. Europace 2016, 18, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Mathias, A.; Moss, A.J.; McNitt, S.; Zareba, W.; Goldenberg, I.; Solomon, S.D.; Kutyifa, V. Clinical Implications of Complete Left-Sided Reverse Remodeling with Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2016, 68, 1268–1276. [Google Scholar] [CrossRef] [PubMed]





| Patient Population (n = 328) | |
|---|---|
| Demographics | |
| Male gender, % (n) | 64 (210) |
| Age, years | 69 ± 12 |
| BMI, kg/m2 | 27.8 ± 4.7 |
| Weight, kg | 84 ± 15 |
| Height, cm | 174 ± 9 |
| Medical history | |
| Hypertension, % (n) | 43 (142) |
| Diabetes mellitus, % (n) | 26 (84) |
| Coronary artery disease, % (n) | 50 (164) |
| Myocardial infarction, % (n) | 38 (125) |
| CABG, % (n) | 17 (57) |
| Dilated cardiomyopathy, % (n) | 52 (171) |
| History of atrial fibrillation, % (n) | 20 (66) |
| Clinical profile | |
| Systolic blood pressure, mmHg | 119 ± 20 |
| Diastolic blood pressure, mmHg | 72 ± 11 |
| NYHA class, % (n) | |
| I | 3 (10) |
| II | 69 (226) |
| III | 27 (89) |
| IV | 1 (3) |
| ECG | |
| Heart rate, bpm | 73 ± 14 |
| PQ duration, ms | 186 ± 41 |
| QRS duration, ms | 159 ± 20 |
| Echocardiography | |
| LV ejection fraction, % | 25 ± 8 |
| LV end diastolic diameter, mm | 61 ± 9 |
| LV end-systolic diameter, mm | 51 ± 10 |
| LV end diastolic volume, mL | 191 ± 78 |
| LV the end-systolic volume, mL | 142 ± 67 |
| LA the end-systolic volume, mL | 74 ± 26 |
| LA volume index †, mL/m2 | 38 ± 13 |
| Mitral regurgitation *, % (n) | 51 (168) |
| Tricuspid regurgitation *, % (n) | 27 (87) |
| Implantation | |
| Upgrade, % (n) | 22 (73) |
| CRT-P, % (n) | 6 (20) |
| CRT-D, % (n) | 94 (308) |
| Lead position | |
| Anterolateral | 2 (8) |
| Lateral | 12 (38) |
| Posterolateral | 72 (235) |
| Posterior | 11 (36) |
| Transvenous LV lead, % (n) | 91 (298) |
| Medication use | |
| β-blocker, % (n) | 89 (292) |
| ACE inhibitor, % (n) | 66 (217) |
| ARB, % (n) | 27 (117) |
| Diuretics, % (n) | 77 (251) |
| Statin, % (n) | 51 (167) |
| OAC, % (n) | 44 (145) |
| ASA, % (n) | 38 (124) |
| Laboratory values | |
| NTproBNP (ng/mL) | 1267 (509–2410) |
| Hb (mmol/L) | 8.4 (7.9–9.0) |
| Creatinine (umol/L) | 96 (78–119) |
| eGFR (mL/min/1.73 m2) | 64 (47–81) |
| Patient Population (N = 328) | |
|---|---|
| IEGM | |
| Atrial sensing (ms) | 194 ± 50 |
| Atrial pacing (ms) | 269 ± 59 |
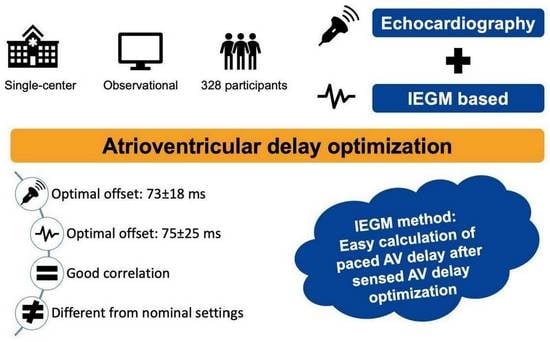
| Offset IEGM (ms) | 75 ± 25 |
| Echocardiography | |
| Atrial sensing (ms) | 75 ± 26 |
| Atrial pacing (ms) | 148 ± 32 |
| Offset Echo (ms) | 73 ± 18 |
| Nominal AV Settings for Different Manufacturers | ||||
|---|---|---|---|---|
| Sensed AV Delay (ms) | Paced AV Delay (ms) | Offset (ms) | p-Value * | |
| Biotronik | 150/120 | 190/160 | 40 | <0.001 |
| Boston Scientific | 120 | 180 | 60 | <0.001 |
| LivaNova | 125/80 | 190/145 | 65 | <0.001 |
| Medtronic | 100 | 130 | 30 | <0.001 |
| Abbott | 150 | 200 | 50 | <0.001 |
| AV Offset Dispersion in Our Patient Cohort | ||||
| AV Offset (ms) | Population (%) | |||
| ≤20 | 0.6 | |||
| 21–40 | 3.0 | |||
| 41–60 | 28.7 | |||
| 61–80 | 46.6 | |||
| 81–100 | 18.6 | |||
| 101–120 | 1.5 | |||
| 121–140 | 0.3 | |||
| >140 | 0.6 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloosterman, M.; Daniëls, F.; Roseboom, E.; Rienstra, M.; Maass, A.H. Cardiac Resynchronization Therapy beyond Nominal Settings: An IEGM-Based Approach for Paced and Sensed Atrioventricular Delay Offset Optimization in Daily Clinical Practice. J. Clin. Med. 2023, 12, 4138. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12124138
Kloosterman M, Daniëls F, Roseboom E, Rienstra M, Maass AH. Cardiac Resynchronization Therapy beyond Nominal Settings: An IEGM-Based Approach for Paced and Sensed Atrioventricular Delay Offset Optimization in Daily Clinical Practice. Journal of Clinical Medicine. 2023; 12(12):4138. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12124138
Chicago/Turabian StyleKloosterman, Mariëlle, Fenna Daniëls, Eva Roseboom, Michiel Rienstra, and Alexander H. Maass. 2023. "Cardiac Resynchronization Therapy beyond Nominal Settings: An IEGM-Based Approach for Paced and Sensed Atrioventricular Delay Offset Optimization in Daily Clinical Practice" Journal of Clinical Medicine 12, no. 12: 4138. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12124138