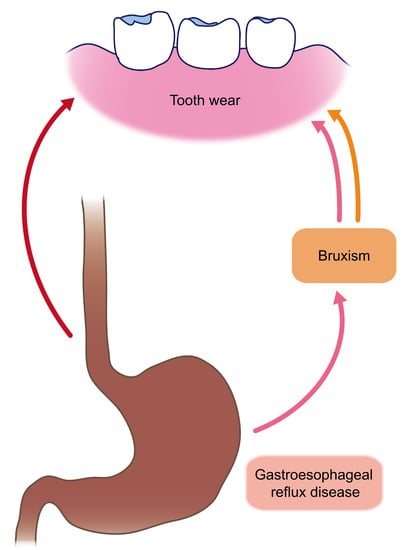
Associations among Bruxism, Gastroesophageal Reflux Disease, and Tooth Wear
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design and Participants
2.2. Data Measurement
2.3. Efforts to Minimize Bias
2.4. Statistical Analysis
3. Results
3.1. Case-Control Study
3.2. Cross-Sectional Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shellis, R.P.; Addy, M. The interactions between attrition, abrasion and erosion in tooth wear. Monogr. Oral Sci. 2014, 25, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Shah, P. A critical review of non-carious cervical (wear) lesions and the role of abfraction, erosion, and abrasion. J. Dent. Res. 2006, 85, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.J.; Khoury, S.; Abe, S.; Yamaguchi, T.; Raphael, K. Bruxism physiology and pathology: An overview for clinicians. J. Oral Rehabil. 2008, 35, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.R.; De Faria, D.L.; De Oliveira, E.; Sobral, M.A. Morphological and mineral analysis of dental enamel after erosive challenge in gastric juice and orange juice. Microsc. Res. Tech. 2011, 74, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Coward, P.Y. Comparison of the erosive potential of gastric juice and a carbonated drink in vitro. J. Oral Rehabil. 2001, 28, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Furuta, K.; Ueno, M.; Egawa, M.; Yoshino, A.; Kondo, S.; Nariai, Y.; Ishibashi, H.; Kinoshita, Y.; Sekine, J. Oral symptoms including dental erosion in gastroesophageal reflux disease are associated with decreased salivary flow volume and swallowing function. J. Gastroenterol. 2012, 47, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kaidonis, J.A. Oral diagnosis and treatment planning: Part 4. Non-carious tooth surface loss and assessment of risk. Br. Dent. J. 2012, 213, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Shellis, R.P.; Featherstone, J.D.; Lussi, A. Understanding the chemistry of dental erosion. Monogr. Oral Sci. 2014, 25, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nakatani, E.; Yoshikawa, H.; Kanno, T.; Nariai, Y.; Yoshino, A.; Vieth, M.; Kinoshita, Y.; Sekine, J. Oral soft tissue disorders are associated with gastroesophageal reflux disease: Retrospective study. BMC Gastroenterol. 2017, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Mengatto, C.M.; Dalberto Cda, S.; Scheeren, B.; Barros, S.G. Association between sleep bruxism and gastroesophageal reflux disease. J. Prosthet. Dent. 2013, 110, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Ohmure, H.; Kanematsu-Hashimoto, K.; Nagayama, K.; Taguchi, H.; Ido, A.; Tominaga, K.; Arakawa, T.; Miyawaki, S. Evaluation of a proton pump inhibitor for sleep bruxism: A randomized clinical trial. J. Dent. Res. 2016, 95, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, F.; Niu, L.; Long, Y.; Tay, F.R.; Chen, J. Association between bruxism and symptomatic gastroesophageal reflux disease: A case-control study. J. Dent. 2018, 77, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T. Dental Erosion and Its Clinical Management; Springer International Publishing: Cham, Switzerland, 2015; pp. 35–96. [Google Scholar]
- Bartlett, D.W.; Fares, J.; Shirodaria, S.; Chiu, K.; Ahmad, N.; Sherriff, M. The association of tooth wear, diet and dietary habits in adults aged 18–30 years old. J. Dent. 2011, 39, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Wild, Y.K.; Heyman, M.B.; Vittinghoff, E.; Dalal, D.H.; Wojcicki, J.M.; Clark, A.L.; Rechmann, B.; Rechmann, P. Gastroesophageal reflux is not associated with dental erosion in children. Gastroenterology 2011, 141, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.G.; Knight, J.K. An index for measuring the wear of teeth. Br. Dent. J. 1984, 156, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Carvalho, T.S. Erosive tooth wear: A multifactorial condition of growing concern and increasing knowledge. Monogr. Oral Sci. 2014, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Meurer, W.J.; Tolles, J. Logistic regression diagnostics: Understanding how well a model predicts outcomes. JAMA 2017, 317, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Hesselbacher, S.; Subramanian, S.; Rao, S.; Casturi, L.; Surani, S. Self-reported sleep bruxism and nocturnal gastroesophageal reflux disease in patients with obstructive sleep apnea: Relationship to gender and ethnicity. Open Respir. Med. J. 2014, 8, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tsiggos, N.; Tortopidis, D.; Hatzikyriakos, A.; Menexes, G. Association between self-reported bruxism activity and occurrence of dental attrition, abfraction, and occlusal pits on natural teeth. J. Prosthet. Dent. 2008, 100, 41–46. [Google Scholar] [CrossRef]
- Okura, K.; Shigemoto, S.; Suzuki, Y.; Noguchi, N.; Omoto, K.; Abe, S.; Matsuka, Y. Mandibular movement during sleep bruxism associated with current tooth attrition. J. Prosthodont. Res. 2017, 61, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Scaffa, P.; Carrilho, M.; Tjaderhane, L.; Di Lenarda, R.; Polimeni, A.; Tezvergil-Mutluay, A.; Tay, F.R.; Pashley, D.H.; Breschi, L. Effects of etch-and-rinse and self-etch adhesives on dentin MMP-2 and MMP-9. J. Dent. Res. 2013, 92, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.T.; Zero, D.T. The potential of saliva in protecting against dental erosion. Monogr. Oral Sci. 2014, 25, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Eisenburger, M.; Addy, M. Erosion and attrition of human enamel in vitro part II: Influence of time and loading. J. Dent. 2002, 30, 349–352. [Google Scholar] [CrossRef]
- Schlueter, N.; Luka, B. Erosive tooth wear—A review on global prevalence and on its prevalence in risk groups. Br. Dent. J. 2018, 224, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, C.H.; Lussi, A. Dental erosions in patients with silent gastro-oesophageal reflux disease (GERD): Characteristics and clinical course with proton pump inhibitor treatment. Gastroenterology 2012, 142, S411. [Google Scholar] [CrossRef]
- Valena, V.; Young, W.G. Dental erosion patterns from intrinsic acid regurgitation and vomiting. Aust. Dent. J. 2002, 47, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Takehara, J.; Takano, T.; Akhter, R.; Morita, M. Correlations of noncarious cervical lesions and occlusal factors determined by using pressure-detecting sheet. J. Dent. 2008, 36, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Sawlani, K.; Lawson, N.C.; Burgess, J.O.; Lemons, J.E.; Kinderknecht, K.E.; Givan, D.A.; Ramp, L. Factors influencing the progression of noncarious cervical lesions: A 5-year prospective clinical evaluation. J. Prosthet. Dent. 2016, 115, 571–577. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, H.; Peters, D.; Zollman, C. Closing the evidence gap in integrative medicine. BMJ 2009, 339. [Google Scholar] [CrossRef] [PubMed]


| Variables | Bruxism (n = 363) 1 | Non-Bruxism (n = 363) 1 | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Model 1 2 | Model 2 3 | |||||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| GERD | 68 (18.7) | 13 (3.6) | 6.21 (3.36–11.46) | <0.001 | 5.30 (2.62–10.70) | <0.001 | 3.84 (1.86–7.95) | <0.001 |
| GERD duration | ||||||||
| ≤5 years | 28 (7.7) | 8 (2.2) | 4.15 (1.86–9.25) | <0.001 | 3.38 (1.38–8.24) | 0.008 | 2.49 (0.98–6.29) | 0.054 |
| >5 years | 40 (11.0) | 5 (1.4) | 9.49 (3.70–24.36) | <0.001 | 8.73 (2.97–25.63) | <0.001 | 6.27 (2.07–18.97) | 0.001 |
| Variables | With Severe Tooth Wear (n = 224) 1 | Without Severe Tooth Wear (n = 502) 1 | Univariate Analysis | Multivariable Analysis 2 | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Non-bruxism | 76 (33.9) | 287 (57.2) | Reference | Reference | ||
| Sleep bruxism | 128 (57.1) | 191 (38.0) | 2.53 (1.81–3.55) | <0.001 | 2.77 (1.82–4.21) | <0.001 |
| Awake bruxism | 6 (2.7) | 8 (1.6) | 2.83 (0.95–8.41) | 0.061 | 1.98 (0.55–7.17) | 0.296 |
| Overlap of sleep and awake bruxism | 14 (6.3) | 16 (3.2) | 3.30 (1.54–7.07) | 0.002 | 3.28 (1.26–8.54) | 0.015 |
| Variables | With Severe Tooth Wear (n = 224) 1 | Without Severe Tooth Wear (n = 502) 1 | Univariate Analysis | Multivariable Analysis 2 | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Non-bruxism | 76 (33.9) | 287 (57.2) | Reference | Reference | ||
| Bruxism without GERD | 115 (51.3) | 180 (35.9) | 2.41 (1.71–3.51) | <0.001 | 2.89 (1.90–4.39) | <0.001 |
| Bruxism with GERD ≤5 years | 10 (4.5) | 18 (3.6) | 2.10 (0.93–4.73) | 0.074 | 2.04 (0.77–5.43) | 0.153 |
| Bruxism with GERD >5 years | 23 (10.3) | 17 (3.4) | 5.11 (2.60–10.04) | <0.001 | 4.70 (2.04–10.83) | <0.001 |
| Variables | Occlusal/Incisal | Palatal/Lingual | Buccal/Labial | Cervical | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Non-bruxism | Reference | Reference | Reference | Reference | ||||
| Bruxism without GERD | 2.47 (1.74–3.49) | <0.001 | 3.30 (1.16–9.37) | 0.025 | 1.78 (0.67–4.75) | 0.246 | 1.03 (0.44–2.41) | 0.952 |
| Bruxism with GERD ≤5 years | 2.21 (0.98–4.98) | 0.057 | 8.59 (1.94–38.04) | 0.005 | 1.78 (0.22–15.87) | 0.560 | 2.25 (0.48–10.59) | 0.305 |
| Bruxism with GERD >5 years | 4.86 (2.48–9.52) | <0.001 | 15.19 (4.57–50.51) | <0.001 | 1.30 (0.16–10.88) | 0.806 | 2.37 (0.64–8.79) | 0.196 |
| Variables | Occlusal/Incisal | Palatal/Lingual | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Non-bruxism | Reference | Reference | ||
| Bruxism without GERD | 2.92 (1.92–4.45) | <0.001 | 2.72 (0.92–8.10) | 0.072 |
| Bruxism with GERD ≤5 years | 2.15 (0.81–5.74) | 0.125 | 8.52 (1.66–43.71) | 0.010 |
| Bruxism with GERD >5 years | 4.23 (1.84–9.75) | 0.001 | 8.57 (2.82–32.21) | 0.001 |
| Variables | Upper | Lower | Anterior | Posterior | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Non-bruxism | Reference | Reference | Reference | Reference | ||||
| Bruxism without GERD | 2.57 (1.75–3.76) | <0.001 | 2.33 (1.63–3.32) | <0.001 | 2.63 (1.77–3.92) | <0.001 | 2.08 (1.44–2.99) | <0.001 |
| Bruxism with GERD ≤5 years | 1.95 (0.79–4.81) | 0.148 | 2.06 (0.89–4.74) | 0.091 | 3.19 (1.36–7.45) | 0.008 | 2.17 (0.94–5.02) | 0.069 |
| Bruxism with GERD >5 years | 5.29 (2.67–10.50) | <0.001 | 4.34 (2.21–8.51) | <0.001 | 4.97 (2.47–9.99) | <0.001 | 4.59 (2.33–9.01) | <0.001 |
| Variables | Upper | Lower | Anterior | Posterior | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Non-bruxism | Reference | Reference | Reference | Reference | ||||
| Bruxism without GERD | 2.61 (1.67–4.06) | <0.001 | 2.68 (1.75–4.11) | <0.001 | 2.66 (1.68–4.21) | <0.001 | 2.18 (1.42–3.36) | <0.001 |
| Bruxism with GERD ≤5 years | 1.55 (0.55–4.37) | 0.413 | 2.02 (0.74–5.51) | 0.171 | 3.10 (1.14–8.45) | 0.027 | 2.06 (0.77–5.55) | 0.153 |
| Bruxism with GERD >5 years | 3.74 (1.63–8.58) | 0.002 | 3.71 (1.60–8.61) | 0.002 | 3.16 (1.37–7.32) | 0.007 | 3.92 (1.69–9.05) | 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yu, F.; Niu, L.; Hu, W.; Long, Y.; Tay, F.R.; Chen, J. Associations among Bruxism, Gastroesophageal Reflux Disease, and Tooth Wear. J. Clin. Med. 2018, 7, 417. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm7110417
Li Y, Yu F, Niu L, Hu W, Long Y, Tay FR, Chen J. Associations among Bruxism, Gastroesophageal Reflux Disease, and Tooth Wear. Journal of Clinical Medicine. 2018; 7(11):417. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm7110417
Chicago/Turabian StyleLi, Yuanyuan, Fan Yu, Lina Niu, Wei Hu, Yong Long, Franklin R. Tay, and Jihua Chen. 2018. "Associations among Bruxism, Gastroesophageal Reflux Disease, and Tooth Wear" Journal of Clinical Medicine 7, no. 11: 417. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm7110417