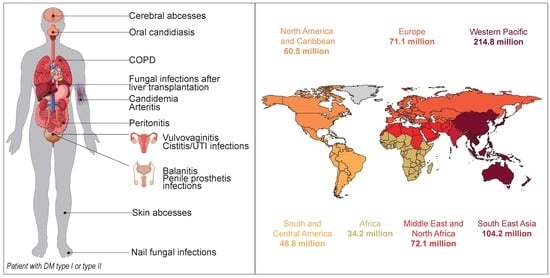
Candida sp. Infections in Patients with Diabetes Mellitus
Abstract
:1. Introduction
2. Particular Features of Candida sp. that Increase the Incidence of Candidiasis in Diabetic Patients
2.1. Enzymatic Activity
2.2. Biofilm Formation
2.3. Hydrophobicity
3. Candidiasis and DM
3.1. Oral Diseases
3.1.1. Oral Candidiasis
3.1.2. Antifungal Treatment of Oral Candidiasis
3.1.3. Periodontal Diseases
3.1.4. Denture Prosthetics and Candidiasis
3.1.5. Co-Occurrence of Dental Plaque, Periodontitis, and Gingivitis
3.1.6. Esophagitis and Oropharyngeal Candidiasis
3.2. Vulvovaginal Candidiasis
3.3. Urinary Tract Candidiasis
3.4. Systemic Candidiasis
3.5. Other Candidiasis
4. Diabetes Mellitus In Vivo Models
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Willis, A.M.; Coulter, W.A.; Fulton, C.R.; Hayes, J.R.; Bell, P.M.; Lamey, P.J. Oral candidal carriage and infection in insulin-treated diabetic patients. Diabet. Med. J. Br. Diabet. Assoc. 1999, 16, 675–679. [Google Scholar] [CrossRef]
- Karaa, A.; Goldstein, A. The spectrum of clinical presentation, diagnosis, and management of mitochondrial forms of diabetes. Pediatr. Diabetes 2015, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Calvet, H.M.; Yoshikawa, T.T. Infections in diabetes. Infect. Dis. Clin. N. Am. 2001, 15, 407–421. [Google Scholar] [CrossRef]
- Type 2 Diabetes: Prevention in People at High Risk|NICE Public Health Guideline 38—NICE. Available online: https://www.nice.org.uk/guidance/ph38/resources/type-2-diabetes-prevention-in-people-at-high-risk-pdf-1996304192197 (accessed on 11 September 2018).
- Blake, R.; Trounce, I.A. Mitochondrial dysfunction and complications associated with diabetes. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Luo, Y.-X.; Chen, H.-Z.; Liu, D.-P. Mitochondria, endothelial cell function, and vascular diseases. Front. Physiol. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.D.; McGee, S.L. The role of mitochondria in the aetiology of insulin resistance and type 2 diabetes. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1303–1312. [Google Scholar] [CrossRef]
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef]
- Agarwal, S.; Raman, R.; Paul, P.G.; Rani, P.K.; Uthra, S.; Gayathree, R.; McCarty, C.; Kumaramanickavel, G.; Sharma, T. Sankara Nethralaya—Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN—DREAMS 1): Study Design and Research Methodology. Ophthalmic Epidemiol. 2005, 12, 143–153. [Google Scholar] [CrossRef]
- De Resende, M.A.; de Sousa, L.V.N.F.; de Oliveira, R.C.B.W.; Koga-Ito, C.Y.; Lyon, J.P. Prevalence and Antifungal Susceptibility of Yeasts Obtained from the Oral Cavity of Elderly Individuals. Mycopathologia 2006, 162, 39–44. [Google Scholar] [CrossRef]
- Guimarães, T.; Nucci, M.; Mendonça, J.S.; Martinez, R.; Brito, L.R.; Silva, N.; Moretti, M.L.; Salomão, R.; Colombo, A.L. Epidemiology and predictors of a poor outcome in elderly patients with candidemia. Int. J. Infect. Dis. 2012, 16, 442–447. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Yarahmadi, S.; Baiat, M.; Shokri, H.; Pourkabireh, M. Factors affecting the prevalence of yeasts in the oral cavity of patients with diabetes mellitus. J. Mycol. Médicale J. Med. Mycol. 2008, 18, 83–88. [Google Scholar] [CrossRef]
- Tang, H.J.; Liu, W.L.; Lin, H.L.; Lai, C.C. Epidemiology and prognostic factors of candidemia in elderly patients. Geriatr. Gerontol. Int. 2015, 15, 688–693. [Google Scholar] [CrossRef]
- Belazi, M.; Velegraki, A.; Fleva, A.; Gidarakou, I.; Papanaum, L.; Baka, D.; Daniilidou, N.; Karamitsos, D. Candidal overgrowth in diabetic patients: Potential predisposing factors. Mycoses 2005, 48, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Darwazeh, A.M.G.; Lamey, P.-J.; Samaranayake, L.P.; Macfarlane, T.W.; Fisher, B.M.; Macrury, S.M.; Maccuish, A.C. The relationship between colonisation, secretor status and in-vitro adhesion of Candida albicans to buccal epithelial cells from diabetics. J. Med. Microbiol. 1990, 33, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, R.H.P.; Miranda, E.T.; Zaia, J.E.; Giannini, M.J.S.M. Species diversity of yeast in oral colonization of insulin-treated diabetes mellitus patients. Mycopathologia 2006, 162, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Gudlaugsson, O.; Gillespie, S.; Lee, K.; Vande Berg, J.; Hu, J.; Messer, S.; Herwaldt, L.; Pfaller, M.; Diekema, D. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 2003, 37, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Padshetty, N.S.; Bai, K.Y.; Rao, M.S. Prevalence of Candida in the oral cavity of diabetic subjects. J. Assoc. Physicians India 2005, 53, 599–602. [Google Scholar] [PubMed]
- Davenport, J.C. The oral distribution of candida in denture stomatitis. Br. Dent. J. 1970, 129, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Flier, J.S.; Underhill, L.H.; Brownlee, M.; Cerami, A.; Vlassara, H. Advanced Glycosylation End Products in Tissue and the Biochemical Basis of Diabetic Complications. N. Engl. J. Med. 1988, 318, 1315–1321. [Google Scholar] [CrossRef]
- Kadir, T.; Pisiriciler, R.; Akyüz, S.; Yarat, A.; Emekli, N.; Ipbüker, A. Mycological and cytological examination of oral candidal carriage in diabetic patients and non-diabetic control subjects: Thorough analysis of local aetiologic and systemic factors. J. Oral Rehabil. 2002, 29, 452–457. [Google Scholar] [CrossRef]
- Wilson, R.M.; Reeves, W.G. Neutrophil phagocytosis and killing in insulin-dependent diabetes. Clin. Exp. Immunol. 1986, 63, 478–484. [Google Scholar] [PubMed]
- Duggan, S.; Essig, F.; Hünniger, K.; Mokhtari, Z.; Bauer, L.; Lehnert, T.; Brandes, S.; Häder, A.; Jacobsen, I.D.; Martin, R.; et al. Neutrophil activation by Candida glabrata but not Candida albicans promotes fungal uptake by monocytes. Cell. Microbiol. 2015, 17, 1259–1276. [Google Scholar] [CrossRef] [PubMed]
- Motta-Silva, A.C.; Aleva, N.A.; Chavasco, J.K.; Armond, M.C.; França, J.P.; Pereira, L.J. Erythematous Oral Candidiasis in Patients with Controlled Type II Diabetes Mellitus and Complete Dentures. Mycopathologia 2010, 169, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Calderone, R.A.; Fonzi, W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001, 9, 327–335. [Google Scholar] [CrossRef]
- Pinto, E.; Ribeiro, I.C.; Ferreira, N.J.; Fortes, C.E.; Fonseca, P.A.; Figueiral, M.H. Correlation between enzyme production, germ tube formation and susceptibility to fluconazole in Candida species isolated from patients with denture-related stomatitis and control individuals. J. Oral Pathol. Med. 2008, 37, 587–592. [Google Scholar] [CrossRef]
- Dorko, E.; Baranová, Z.; Jenča, A.; Kizek, P.; Pilipčinec, E.; Tkáčiková, L. Diabetes mellitus and candidiases. Folia Microbiol. 2005, 50, 255–261. [Google Scholar] [CrossRef]
- Nowakowska, D.; Kurnatowska, A.; Stray-Pedersen, B.; Wilczyński, J. Species distribution and influence of glycemic control on fungal infections in pregnant women with diabetes. J. Infect. 2004, 48, 339–346. [Google Scholar] [CrossRef]
- Hammad, M.M.; Darwazeh, A.M.G.; Idrees, M.M. The effect of glycemic control on Candida colonization of the tongue and the subgingival plaque in patients with type II diabetes and periodontitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 321–326. [Google Scholar] [CrossRef]
- Al Mubarak, S.; Robert, A.A.; Baskaradoss, J.K.; Al-Zoman, K.; Al Sohail, A.; Alsuwyed, A.; Ciancio, S. The prevalence of oral Candida infections in periodontitis patients with type 2 diabetes mellitus. J. Infect. Public Health 2013, 6, 296–301. [Google Scholar]
- Balan, P.; Castelino, R.L.; Fazil Areekat, B.K. Candida Carriage Rate and Growth Characteristics of Saliva in Diabetes Mellitus Patients: A Case‒Control Study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2015, 9, 274–279. [Google Scholar] [CrossRef]
- Gürsoy, S.; Koçkar, T.; Atik, S.U.; Önal, Z.; Önal, H.; Adal, E. Autoimmunity and intestinal colonization by Candida albicans in patients with type 1 diabetes at the time of the diagnosis. Korean J. Pediatr. 2018, 61, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Bommanavar, S.; Gugwad, S.; Malik, N. Phenotypic switch: The enigmatic white-gray-opaque transition system of Candida albicans. J. Oral Maxillofac. Pathol. 2017, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O.; Atkinson, M.A.; Neu, J. The “Perfect Storm” for Type 1 Diabetes: The Complex Interplay Between Intestinal Microbiota, Gut Permeability, and Mucosal Immunity. Diabetes 2008, 57, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; de Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Cartenì, M.; Generoso, M.; et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Gosiewski, T.; Salamon, D.; Szopa, M.; Sroka, A.; Malecki, M.T.; Bulanda, M. Quantitative evaluation of fungi of the genus Candida in the feces of adult patients with type 1 and 2 diabetes—A pilot study. Gut Pathog. 2014, 6, 43. [Google Scholar] [CrossRef]
- Soyucen, E.; Gulcan, A.; Aktuglu-Zeybek, A.C.; Onal, H.; Kiykim, E.; Aydin, A. Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatr. Int. 2014, 56, 336–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalewska, B.; Zorena, K.; Szmigiero-Kawko, M.; Wąż, P.; Myśliwiec, M. High Interleukin-12 Levels May Prevent an Increase in the Amount of Fungi in the Gastrointestinal Tract during the First Years of Diabetes Mellitus Type 1. Dis. Mark. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Abelson, J.A.; Moore, T.; Bruckner, D.; Deville, J.; Nielsen, K. Frequency of Fungemia in Hospitalized Pediatric Inpatients Over 11 Years at a Tertiary Care Institution. Pediatrics 2005, 116, 61–67. [Google Scholar] [CrossRef]
- Costa, S.F.; Marinho, I.; Araújo, E.A.; Manrique, A.E.; Medeiros, E.A.; Levin, A.S. Nosocomial fungaemia: A 2-year prospective study. J. Hosp. Infect. 2000, 45, 69–72. [Google Scholar] [CrossRef]
- Lopes Colombo, A.; Nucci, M.; Salomão, R.; Branchini, M.L.M.; Richtmann, R.; Derossi, A.; Wey, S.B. High rate of non-albicans candidemia in Brazilian tertiary care hospitals. Diagn. Microbiol. Infect. Dis. 1999, 34, 281–286. [Google Scholar] [CrossRef]
- Wang, H.; Liu, N.; Yin, M.; Han, H.; Yue, J.; Zhang, F.; Shan, T.; Guo, H.; Wu, D. The epidemiology, antifungal use and risk factors of death in elderly patients with candidemia: A multicentre retrospective study. BMC Infect. Dis. 2014, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.; Silva, S.; Henriques, M. Candida glabrata Biofilms: How Far Have We Come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef]
- Silva, S.; Rodrigues, C.F.; Araújo, D.; Rodrigues, M.; Henriques, M. Candida Species Biofilms’ Antifungal Resistance. J. Fungi 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.T.; Tavakoli, M.; Zakavi, F.; Shokohi, T.; Mofarrah, R.; Ansari, S.; Armaki, M.T. In vitro antifungal susceptibility of Candida speciesisolated from diabetic patients. Rev. Soc. Bras. Med. Trop. 2018, 51, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Puig-Asensio, M.; Padilla, B.; Garnacho-Montero, J.; Zaragoza, O.; Aguado, J.M.; Zaragoza, R.; Montejo, M.; Muñoz, P.; Ruiz-Camps, I.; Cuenca-Estrella, M.; et al. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: A population-based surveillance in Spain. Clin. Infect. Dis. 2014, 20, O245–O254. [Google Scholar] [CrossRef] [PubMed]
- Meunier-Carpentier, F.; Kiehn, T.E.; Armstrong, D. Fungemia in the immunocompromised host. Changing patterns, antigenemia, high mortality. Am. J. Med. 1981, 71, 363–370. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Karabinis, A.; Samonis, G.; Falagas, M.E. Candidemia in immunocompromised and immunocompetent critically ill patients: A prospective comparative study. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 377–384. [Google Scholar] [CrossRef]
- Man, A.; Ciurea, C.N.; Pasaroiu, D.; Savin, A.-I.; Toma, F.; Sular, F.; Santacroce, L.; Mare, A. New perspectives on the nutritional factors influencing growth rate of Candida albicans in diabetics. An in vitro study. Mem. Inst. Oswaldo Cruz 2017, 112, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Barnett, J.A. The Utilization of Disaccharides and Some Other Sugars RY Yeasts. Adv. Carbohydr. Chem. Biochem. 1981, 39, 347–404. [Google Scholar]
- Rodrigues, C.F.; Henriques, M. Oral mucositis caused by Candida glabrata biofilms: Failure of the concomitant use of fluconazole and ascorbic acid. Ther. Adv. Infect. Dis. 2017, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Mahata, D.; Migliolo, L.; Parekh, A.; Addy, P.S.; Mandal, M.; Basak, A. Glucose directly promotes antifungal resistance in the fungal pathogen, Candida spp. J. Biol. Chem. 2014, 289, 25468–25473. [Google Scholar] [CrossRef] [PubMed]
- Rodaki, A.; Bohovych, I.M.; Enjalbert, B.; Young, T.; Odds, F.C.; Gow, N.A.R.; Brown, A.J.P. Glucose Promotes Stress Resistance in the Fungal Pathogen Candida albicans. Mol. Biol. Cell 2009, 20, 4845–4855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramono, K.; Yamazaki, M.; Tsuboi, R.; Ogawa, H. Comparison of proteinase, lipase and alpha-glucosidase activities from the clinical isolates of Candida species. Jpn. J. Infect. Dis. 2006, 59, 73–76. [Google Scholar]
- Ingham, C.J.; Boonstra, S.; Levels, S.; de Lange, M.; Meis, J.F.; Schneeberger, P.M. Rapid Susceptibility Testing and Microcolony Analysis of Candida spp. Cultured and Imaged on Porous Aluminum Oxide. PLoS ONE 2012, 7, e33818. [Google Scholar] [CrossRef]
- Manfredi, M.; McCullough, M.J.; Al-Karaawi, Z.M.; Hurel, S.J.; Porter, S.R. The isolation, identification and molecular analysis of Candida spp. isolated from the oral cavities of patients with diabetes mellitus. Oral Microbiol. Immunol. 2002, 17, 181–185. [Google Scholar] [CrossRef]
- Soysa, N.S.; Samaranayake, L.P.; Ellepola, A.N.B. Diabetes mellitus as a contributory factor in oral candidosis. Diabet. Med. 2006, 23, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.S.P.; Chu, F.C.S.; Leung, W.K.; Jin, L.J.; Samaranayake, L.P.; Siu, S.C. Phospholipase, proteinase and haemolytic activities of Candida albicans isolated from oral cavities of patients with type 2 diabetes mellitus. J. Med. Microbiol. 2007, 56, 1393–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manns, J.M.; Mosser, D.M.; Buckley, H.R. Production of a hemolytic factor by Candida albicans. Infect. Immun. 1994, 62, 5154–5156. [Google Scholar]
- Fatahinia, M.; Poormohamadi, F.; Mahmoudabadi, A.Z. Comparative study of esterase and hemolytic activities in clinically important Candida species, isolated from oral cavity of diabetic and non-diabetic individuals. Jundishapur J. Microbiol. 2015, 8, 3–6. [Google Scholar] [CrossRef]
- Shimizu, M.T.; Almeida, N.Q.; Fantinato, V.; Unterkircher, C.S. Studies on hyaluronidase, chondroitin sulphatase, proteinase and phospholipase secreted by Candida species. Mycoses 1996, 39, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.; Albrecht, A.; Bader, O.; Hube, B. Candida albicans proteinases and host/pathogen interactions. Cell. Microbiol. 2004, 6, 915–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminishi, H.; Tanaka, M.; Cho, T.; Maeda, H.; Hagihara, Y. Activation of the plasma kallikrein-kinin system by Candida albicans proteinase. Infect. Immun. 1990, 58, 2139–2143. [Google Scholar] [PubMed]
- Kaminishi, H.; Miyaguchi, H.; Tamaki, T.; Suenaga, N.; Hisamatsu, M.; Mihashi, I.; Matsumoto, H.; Maeda, H.; Hagihara, Y. Degradation of humoral host defense by Candida albicans proteinase. Infect. Immun. 1995, 63, 984–988. [Google Scholar]
- Ghannoum, M.A. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 2000, 13, 122–143. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64. [Google Scholar] [CrossRef]
- Naglik, J.R.; König, A.; Hube, B.; Gaffen, S.L. Candida albicans–epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Donlan, R.; Costerton, J. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Fonseca, E.; Silva, S.; Rodrigues, C.F.; Alves, C.; Azeredo, J.; Henriques, M. Effects of fluconazole on Candida glabrata biofilms and its relationship with ABC transporter gene expression. Biofouling 2014, 30, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Silva, S.; Henriques, M. Candida glabrata: A review of its features and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P.K. Candida Biofilms: Development, Architecture, and Resistance. Microbiol. Spectr. 2015, 3, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.E.M.; Darouiche, R.O.R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Roussos, N.; Vardakas, K.Z. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: A systematic review. Int. J. Infect. Dis. 2010, 14, e954–e966. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Jin, L.; Samaranayake, L.P. Biofilm lifestyle of Candida: A mini review. Oral Dis. 2008, 14, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.A.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E.; Betts, R.F.; Dupont, B.F.; Wu, C.; Buell, D.N.; Kovanda, L.; Lortholary, O. Early Removal of Central Venous Catheter in Patients with Candidemia Does Not Improve Outcome: Analysis of 842 Patients from 2 Randomized Clinical Trials. Clin. Infect. Dis. 2010, 51, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, J.; Kuhn, D.; Mukherjee, P.; Hoyer, L.; McCormick, T.; Ghannoum, M. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Saville, S.P.; Thomas, D.P.; López-Ribot, J.L. Candida biofilms: An update. Eukaryot. Cell 2005, 4, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Nett, J.; Oschel, P.; Albrecht, R.; Marchillo, K.; Pitula, A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 2004, 72, 6023–6031. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J. Candida biofilm resistance. Drug Resist. Updat. 2004, 7, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.; Lepak, A.; Marchillo, K.; Andes, D. Time course global gene expression analysis of an in vivo Candida biofilm. J. Infect. Dis. 2009, 200, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.; Vila, T.; Romo, J.; Montelongo-Jauregui, D.; Wall, G.; Ramasubramanian, A.; Lopez-Ribot, J. The Candida albicans Biofilm Matrix: Composition, Structure and Function. J. Fungi 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Robertson, D.P.; Hodge, P.J.; Lappin, D.F.; Ramage, G. Hydrolytic Enzyme Production is Associated with Candida Albicans Biofilm Formation from Patients with Type 1 Diabetes. Mycopathologia 2010, 170, 229–235. [Google Scholar] [CrossRef]
- Hoyer, L.L.; Cota, E. Candida albicans agglutinin-like sequence (Als) family vignettes: A review of als protein structure and function. Front. Microbiol. 2016, 7, 280. [Google Scholar] [CrossRef]
- Rauceo, J.M.; Gaur, N.K.; Lee, K.-G.; Edwards, J.E.; Klotz, S.A.; Lipke, P.N. Global Cell Surface Conformational Shift Mediated by a Candida albicans Adhesin. Infect. Immun. 2004, 72, 4948–4955. [Google Scholar] [CrossRef] [Green Version]
- Zakikhany, K.; Naglik, J.R.; Schmidt-Westhausen, A.; Holland, G.; Schaller, M.; Hube, B. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell. Microbiol. 2007, 9, 2938–2954. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Oh, S.-H.; Cheng, G.; Green, C.B.; Nuessen, J.A.; Yeater, K.; Leng, R.P.; Brown, A.J.P.; Hoyer, L.L. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology 2004, 150, 2415–2428. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Oh, S.-H.; Yeater, K.M.; Hoyer, L.L. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family. Microbiology 2005, 151, 1619–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Oh, S.-H.; Hoyer, L.L. Deletion of ALS5, ALS6 or ALS7 increases adhesion of Candida albicans to human vascular endothelial and buccal epithelial cells. Med. Mycol. 2007, 45, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Ho, J.; Naglik, J. Candida–Epithelial Interactions. J. Fungi 2018, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- De Las Peñas, A.; Pan, S.-J.; Castaño, I.; Alder, J.; Cregg, R.; Cormack, B.P. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 2003, 17, 2245–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castano, I.; Pan, S.; Zupancic, M.; Hennequin, C.; Dujon, B.; Cormack, B. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol. Microbiol. 2005, 55, 1246–1258. [Google Scholar] [CrossRef]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.W.J.; Kraneveld, E.A.; Yin, Q.Y.; Dekker, H.L.; Gross, U.; Crielaard, W.; de Koster, C.G.; Bader, O.; Klis, F.M.; Weig, M. The cell wall of the human pathogen Candida glabrata: Differential incorporation of novel adhesin-like wall proteins. Eukaryot. Cell 2008, 7, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, A.; Odahara, K.; Toume, M.; Watanabe, T.; Mikami, T.; Matsumoto, T. Change in the respiration system of Candida albicans in the lag and log growth phase. Biol. Pharm. Bull. 2006, 29, 448–450. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Duque, C.; Höfling, J.F.; Gonçalves, R.B. Genetic and phenotypic evaluation of Candida albicans strains isolated from subgingival biofilm of diabetic patients with chronic periodontitis. Med. Mycol. 2012, 50, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Calderone, R.A.; Braun, P.C. Adherence and receptor relationships of Candida albicans. Microbiol. Rev. 1991, 55, 1–20. [Google Scholar]
- Silva, T.M.; Glee, P.M.; Hazen, K.C. Influence of cell surface hydrophobicity on attachment of Candida albicans to extracellular matrix proteins. J. Med. Vet. Mycol. 1995, 33, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.G.; Mårdh, P.A.; Pina-Vaz, C.; Martinez-de-Oliveira, J.; Fonseca, A.F. Germ tube formation changes surface hydrophobicity of Candida cells. Infect. Dis. Obstet. Gynecol. 1999, 7, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neville, B.W.; Damm, D.D.; Allen, C.M.; Chi, A.C. Oral and maxillofacial pathology. In In Fungal and Protozoal Diseases; Elsevier: London, UK, 2011; pp. 213–221. [Google Scholar]
- Regezi, J.A.; Sciubba, J.J.; Jordan, R.C.K. Oral pathology clinical pathologic correlations. In White Lesions; Elsevier: St. Louis, MO, USA, 2008; pp. 98–102. [Google Scholar]
- Lott, T.J.; Holloway, B.P.; Logan, D.A.; Fundyga, R.; Arnold, J. Towards understanding the evolution of the human commensal yeast Candida albicans. Microbiology 1999, 145, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Meer, J.W.M.; van de Veerdonk, F.L.; Joosten, L.A.B.; Kullberg, B.-J.; Netea, M.G. Severe Candida spp. infections: New insights into natural immunity. Int. J. Antimicrob. Agents 2010, 36, S58–S62. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Klingspor, L.; Sundin, U.; Altamash, M.; Klinge, B.; Engström, P.-E. Periodontal conditions, oral Candida albicans and salivary proteins in type 2 diabetic subjects with emphasis on gender. BMC Oral Health 2009, 9, 12. [Google Scholar] [CrossRef]
- Lamey, P.J.; Darwaza, A.; Fisher, B.M.; Samaranayake, L.P.; Macfarlane, T.W.; Frier, B.M. Secretor status, candidal carriage and candidal infection in patients with diabetes mellitus. J. Oral Pathol. 1988, 17, 354–357. [Google Scholar] [CrossRef]
- Mulu, A.; Kassu, A.; Anagaw, B.; Moges, B.; Gelaw, A.; Alemayehu, M.; Belyhun, Y.; Biadglegne, F.; Hurissa, Z.; Moges, F.; et al. Frequent detection of ‘azole’ resistant Candida species among late presenting AIDS patients in northwest Ethiopia. BMC Infect. Dis. 2013, 13, 82. [Google Scholar] [CrossRef]
- Goregen, M.; Miloglu, O.; Buyukkurt, M.C.; Caglayan, F.; Aktas, A.E. Median rhomboid glossitis: A clinical and microbiological study. Eur. J. Dent. 2011, 5, 367–372. [Google Scholar]
- Arendorf, T.M.; Walker, D.M. Tobacco smoking and denture wearing as local aetiological factors in median rhomboid glossitis. Int. J. Oral Surg. 1984, 13, 411–415. [Google Scholar] [CrossRef]
- Flaitz, C.M.; Nichols, C.M.; Hicks, M.J. An overview of the oral manifestations of AIDS-related Kaposi’s sarcoma. Compend. Contin. Educ. Dent. 1995, 16, 136–138. [Google Scholar] [PubMed]
- Sanitá, P.V.; Zago, C.E.; Pavarina, A.C.; Jorge, J.H.; Machado, A.L.; Vergani, C.E. Enzymatic activity profile of a Brazilian culture collection of Candida albicans isolated from diabetics and non-diabetics with oral candidiasis. Mycoses 2014, 57, 351–357. [Google Scholar] [PubMed]
- Samaranayake, L.P.; Raeside, J.M.; MacFarlane, T.W. Factors affecting the phospholipase activity of Candida species in vitro. Sabouraudia 1984, 22, 201–207. [Google Scholar] [CrossRef]
- Lyon, J.P.; de Resende, M.A. Correlation between adhesion, enzyme production, and susceptibility to fluconazole in Candida albicans obtained from denture wearers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 632–638. [Google Scholar] [CrossRef]
- Arslan, S.; Koç, A.N.; Şekerci, A.E.; Tanriverdi, F.; Sav, H.; Aydemir, G.; Diri, H. Genotypes and virulence factors of Candida species isolated from oralcavities of patients with type 2 diabetes mellitus. Turkish J. Med. Sci. 2016, 46, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Koga-Ito, C.Y.; Lyon, J.P.; Vidotto, V.; de Resende, M.A. Virulence Factors and Antifungal Susceptibility of Candida albicans Isolates from Oral Candidosis Patients and Control Individuals. Mycopathologia 2006, 161, 219–223. [Google Scholar] [CrossRef] [PubMed]
- D’Eça Júnior, A.; Silva, A.F.; Rosa, F.C.; Monteiro, S.G.; de Maria Silva Figueiredo, P.; de Andrade Monteiro, C. In vitro differential activity of phospholipases and acid proteinases of clinical isolates of Candida. Rev. Soc. Bras. Med. Trop. 2011, 44, 334–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfredi, M.; McCullough, M.J.; Al-Karaawi, Z.M.; Vescovi, P.; Porter, S.R. In vitro evaluation of virulence attributes of Candida spp. isolated from patients affected by diabetes mellitus. Oral Microbiol. Immunol. 2006, 21, 183–189. [Google Scholar] [CrossRef]
- De Menezes Thiele, M.C.; de Paula E Carvalho, A.; Gursky, L.C.; Rosa, R.T.; Samaranayake, L.P.; Rosa, E.A.R. The role of candidal histolytic enzymes on denture-induced stomatitis in patients living in retirement homes. Gerodontology 2008, 25, 229–236. [Google Scholar] [CrossRef]
- Negri, M.; Martins, M.; Henriques, M.; Svidzinski, T.I.E.; Azeredo, J.; Oliveira, R. Examination of Potential Virulence Factors of Candida tropicalis Clinical Isolates From Hospitalized Patients. Mycopathologia 2010, 169, 175–182. [Google Scholar] [CrossRef]
- Sánchez-Vargas, L.O.; Estrada-Barraza, D.; Pozos-Guillen, A.J.; Rivas-Caceres, R. Biofilm formation by oral clinical isolates of Candida species. Arch. Oral Biol. 2013, 58, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.; Henriques, M. Susceptibility of Candida glabrata biofilms to echinocandins: Alterations in the matrix composition. Biofouling 2018, 34, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Henriques, M. Portrait of Matrix Gene Expression in Candida glabrata Biofilms with Stress Induced by Different Drugs. Genes 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Gow, N.A.R.; Munro, C.A. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob. Agents Chemother. 2013, 57, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P.; Hughes, A.; Weetman, D.A.; MacFarlane, T.W. Growth and acid production of Candida species in human saliva supplemented with glucose. J. Oral Pathol. 1986, 15, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P.; MacFarlane, T.W. An in-vitro study of the adherence of Candida albicans to acrylic surfaces. Arch. Oral Biol. 1980, 25, 603–609. [Google Scholar] [CrossRef]
- Pallavan, B.; Ramesh, V.; Dhanasekaran, B.P.; Oza, N.; Indu, S.; Govindarajan, V. Comparison and correlation of candidal colonization in diabetic patients and normal individuals. J. Diabetes Metab. Disord. 2014, 13, 66. [Google Scholar] [CrossRef] [Green Version]
- Zomorodian, K.; Kavoosi, F.; Pishdad, G.R.; Mehriar, P.; Ebrahimi, H.; Bandegani, A.; Pakshir, K. Prevalence of oral Candida colonization in patients with diabetes mellitus. J. Mycol. Med. 2016, 26, 103–110. [Google Scholar] [CrossRef]
- Buysschaert, M.; Medina, J.L.; Bergman, M.; Shah, A.; Lonier, J. Prediabetes and associated disorders. Endocrine 2015, 48, 371–393. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Mehmood, A.; Saeed, A.; Al-Hezaimi, K.; Samaranayake, L.P. Association between glycemic status and oral Candida carriage in patients with prediabetes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 53–58. [Google Scholar] [CrossRef]
- Darwazeh, A.M.; MacFarlane, T.W.; McCuish, A.; Lamey, P.J. Mixed salivary glucose levels and candidal carriage in patients with diabetes mellitus. J. Oral Pathol. Med. 1991, 20, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Yar Ahmadi, S.; Khosravi, A.; Larijani, B.; Baiat, M.; Mahmoudi, M.; Baradar Jalili, R. Assessment of the fungal flora and the prevalence of fungal infections in the mouth of diabetics. Iran. J. Endocrinol. Metab. 2002, 4, 105–109. [Google Scholar]
- Fisher, B.M.; Lamey, P.J.; Samaranayake, L.P.; MacFarlane, T.W.; Frier, B.M. Carriage of Candida species in the oral cavity in diabetic patients: Relationship to glycaemic control. J. Oral Pathol. 1987, 16, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Oksuz, S.; Sencan, I.; Gulcan, A.; Karabay, O.; Gulcan, E.; Yildiz, O. Prevalance and risk factors for yeast colonization in adult diabetic patients. Ethiop. Med. J. 2005, 43, 103–109. [Google Scholar] [PubMed]
- Kjellman, O. Secretion rate and buffering action of whole mixed saliva in subjects with insulin-treated diabetes mellitus. Odontol. Revy 1970, 21, 159–168. [Google Scholar]
- Costa, A.L.; Silva, B.M.A.; Soares, R.; Mota, D.; Alves, V.; Mirante, A.; Ramos, J.C.; Maló de Abreu, J.; Santos-Rosa, M.; Caramelo, F.; et al. Type 1 diabetes in children is not a predisposing factor for oral yeast colonization. Med. Mycol. 2016, 55, 358–367. [Google Scholar] [CrossRef]
- Reinhart, H.; Muller, G.; Sobel, J.D. Specificity and mechanism of in vitro adherence of Candida albicans. Ann. Clin. Lab. Sci. 1985, 15, 406–413. [Google Scholar]
- Samaranayake, L.P.; Macfarlane, T.W. The Effect of Dietary Carbohydrates on the In-vitro Adhesion of Candida Albicans to Epithelial Cells. J. Med. Microbiol. 1982, 15, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Naik, R.; Ahmed Mujib, B.R.; Raaju, U.R.; Telagi, N. Assesing oral candidal carriage with mixed salivary glucose levels as non-invasive diagnostic tool in type-2 Diabetics of Davangere, Karnataka, India. J. Clin. Diagn. Res. 2014, 8, 69–72. [Google Scholar]
- Sashikumar, R.; Kannan, R. Salivary glucose levels and oral candidal carriage in type II diabetics. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 706–711. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, D. The physiology and biology of saliva. In Color Atlas and Text of Salivary Gland: Disease, Disorders and Surgery; deBurgh Norman, J., McGurk, M., Eds.; Mosby-Wolfe: London, UK, 1995; pp. 40–48. [Google Scholar]
- Panchbhai, A.S.; Degwekar, S.S.; Bhowte, R.R. Estimation of salivary glucose, salivary amylase, salivary total protein and salivary flow rate in diabetics in India. J. Oral Sci. 2010, 52, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorocka-Bobkowska, B.; Budtz-Jörgensen, E.; Włoch, S. Non-insulin-dependent diabetes mellitus as a risk factor for denture stomatitis. J. Oral Pathol. Med. 1996, 25, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P.; MacFarlane, T.W. Factors affecting the in-vitro adherence of the fungal oral pathogen Candida albicans to epithelial cells of human origin. Arch. Oral Biol. 1982, 27, 869–873. [Google Scholar] [CrossRef]
- Mantri, S.P.S.S.P.; Parkhedkar, R.D.; Mantri, S.P.S.S.P. Candida colonisation and the efficacy of chlorhexidine gluconate on soft silicone-lined dentures of diabetic and non-diabetic patients. Gerodontology 2013, 30, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Knight, L.; Fletcher, J. Growth of Candida albicans in saliva: Stimulation by glucose associated with antibiotics, corticosteroids, and diabetes mellitus. J. Infect. Dis. 1971, 123, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Malic, S.; Hill, K.E.; Ralphs, J.R.; Hayes, A.; Thomas, D.W.; Potts, A.J.; Williams, D.W. Characterization of Candida albicans infection of an in vitro oral epithelial model using confocal laser scanning microscopy. Oral Microbiol. Immunol. 2007, 22, 188–194. [Google Scholar] [CrossRef]
- Wang, J.; Ohshima, T.; Yasunari, U.; Namikoshi, S.; Yoshihara, A.; Miyazaki, H.; Maeda, N. The carriage of Candida species on the dorsal surface of the tongue: The correlation with the dental, periodontal and prosthetic status in elderly subjects. Gerodontology 2006, 23, 157–163. [Google Scholar] [CrossRef]
- Maria Beatriz Ribeiro Cardoso; Eliana Campêlo Lago. Oral Changes in Elderly from an Association Center. Rev. Para. Med. V 2010, 24, 35–41. [Google Scholar]
- Bianchi, C.M.; Bianchi, H.A.; Tadano, T.; Depaula, C.R.; Hoffmann-Santos, H.D.; Leite, D.P.; Hahn, R.C. Factors related to oral candidiasis in elderly users and non-users of removable dental prostheses. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 6–10. [Google Scholar] [CrossRef]
- Prado Leite, D.; Rabello Piva, M.; Ricardo Saquete Martins-Filho, P. Identification of Candida species in patients with denture stomatitis and evaluation of susceptibility to miconazole and photodynamic therapy. Rev. Odontol. UNESP 2015, 44, 12–17. [Google Scholar]
- Da Silva Santos, J.; Batista, S.A.; Silva, A., Jr.; Ferreira, M.F.; Agostini, M.; Torres, S.R. Oral candidiasis in patients admitted to ICU. Rev. Bras. Odontol. 2014, 71, 176–179. [Google Scholar]
- Scalercio, M.; Valente, T.; Israel, M.S.; Ramos, M.E. Denture stomatitis associated with candidiasis: Diagnosis and treatment. RGO 2007, 44, 395–398. [Google Scholar]
- Khovidhunkit, S.P.; Suwantuntula, T.; Thaweboon, S.; Mitrirattanakul, S.; Chomkhakhai, U.; Khovidhunkit, W. Xerostomia, hyposalivation, and oral microbiota in type 2 diabetic patients: A preliminary study. J. Med. Assoc. Thail. 2009, 92, 1220–1228. [Google Scholar]
- Sudbery, P.; Gow, N.; Berman, J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004, 12, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Aitken-Saavedra, J.; Lund, R.G.; González, J.; Huenchunao, R.; Perez-Vallespir, I.; Morales-Bozo, I.; Urzúa, B.; Tarquinio, S.C.; Maturana-Ramírez, A.; Martos, J.; et al. Diversity, frequency and antifungal resistance of Candida species in patients with type 2 diabetes mellitus. Acta Odontol. Scand. 2018, 76, 580–586. [Google Scholar] [CrossRef]
- Lydia Rajakumari, M.; Saravana Kumari, P. Prevalence of Candida species in the buccal cavity of diabetic and non-diabetic individuals in and around Pondicherry. J. Mycol. Med. 2016, 26, 359–367. [Google Scholar] [CrossRef]
- Premkumar, J.; Ramani, P.; Chandrasekar, T.; Natesan, A.; Premkumar, P. Detection of species diversity in oral candida colonization and anti-fungal susceptibility among non-oral habit adult diabetic patients. J. Nat. Sci. Biol. Med. 2014, 5, 148. [Google Scholar] [CrossRef] [Green Version]
- Bremenkamp, R.M.; Caris, A.R.; Jorge, A.O.C.; Back-Brito, G.N.; Mota, A.J.; Balducci, I.; Brighenti, F.L.; Koga-Ito, C.Y. Prevalence and antifungal resistance profile of Candida spp. oral isolates from patients with type 1 and 2 diabetes mellitus. Arch. Oral Biol. 2011, 56, 549–555. [Google Scholar] [CrossRef]
- Manfredi, M.; McCullough, M.J.; Polonelli, L.; Conti, S.; Al-Karaawi, Z.M.; Vescovi, P.; Porter, S.R. In vitro antifungal susceptibility to six antifungal agents of 229 Candida isolates from patients with diabetes mellitus. Oral Microbiol. Immunol. 2006, 21, 177–182. [Google Scholar] [CrossRef]
- Sanitá, P.V.; De Oliveira Mima, E.G.; Pavarina, A.C.; Jorge, J.H.; Machado, A.L.; Vergani, C.E. Susceptibility profile of a Brazilian yeast stock collection of Candida species isolated from subjects with Candida-associated denture stomatitis with or without diabetes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 562–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Attas, S.; Amro, S. Candidal colonization, strain diversity, and antifungal susceptibility among adult diabetic patients. Ann. Saudi Med. 2010, 30, 101. [Google Scholar] [CrossRef] [PubMed]
- Indira, P.; Kumar, P.M.; Shalini, S.; Vaman, K. Opportunistic infections among People Living with HIV (PLHIV) with Diabetes Mellitus (DM) attending a tertiary care hospital in coastal city of South India. PLoS ONE 2015, 10, 4–11. [Google Scholar] [CrossRef]
- Scully, C.; Monteil, R.; Sposto, M.R. Infectious and tropical diseases affecting the human mouth. Periodontology 2000 1998, 18, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Stanford, T.W.; Rivera-Hidalgo, F. Oral mucosal lesions caused by infective microorganisms. II. Fungi and parasites. Periodontology 2000 1999, 21, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Löe, H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, M.; Ford, P.; Seymour, G. Periodontal disease and systemic health: Current status. Aust. Dent. J. 2009, 54, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Olczak-Kowalczyk, D.; Pyrżak, B.; Dąbkowska, M.; Pańczyk-Tomaszewska, M.; Miszkurka, G.; Rogozińska, I.; Swoboda-Kopeć, E.; Gozdowski, D.; Kalińska, A.; Piróg, A.; et al. Candida spp. and gingivitis in children with nephrotic syndrome or type 1 diabetes. BMC Oral Health 2015, 15, 57. [Google Scholar] [CrossRef]
- Lotfi-Kamran, M.H.; Jafari, A.A.; Falah-Tafti, A.; Tavakoli, E.; Falahzadeh, M.H. Candida Colonization on the Denture of Diabetic and Non-diabetic Patients. Dent. Res. J. 2009, 6, 23–27. [Google Scholar]
- Urzúa, B.; Hermosilla, G.; Gamonal, J.; Morales-Bozo, I.; Canals, M.; Barahona, S.; Cóccola, C.; Cifuentes, V. Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Med. Mycol. 2008, 46, 783–793. [Google Scholar] [CrossRef]
- Sardi, J.; Duque, C.; Mariano, F.; Peixoto, I.; Hofling, J.; Gonçalves, R.B. Candida spp. in periodontal disease: A brief review. J. Oral Sci. 2010, 52, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Järvensivu, A.; Hietanen, J.; Rautemaa, R.; Sorsa, T.; Richardson, M. Candida yeasts in chronic periodontitis tissues and subgingival microbial biofilms in vivo. Oral Dis. 2004, 10, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Rams, T.E.; Listgarten, M.A. Yeasts, enteric rods and pseudomonads in the subgingival flora of severe adult periodontitis. Oral Microbiol. Immunol. 1988, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, A.H.; Nygaard-Østby, B.; Bøygard, G.K.; Eribe, E.R.; Olsen, I.; Gjermo, P. Yeasts in periodontal pockets. J. Clin. Periodontol. 2001, 28, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Ergun, S.; Cekici, A.; Topcuoglu, N.; Migliari, D.-A.; Külekçi, G.; Tanyeri, H.; Isik, G. Oral status and Candida colonization in patients with Sjögren’s Syndrome. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e310–e315. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Duque, C.; Camargo, G.A.C.G.; Hofling, J.F.; Gonçalves, R.B. Periodontal conditions and prevalence of putative periodontopathogens and Candida spp. in insulin-dependent type 2 diabetic and non-diabetic patients with chronic periodontitis—A pilot study. Arch. Oral Biol. 2011, 56, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.M.; Boriollo, M.F.G.; Alves, A.C.B.A.; Klein, M.I.; Gonçalves, R.B.; Höfling, J.F. Genetic diversity and exoenzyme activities of Candida albicans and Candida dubliniensis isolated from the oral cavity of Brazilian periodontal patients. Arch. Oral Biol. 2008, 53, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Thafeed AlGhamdi, A.S.; Mikami, T.; Mehmood, A.; Ahmed, H.B.; Samaranayake, L.P.; Tenenbaum, H.C. Effect of Glycemic Control on Self-Perceived Oral Health, Periodontal Parameters, and Alveolar Bone Loss Among Patients With Prediabetes. J. Periodontol. 2014, 85, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Samaranayake, L.P.; Al-Askar, M.; Al-Hezaimi, K. Periodontal Disease in Habitual Cigarette Smokers and Nonsmokers With and Without Prediabetes. Am. J. Med. Sci. 2013, 345, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Tenenbaum, H.C.; Nogueira-Filho, G.; Nooh, N.; O’Bello Correa, F.; Warnakulasuriya, S.; Dasanayake, A.P.; Al-Hezaimi, K. Periodontal Inflammatory Conditions Among Gutka Chewers and Non-chewers With and Without Prediabetes. J. Periodontol. 2013, 84, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Al-Askar, M.; Al-Rasheed, A.; Al-Hezaimi, K.; Babay, N.; Galindo-Moreno, P. Comparison of Self-Perceived Oral Health, Periodontal Inflammatory Conditions and Socioeconomic Status in Individuals With and Without Prediabetes. Am. J. Med. Sci. 2012, 344, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Näsström, K.; Benchimol, D.; Altamash, M.; Klinge, B.; Engström, P.-E. Comparison of Periodontal and Socioeconomic Status Between Subjects With Type 2 Diabetes Mellitus and Non-Diabetic Controls. J. Periodontol. 2007, 78, 2112–2119. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. Impact of Diabetes Mellitus and Glycemic Control on the Osseointegration of Dental Implants: A Systematic Literature Review. J. Periodontol. 2009, 80, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.S.; Hinthorn, D.; Lai, S.M.; Ellerbeck, E.F. Hyperglycaemia and mortality of diabetic patients with candidaemia. Diabet. Med. 2005, 22, 1252–1257. [Google Scholar] [CrossRef]
- Oztürkcan, S.; Oztürkcan, S.; Topçu, S.; Akinci, S.; Bakici, M.Z.; Yalçin, N. Incidence of oral candidiasis in diabetic patients. Mikrobiyol. Bülteni 1993, 27, 352–356. [Google Scholar]
- Rodero, L.; Davel, G.; Soria, M.; Vivot, W.; Córdoba, S.; Canteros, C.E.; Saporiti, A.; EMIFN. [Multicenter study of fungemia due to yeasts in Argentina]. Rev. Argent. Microbiol. 2005, 37, 189–195. [Google Scholar] [PubMed]
- Saes Busato, I.M.; Bittencourt, M.S.; Machado, M.Â.N.; Grégio, A.M.T.; Azevedo-Alanis, L.R. Association between metabolic control and oral health in adolescents with type 1 diabetes mellitus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e51–e56. [Google Scholar] [CrossRef]
- Weykamp, C. HbA1c: A review of analytical and clinical aspects. Ann. Lab. Med. 2013, 33, 393–400. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbial Ecology of Dental Plaque and its Significance in Health and Disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef]
- Brook, I. Bacterial Interference. Crit. Rev. Microbiol. 1999, 25, 155–172. [Google Scholar] [CrossRef]
- He, X.; McLean, J.S.; Guo, L.; Lux, R.; Shi, W. The social structure of microbial community involved in colonization resistance. ISME J. 2014, 8, 564–574. [Google Scholar] [CrossRef]
- Roberts, F.A.; Darveau, R.P. Microbial protection and virulence in periodontal tissue as a function of polymicrobial communities: Symbiosis and dysbiosis. Periodontology 2000 2015, 69, 18–27. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Mima, E.G.G.; Vergani, C.E.E.; Machado, A.L.L.; Massucato, E.M.S.M.S.; Colombo, A.L.L.; Bagnato, V.S.S.; Pavarina, A.C.C. Comparison of Photodynamic Therapy versus conventional antifungal therapy for the treatment of denture stomatitis: A randomized clinical trial. Clin. Microbiol. Infect. 2012, 18, E380–E388. [Google Scholar] [CrossRef] [PubMed]
- Sanit, P.V.; Pavarina, A.C.; Giampaolo, E.T.; Silva, M.M.; De Oliveira Mima, E.G.; Ribeiro, D.G.; Vergani, C.E. Candida spp. prevalence in well controlled type 2 diabetic patients with denture stomatitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, 726–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanita, P.V.; Machado, A.L.; Pavarina, A.C.; Massucato, E.M.S.; Colombo, A.L.; Vergani, C.E. Microwave denture disinfection versus nystatin in treating patients with well-controlled type 2 diabetes and denture stomatitis: A randomized clinical trial. Int. J. Prosthodont. 2012, 25, 232–244. [Google Scholar]
- Silva, M.M.; Mima, E.G.; Colombo, A.L.; Sanitá, P.V.; Jorge, J.H.; Massucato, E.M.S.; Vergani, C.E. Comparison of denture microwave disinfection and conventional antifungal therapy in the treatment of denture stomatitis: A randomized clinical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 469–479. [Google Scholar] [CrossRef]
- Melo, A.S.; Bizerra, F.C.; Freymüller, E.; Arthington-Skaggs, B.A.; Colombo, A.L. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med. Mycol. 2011, 49, 253–262. [Google Scholar] [CrossRef]
- Iacopino, A.M.; Wathen, W.F. Oral candidal infection and denture stomatitis: A comprehensive review. J. Am. Dent. Assoc. 1992, 123, 46–51. [Google Scholar] [CrossRef]
- Artico, G.; Freitas, R.; Santos Filho, A.; Benard, G.; Romiti, R.; Migliari, D. Prevalence of Candida spp., xerostomia, and hyposalivation in oral lichen planus—A controlled study. Oral Dis. 2014, 20, e36–e41. [Google Scholar] [CrossRef]
- Willis, A.M.; Coulter, W.A.; Sullivan, D.J.; Coleman, D.C.; Hayes, J.R.; Bell, P.M.; Lamey, P.J. Isolation of C. dubliniensis from insulin-using diabetes mellitus patients. J. Oral Pathol. Med. 2000, 29, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Coco, B.J.; Bagg, J.; Cross, L.J.; Jose, A.; Cross, J.; Ramage, G. Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral Microbiol. Immunol. 2008, 23, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Arias, C.; Vicente, J.L.; Sahand, I.H.; Eguia, A.; De-Juan, A.; Madariaga, L.; Aguirre, J.M.; Eraso, E.; Quindós, G. Isolation of Candida dubliniensis in denture stomatitis. Arch. Oral Biol. 2009, 54, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Vanden Abbeele, A.; de Meel, H.; Ahariz, M.; Perraudin, J.-P.; Beyer, I.; Courtois, P. Denture contamination by yeasts in the elderly. Gerodontology 2008, 25, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.C.; Thomas, C.J.; Whittle, T. A 2-year study of Candida-associated denture stomatitis treatment in aged care subjects. Gerodontology 2005, 22, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Fongsmut, T.; Deerochanawong, C.; Prachyabrued, W. Intraoral candida in Thai diabetes patients. J. Med. Assoc. Thail. 1998, 81, 449–453. [Google Scholar]
- Darwazeh, A.-G.; Hammad, M.; Al-Jamaei, A. The relationship between oral hygiene and oral colonization with Candida species in healthy adult subjects. Int. J. Dent. Hyg. 2010, 8, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.G.; Komiyama, E.Y.; Dos Santos, S.S.F.; Jorge, A.O.C.; Brighenti, F.L.; Koga-Ito, C.Y. In vitro adherence of Candida albicans isolated from patients with chronic periodontitis. J. Appl. Oral Sci. 2011, 19, 384–387. [Google Scholar] [CrossRef] [Green Version]
- Rosa, E.A.R.; Rached, R.N.; Ignacio, S.A.; Rosa, R.T.; Jose da Silva, W.; Yau, J.Y.Y.; Samaranayake, L.P. Phenotypic evaluation of the effect of anaerobiosis on some virulence attributes of Candida albicans. J. Med. Microbiol. 2008, 57, 1277–1281. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Nagata, N.; Shimbo, T.; Nishijima, T.; Watanabe, K.; Aoki, T.; Sekine, K.; Okubo, H.; Watanabe, K.; Sakurai, T.; et al. Long-term trends in esophageal candidiasis prevalence and associated risk factors with or without HIV infection: Lessons from an endoscopic study of 80,219 patients. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Mojazi Amiri, H.; Frandah, W.; Colmer-Hamood, J.; Raj, R.; Nugent, K. Risk factors of Candida colonization in the oropharynx of patients admitted to an intensive care unit. J. Mycol. Med. 2012, 22, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Owotade, F.J.; Patel, M.; Ralephenya, T.R.M.D.R.; Vergotine, G. Oral candida colonization in HIV-positive women: Associated factors and changes following antiretroviral therapy. J. Med. Microbiol. 2013, 62, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Terayama, Y.; Matsuura, T.; Uchida, M.; Narama, I.; Ozaki, K. Probiotic (yogurt) containing Lactobacillus gasseri OLL2716 is effective for preventing Candida albicans-induced mucosal inflammation and proliferation in the forestomach of diabetic rats. Histol. Histopathol. 2016, 31, 689–697. [Google Scholar] [PubMed]
- Malazy, O.T.; Shariat, M.; Heshmat, R.; Majlesi, F.; Alimohammadian, M.; Tabari, N.K.; Larijani, B. Vulvovaginal candidiasis and its related factors in diabetic women. Taiwan J. Obstet. Gynecol. 2007, 46, 399–404. [Google Scholar] [CrossRef]
- Atabek, M.E.; Akyürek, N.; Eklioglu, B.S. Frequency of Vaginal Candida Colonization and Relationship between Metabolic Parameters in Children with Type 1 Diabetes Mellitus. J. Pediatr. Adolesc. Gynecol. 2013, 26, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, N.J. V Treatment of Vulvovaginal Candidiasis in Patients With Diabetes. Diabetes Care 1998, 21, 451. [Google Scholar] [CrossRef] [PubMed]
- De Leon, E.M.; Jacober, S.J.; Sobel, J.D.; Foxman, B. Prevalence and risk factors for vaginal Candida colonization in women with type 1 and type 2 diabetes. BMC Infect. Dis. 2002, 2, 1. [Google Scholar] [CrossRef]
- Reed, B.D. Risk factors for Candida vulvovaginitis. Obstet. Gynecol. Surv. 1992, 47, 551–560. [Google Scholar] [CrossRef]
- Hoeltge, G. Clinical Laboratory Technical Procedure Manuals, 3rd ed.; NCCLS: Wayne, PA, USA, 1996; ISBN 9781562383152. [Google Scholar]
- Saporiti, A.M.; Gómez, D.; Levalle, S.; Galeano, M.; Davel, G.; Vivot, W.; Rodero, L. [Vaginal candidiasis: Etiology and sensitivity profile to antifungal agents in clinical use]. Rev. Argent. Microbiol. 2001, 33, 217–222. [Google Scholar]
- Sobel, J.D.; Chaim, W. Treatment of Torulopsis glabrata vaginitis: Retrospective review of boric acid therapy. Clin. Infect. Dis. 1997, 24, 649–652. [Google Scholar] [CrossRef]
- Goswami, R.; Dadhwal, V.; Tejaswi, S.; Datta, K.; Paul, A.; Haricharan, R.N.; Banerjee, U.; Kochupillai, N.P. Species-specific prevalence of vaginal candidiasis among patients with diabetes mellitus and its relation to their glycaemic status. J. Infect. 2000, 41, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Nagesha, C.N.; Ananthakrishna, N.C. Clinical and laboratory study of monilial vaginitis. Am. J. Obstet. Gynecol. 1970, 107, 1267–1268. [Google Scholar] [CrossRef]
- Grigoriou, O.; Baka, S.; Makrakis, E.; Hassiakos, D.; Kapparos, G.; Kouskouni, E. Prevalence of clinical vaginal candidiasis in a university hospital and possible risk factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 126, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Achkar, J.M.; Fries, B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010, 23, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, S.C.; Saini, S.; Mathew, S. Non-albicans Candida Infection: An Emerging Threat. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 615958. [Google Scholar] [CrossRef] [PubMed]
- Lattif, A.A.; Mukhopadhyay, G.; Banerjee, U.; Goswami, R.; Prasad, R. Molecular typing and in vitro fluconazole susceptibility of Candida species isolated from diabetic and nondiabetic women with vulvovaginal candidiasis in India. J. Microbiol. Immunol. Infect. 2011, 44, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Gunther, L.S.A.; Martins, H.P.R.; Gimenes, F.; De Abreu, A.L.P.; Consolaro, M.E.L.; Svidzinski, T.I.E. Prevalence of Candida albicans and non-albicans isolates from vaginal secretions: Comparative evaluation of colonization, vaginal candidiasis and recurrent vaginal candidiasis in diabetic and non-diabetic women. Sao Paulo Med. J. 2014, 132, 116–120. [Google Scholar] [CrossRef]
- Sherry, L.; Kean, R.; McKloud, E.; O’Donnell, L.E.; Metcalfe, R.; Jones, B.L.; Ramage, G. Biofilms Formed by Isolates from Recurrent Vulvovaginal Candidiasis Patients Are Heterogeneous and Insensitive to Fluconazole. Antimicrob. Agents Chemother. 2017, 61, e01065-17. [Google Scholar] [CrossRef]
- Ray, D.; Goswami, R.; Banerjee, U.; Dadhwal, V.; Goswami, D.; Mandal, P.; Sreenivas, V.; Kochupillai, N. Prevalence of Candida glabrata and Its Response to Boric Acid Vaginal Suppositories in Comparison With Oral Fluconazole in Patients With Diabetes and Vulvovaginal Candidiasis. Diabetes Care 2007, 30, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Peer, A.K.; Hoosen, A.A.; Seedat, M.A.; van den Ende, J.; Omar, M.A. Vaginal yeast infections in diabetic women. S. Afr. Med. J. 1993, 83, 727–729. [Google Scholar]
- Nyirjesy, P.; Sobel, J.D. Genital Mycotic Infections in Patients With Diabetes. Postgrad. Med. 2013, 125, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Nash, E.E.; Peters, B.M.; Lilly, E.A.; Noverr, M.C.; Fidel, P.L. A Murine Model of Candida glabrata Vaginitis Shows No Evidence of an Inflammatory Immunopathogenic Response. PLoS ONE 2016, 11, e0147969. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, P.R.; David, P.R.; Peres, N.P.; da Cunha, K.C.; de Almeida, M.T.G. [Phenotypic characterization of yeasts isolated from the vaginal mucosa of adult women]. Rev. Bras. Ginecol. Obstet. 2009, 31, 177–181. [Google Scholar]
- Carrara, M.A.; Bazotte, R.B.; Donatti, L.; Svidzinski, T.I.E.; Consolaro, M.E.L.; Patussi, E.V.; Batista, M.R. Effect of experimental diabetes on the development and maintenance of vulvovaginal candidiasis in female rats. Am. J. Obstet. Gynecol. 2009, 200, 659.e1–659.e4. [Google Scholar] [CrossRef] [PubMed]
- Bassyouni, R.H.; Wegdan, A.A.; Abdelmoneim, A.; Said, W.; Aboelnaga, F. Phospholipase and aspartyl proteinase activities of candida species causing vulvovaginal candidiasis in patients with type 2 diabetes mellitus. J. Microbiol. Biotechnol. 2015, 25, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Faraji, R.; Rahimi, A.; Rezvanmadani, F.; Hashemi, M. Prevalence of vaginal candidiasis infection in diabetic women. Afr. J. Microbiol. Res. 2012, 6, 2773–2778. [Google Scholar]
- Yildirim, Z.; Kilic, N.; Kalkanci, A. Fluorometric determination of acid proteinase activity in Candida albicans strains from diabetic patients with vulvovaginal candidiasis. Mycoses 2011, 54, e463–e467. [Google Scholar] [CrossRef]
- Chaffin, W.L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008, 72, 495–544. [Google Scholar] [CrossRef]
- Yang, Y.-L. Virulence factors of Candida species. J. Microbiol. Immunol. Infect. 2003, 36, 223–228. [Google Scholar]
- Samaranayake, Y.H.; Dassanayake, R.S.; Cheung, B.P.K.; Jayatilake, J.A.M.S.; Yeung, K.W.S.; Yau, J.Y.Y.; Samaranayake, L.P. Differential phospholipase gene expression by Candida albicans in artificial media and cultured human oral epithelium. APMIS 2006, 114, 857–866. [Google Scholar] [CrossRef]
- Tilak, R.; Kumari, V.; Banerjee, T.; Kumar, P.; Pandey, S. Emergence of non-albicans Candida among candidal vulvovaginitis cases and study of their potential virulence factors, from a tertiary care center, North India. Indian J. Pathol. Microbiol. 2013, 56, 144. [Google Scholar] [CrossRef] [PubMed]
- Kuştimur, S.; El-Nahi, H.; Altan, N. Virulence of Proteinase-Positive and Proteinase-Negative Candida Albicans to Mouse and Killing of the Yeast by Normal Human Leukocytes. In Candida and Candidamycosis; Springer US: Boston, MA, USA, 1991; pp. 159–166. [Google Scholar]
- Kendirci, M.; Koç, A.N.; Kurtoglu, S.; Keskin, M.; Kuyucu, T. Vulvovaginal candidiasis in children and adolescents with type 1 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2004, 17, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Kelekci, S.; Kelekci, H.; Cetin, M.; Inan, I.; Tokucoglu, S. Glucose tolerance in pregnant women with vaginal candidiasis. Ann. Saudi Med. 2004, 24, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Nyirjesy, P.; Zhao, Y.; Ways, K.; Usiskin, K. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr. Med. Res. Opin. 2012, 28, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Raith, L.; Csató, M.; Dobozy, A. Decreased Candida albicans killing activity of granulocytes from patients with diabetes mellitus. Mykosen 1983, 26, 557–564. [Google Scholar] [CrossRef]
- Paramythiotou, E.; Frantzeskaki, F.; Flevari, A.; Armaganidis, A.; Dimopoulos, G. Invasive Fungal Infections in the ICU: How to Approach, How to Treat. Molecules 2014, 19, 1085–1119. [Google Scholar] [CrossRef] [Green Version]
- Sobel, J.D.; Myers, P.G.; Kaye, D.; Levison, M.E. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J. Infect. Dis. 1981, 143, 76–82. [Google Scholar] [CrossRef]
- Zheng, N.N.; Guo, X.C.; Lv, W.; Chen, X.X.; Feng, G.F. Characterization of the vaginal fungal flora in pregnant diabetic women by 18S rRNA sequencing. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1031–1040. [Google Scholar] [CrossRef]
- Nowakowska, D.; Kurnatowska, A.; Stray-Pedersen, B.; Wilczyński, J. Activity of hydrolytic enzymes in fungi isolated from diabetic pregnant women: Is there any relationship between fungal alkaline and acid phosphatase activity and glycemic control? APMIS 2004, 112, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Guzel, A.B.; Ilkit, M.; Burgut, R.; Urunsak, İ.F.; Ozgunen, F.T. An Evaluation of Risk Factors in Pregnant Women with Candida Vaginitis and the Diagnostic Value of Simultaneous Vaginal and Rectal Sampling. Mycopathologia 2011, 172, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.; Czeizel, A.E. Asymptomatic trichomonas and candida colonization and pregnancy outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Spinillo, A.; Capuzzo, E.; Acciano, S.; De Santolo, A.; Zara, F. Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 1999, 180, 14–17. [Google Scholar] [CrossRef]
- Cotch, M.F.; Hillier, S.L.; Gibbs, R.S.; Eschenbach, D.A. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am. J. Obstet. Gynecol. 1998, 178, 374–380. [Google Scholar] [CrossRef]
- French, W.; Gad, A. The frequency of Candida infections in pregnancy and their treatment with clotrimazole. Curr. Med. Res. Opin. 1977, 4, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.J.; Moore, E.E.; Boyko, E.J.; Scholes, D.; Lin, F.; Vittinghoff, E.; Fihn, S.D. Vaginal symptoms in postmenopausal women. Menopause 2010, 17, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, J.; Polidori, D.; Zhao, Y.; Al, E. Canagliflozin, an inhibitor of sodium glucose co-transporter 2, improves glycemic control, lowers body weight, and improves beta-cell function in subjects with type 2 diabetes on background metformin. In Proceedings of the 46th Annual Meeting of the European Association for the Study of Diabetes, Stockholm, Sweden, 20–24 September 2010. [Google Scholar]
- Rosenstock, J.; Aggarwal, N.; Polidori, D.; Zhao, Y.; Arbit, D.; Usiskin, K.; Capuano, G.; Canovatchel, W. Canagliflozin DIA 2001 Study Group Dose-Ranging Effects of Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor, as Add-On to Metformin in Subjects With Type 2 Diabetes. Diabetes Care 2012, 35, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Gross, J.L.; Pieters, A.; Bastien, A.; List, J.F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 2223–2233. [Google Scholar] [CrossRef]
- Yokoyama, H.; Nagao, A.; Watanabe, S.; Honjo, J. Incidence and risk of vaginal candidiasis associated with sodium-glucose cotransporter 2 inhibitors in real-world practice for women with type 2 diabetes. J. Diabetes Investig. 2018. [Google Scholar] [CrossRef]
- Banerjee, K.; Curtis, E.; San Lazaro, C.; Graham, J.C. Low prevalence of genital candidiasis in children. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 696–698. [Google Scholar] [CrossRef]
- Schaaf, V.M.; Perez-Stable, E.J.; Borchardt, K. The limited value of symptoms and signs in the diagnosis of vaginal infections. Arch. Intern. Med. 1990, 150, 1929–1933. [Google Scholar] [CrossRef]
- Sonck, C.E.; Somersalo, O. The yeast flora of the anogenital region in diabetic girls. Arch. Dermatol. 1963, 88, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Sopian, I.L.; Shahabudin, S.; Ahmed, M.A.; Lung, L.T.T.; Sandai, D. Yeast Infection and Diabetes Mellitus among Pregnant Mother in Malaysia. Malays. J. Med. Sci. 2016, 23, 27–34. [Google Scholar] [PubMed]
- Nowakowska, D.; Kurnatowska, A.; Stray-Pedersen, B.; Wilczynski, J. Prevalence of fungi in the vagina, rectum and oral cavity in pregnant diabetic women: Relation to gestational age and symptoms. Acta Obstet. Gynecol. Scand. 2004, 83, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.N.; Noor, S.M.; Mat Nor, L.A.; Osman, M.; Rahman, M.M. Candida isolates from pregnant women and their antifungal susceptibility in a Malaysian tertiary-care hospital. Pakistan J. Med. Sci. 2015, 31, 658–661. [Google Scholar]
- Mikamo, H.; Yamagishi, Y.; Sugiyama, H.; Sadakata, H.; Miyazaki, S.; Sano, T.; Tomita, T. High glucose-mediated overexpression of ICAM-1 in human vaginal epithelial cells increases adhesion of Candida albicans. J. Obstet. Gynaecol. 2018, 38, 226–230. [Google Scholar] [CrossRef]
- Yismaw, G.; Asrat, D.; Woldeamanuel, Y.; Unakal, C. Prevalence of candiduria in diabetic patients attending Gondar University Hospital, Gondar, Ethiopia. Iran. J. Kidney Dis. 2013, 7, 102–107. [Google Scholar] [PubMed]
- Mnif, M.F.; Kamoun, M.; Kacem, F.H.; Bouaziz, Z.; Charfi, N.; Mnif, F.; Ben Naceur, B.; Rekik, N.; Abid, M. Complicated urinary tract infections associated with diabetes mellitus: Pathogenesis, diagnosis and management. Indian J. Endocrinol. Metab. 2013, 17, 442–445. [Google Scholar]
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903. [Google Scholar] [CrossRef]
- Esmailzadeh, A.; Zarrinfar, H.; Fata, A.; Sen, T. High prevalence of candiduria due to non- albicans Candida species among diabetic patients: A matter of concern? J. Clin. Lab. Anal. 2018, 32, e22343. [Google Scholar] [CrossRef]
- Falahati, M.; Farahyar, S.; Akhlaghi, L.; Mahmoudi, S.; Sabzian, K.; Yarahmadi, M.; Aslani, R. Characterization and identification of candiduria due to Candida species in diabetic patients. Curr. Med. Mycol. 2016, 2, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, M.; Trevisan, R. Genitourinary infections in diabetic patients in the new era of diabetes therapy with sodium-glucose cotransporter-2 inhibitors. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 963–970. [Google Scholar] [CrossRef]
- Jarvis, W.R. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin. Infect. Dis. 1995, 20, 1526–1530. [Google Scholar] [CrossRef]
- Bartkowski, D.P.; Lanesky, J.R. Emphysematous prostatitis and cystitis secondary to Candida albicans. J. Urol. 1988, 139, 1063–1065. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Soni, B.; Hughes, P.; Ramage, G.; Sherry, L.; Singh, G.; Mansour, P. Candida albicans Fungaemia following Traumatic Urethral Catheterisation in a Paraplegic Patient with Diabetes Mellitus and Candiduria Treated by Caspofungin. Case Rep. Infect. Dis. 2013, 2013, 693480. [Google Scholar] [PubMed]
- Huang, J.J.; Tseng, C.C. Emphysematous pyelonephritis: Clinicoradiological classification, management, prognosis, and pathogenesis. Arch. Intern. Med. 2000, 160, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Grupper, M.; Kravtsov, A.; Potasman, I. Emphysematous Cystitis. Medicine 2007, 86, 47–53. [Google Scholar] [CrossRef]
- Alansari, A.; Borras, M.D.; Boma, N. “I have chicken fat in my urine!” A case of Candida tropicalis induced emphysematous pyelitis. Med. Mycol. Case Rep. 2015, 10, 27–28. [Google Scholar] [CrossRef]
- Wang, L.; Ji, X.; Sun, G.; Qin, Y.; Gong, M.; Zhang, J.; Li, N.; Na, Y. Fungus ball and emphysematous cystitis secondary to Candida tropicalis: A case report. Can. Urol. Assoc. J. 2015, 9, E683–E686. [Google Scholar] [CrossRef]
- Garg, V. Comparison of Clinical Presentation and Risk Factors in Diabetic and Non- Diabetic Females with Urinary Tract Infection Assessed as Per the European Association of Urology Classification. J. Clin. Diagnostic Res. 2015, 9, PC12–PC14. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hiramatsu, M.; Fukazawa, M.; Matsumoto, M.; Honda, K.; Suzuki, Y.; Kawabe, Y. Effect of SGLT2 inhibitors in a murine model of urinary tract infection with Candida albicans. Diabetes Obes. Metab. 2014, 16, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Posteraro, B.; Trecarichi, E.; Al, E. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 2007, 45, 1843–1850. [Google Scholar] [CrossRef]
- Michalopoulos, A.; Kriaras, J.; Geroulanos, S. Systemic candidiasis in cardiac surgery patients. Eur. J. Cardiothorac. Surg. 1997, 11, 728–731. [Google Scholar] [CrossRef] [Green Version]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S.; National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-Resistant Pathogens Associated with HealthcareAssociated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [PubMed]
- Muskett, H.; Shahin, J.; Eyres, G.; Harvey, S.; Rowan, K.; Harrison, D. Risk factors for invasive fungal disease in critically ill adult patients: A systematic review. Crit. Care 2011, 15, R287. [Google Scholar] [CrossRef] [PubMed]
- Paphitou, N.I.; Ostrosky-Zeichner, L.; Rex, J.H. Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: Approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med. Mycol. 2005, 43, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, A.S.; Geroulanos, S.; Mentzelopoulos, S.D. Determinants of Candidemia and Candidemia-Related Death in Cardiothoracic ICU Patients. Clin. Investig. Crit. Care 2003, 124, 2244–2255. [Google Scholar] [CrossRef]
- Wu, J.-Q.; Zhu, L.-P.; Ou, X.-T.; Xu, B.; Hu, X.-P.; Wang, X.; Weng, X.-H. Epidemiology and risk factors for non- Candida albicans candidemia in non-neutropenic patients at a Chinese teaching hospital. Med. Mycol. 2010, 49, 1–4. [Google Scholar] [CrossRef]
- Pfaller, M.; Jones, R.; Doern, G.; Fluit, A.; Verhoef, J.; Sader, H.; Messer, S.; Houston, A.; Coffman, S.; Hollis, R. International surveillance of blood stream infections due to Candida species in the European SENTRY Program: Species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. SENTRY Participant Group (Euro). Diagn. Microbiol. Infect. Dis. 1999, 35, 19–25. [Google Scholar] [CrossRef]
- Shekari Ebrahim Abad, H.; Zaini, F.; Kordbacheh, P.; Mahmoudi, M.; Safara, M.; Mortezaee, V. In Vitro Activity of Caspofungin Against Fluconazole-Resistant Candida Species Isolated From Clinical Samples in Iran. Jundishapur J. Microbiol. 2015, 8, 4–7. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Y.; Feng, X.; Liu, Y.; Wang, S.; Zhu, X.; Chen, Q.; Pan, S. Candidemia: Incidence rates, type of species, and risk factors at a tertiary care academic hospital in China. Int. J. Infect. Dis. 2014, 22, 4–8. [Google Scholar] [CrossRef]
- Barchiesi, F.; Spreghini, E.; Tomassetti, S.; Della Vittoria, A.; Arzeni, D.; Manso, E.; Scalise, G. Effects of caspofungin against Candida guilliermondii and Candida parapsilosis. Antimicrob. Agents Chemother. 2006, 50, 2719–2727. [Google Scholar] [CrossRef]
- Zepelin, M.B.-V.; Kunz, L.; Ruchel, R.; Reichard, U.; Weig, M.; Gross, U. Epidemiology and antifungal susceptibilities of Candida spp. to six antifungal agents: Results from a surveillance study on fungaemia in Germany from July 2004 to August 2005. J. Antimicrob. Chemother. 2007, 60, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.H.; Peart-Vigilance, C.; Kao, W.H.; Brancati, F.L. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care 1999, 22, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Desnos-Ollivier, M.; Ragon, M.; Robert, V.; Raoux, D.; Gantier, J.-C.; Dromer, F. Debaryomyces hansenii (Candida famata), a Rare Human Fungal Pathogen Often Misidentified as Pichia guilliermondii (Candida guilliermondii). J. Clin. Microbiol. 2008, 46, 3237–3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savini, V.; Catavitello, C.; Di Marzio, I.; Masciarelli, G.; Astolfi, D.; Balbinot, A.; Bianco, A.; Pompilio, A.; Di Bonaventura, G.; D’Amario, C.; et al. Pan-azole-Resistant Candida guilliermondii from a Leukemia Patient’s Silent Funguria. Mycopathologia 2010, 169, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Savini, V.; Catavitello, C.; Onofrillo, D.; Masciarelli, G.; Astolfi, D.; Balbinot, A.; Febbo, F.; D’Amario, C.; D’Antonio, D. What do we know about Candida guilliermondii? A voyage throughout past and current literature about this emerging yeast. Mycoses 2011, 54, 434–441. [Google Scholar] [CrossRef]
- Hamilton, H.C.; Foxcroft, D. Central venous access sites for the prevention of venous thrombosis, stenosis and infection in patients requiring long-term intravenous therapy. In Cochrane Database of Systematic Reviews; Hamilton, H.C., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2007; p. CD004084. [Google Scholar]
- Ma, X.; Sun, W.; Liu, T. [Clinical characteristics of Candida septicemia seen in a neonatal intensive care unit: Analysis of 9 cases]. Zhonghua er ke za zhi = Chin. J. Pediatr. 2006, 44, 694–697. [Google Scholar]
- Patel, G.P.; Simon, D.; Scheetz, M.; Crank, C.W.; Lodise, T.; Patel, N. The Effect of Time to Antifungal Therapy on Mortality in Candidemia Associated Septic Shock. Am. J. Ther. 2009, 16, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Nucci, M.; Park, B.J.; Nouér, S.A.; Arthington-Skaggs, B.; da Matta, D.A.; Warnock, D.; Morgan, J.; Brazilian Network Candidemia Study, for the B.N.C. Epidemiology of candidemia in Brazil: A nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 2006, 44, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.L.; Neofytos, D.; Anaissie, E.J.; Fishman, J.A.; Steinbach, W.J.; Olyaei, A.J.; Marr, K.A.; Pfaller, M.A.; Chang, C.; Webster, K.M. Epidemiology and Outcomes of Candidemia in 2019 Patients: Data from the Prospective Antifungal Therapy Alliance Registry. Clin. Infect. Dis. 2009, 48, 1695–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Tobón, A.M.; Restrepo, A.; Colombo, A.L. Epidemiology of Opportunistic Fungal Infections in Latin America. Clin. Infect. Dis. 2010, 51, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, T.; Mc Carthy, J.F.; Rady, M.Y.; Serkey, J.; Gordon, S.; Starr, N.J.; Cosgrove, D.M. Early bloodstream infection after cardiopulmonary bypass: Frequency rate, risk factors, and implications. Crit. Care Med. 1997, 25, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Leroy, O.; Gangneux, J.-P.; Montravers, P.; Mira, J.-P.; Gouin, F.; Sollet, J.-P.; Carlet, J.; Reynes, J.; Rosenheim, M.; Regnier, B.; et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: A multicenter, prospective, observational study in France (2005–2006). Crit. Care Med. 2009, 37, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Lazarus, D.R.; Sherner, J.H.; Jackson, W.L.; Morrel, M.; Fraser, V.J.; Kollef, M.H. Do clinical features allow for accurate prediction of fungal pathogenesis in bloodstream infections? Potential implications of the increasing prevalence of non-albicans candidemia. Crit. Care Med. 2007, 35, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Corzo-leon, D.E.; Alvarado-matute, T.; Colombo, A.L.; Cornejo-juarez, P.; Cortes, J.; Echevarria, J.I.; Macias, A.E.; Nucci, M.; Ostrosky-Zeichner, L.; Ponce-de-Leon, A.; et al. Surveillance of Candida spp Bloodstream Infections: Epidemiological Trends and Risk Factors of Death in Two Mexican Tertiary Care Hospitals. PLoS ONE 2014, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of Candidemia in Latin America: A Laboratory-Based Survey. PLoS ONE 2013, 8, e59373. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, A.; Varma, A. Candida glabrata candidemia: An emerging threat in critically ill patients. Indian J. Crit. Care Med. 2015, 19, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Pozzilli, P.; Leslie, R.D. Infections and diabetes: Mechanisms and prospects for prevention. Diabet. Med. 1994, 11, 935–941. [Google Scholar] [CrossRef] [PubMed]
- MacCuish, A.C.; Urbaniak, S.J.; Campbell, C.J.; Duncan, L.J.; Irvine, W.J. Phytohemagglutinin transformation and circulating lymphocyte subpopulations in insulin-dependent diabetic patients. Diabetes 1974, 23, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, M.K. Handicaps to host defense. Effects of hyperglycemia on C3 and Candida albicans. Diabetes 1990, 39, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Padawer, D.; Pastukh, N.; Nitzan, O.; Labay, K.; Aharon, I.; Brodsky, D.; Glyatman, T.; Peretz, A. Catheter-associated candiduria: Risk factors, medical interventions, and antifungal susceptibility. Am. J. Infect. Control 2015, 43, e19–e22. [Google Scholar] [CrossRef] [PubMed]
- Tambyah, P.A.; Halvorson, K.T.; Maki, D.G. A Prospective Study of Pathogenesis of Catheter-Associated Urinary Tract Infections. Mayo Clin. Proc. 1999, 74, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Maki, D.G.; Tambyah, P.A. Engineering out the risk for infection with urinary catheters. Emerg. Infect. Dis. 2001, 7, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; Mikherjee, P.K.; Clark, T.A.; Pujol, C.; Chandra, J.; Hajjeh, R.A.; Warnock, D.W.; Soil, D.R.; Ghannoum, M.A. Candida parapsilosis characterization in an outbreak setting. Emerg. Infect. Dis. 2004, 10, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Khatib, R.; Johnson, L.B.; Fakih, M.G.; Riederer, K.; Briski, L. Current trends in candidemia and species distribution among adults: Candida glabrata surpasses C. albicans in diabetic patients and abdominal sources. Mycoses 2016, 59, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, P.A. Surgical critical care: Fungal infections in surgical patients. Crit. Care Med. 2006, 34, S215–S224. [Google Scholar] [CrossRef]
- Hattori, H.; Maeda, M.; Nagatomo, Y.; Takuma, T.; Niki, Y.; Naito, Y.; Sasaki, T.; Ishino, K. Epidemiology and risk factors for mortality in bloodstream infections: A single-center retrospective study in Japan. Am. J. Infect. Control 2018, 46, e75–e79. [Google Scholar] [CrossRef]
- Zhao, S.J.; Fu, Y.Q.; Zhu, M.X.; Zhou, H.; Xu, M.; Yan, R.L.; Shui, Y.X.; Zhou, J.Y. [Patients of Escherichia coli bloodstream infection: Analysis of antibiotic resistance and predictors of mortality]. Zhonghua Yi Xue Za Zhi 2017, 97, 2496–2500. [Google Scholar]
- Tumbarello, M.; Fiori, B.; Trecarichi, E.M.; Posteraro, P.; Losito, A.R.; de Luca, A.; Sanguinetti, M.; Fadda, G.; Cauda, R.; Posteraro, B. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS ONE 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Bristow, I.R.; Spruce, M.C. Fungal foot infection, cellulitis and diabetes: A review. Diabet. Med. 2009, 26, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Tchernev, G.; Penev, P.K.; Nenoff, P.; Zisova, L.G.; Cardoso, J.C.; Taneva, T.; Ginter-Hanselmayer, G.; Ananiev, J.; Gulubova, M.; Hristova, R.; et al. Onychomycosis: Modern diagnostic and treatment approaches. Wiener Medizinische Wochenschrift 2013, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-J.; Hsu, S.-C.; Tien, K.-J.; Hsiao, J.-Y.; Lin, S.-R.; Chen, H.-C.; Hsieh, M.-C. Metabolic syndrome associated with toenail onychomycosis in Taiwanese with diabetes mellitus. Int. J. Dermatol. 2008, 47, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Banerjee, T.; Chakravarty, J.; Singh, S.; Dwivedi, A.; Tilak, R. Identification, antifungal resistance profile, in vitro biofilm formation and ultrastructural characteristics of Candida species isolated from diabetic foot patients in Northern India. Indian J. Med. Microbiol. 2016, 34, 308. [Google Scholar]
- Raiesi, O.; Siavash, M.; Mohammadi, F.; Chabavizadeh, J.; Mahaki, B.; Maherolnaghsh, M.; Dehghan, P. Frequency of Cutaneous Fungal Infections and Azole Resistance of the Isolates in Patients with Diabetes Mellitus. Adv. Biomed. Res. 2017, 6, 71. [Google Scholar] [PubMed]
- Lugo-Somolinos, A.; Sánchez, J.L. Prevalence of dermatophytosis in patients with diabetes. J. Am. Acad. Dermatol. 1992, 26, 408–410. [Google Scholar] [CrossRef]
- Pierard, G.E.; Pierard-Franchimont, C. The nail under fungal siege in patients with type II diabetes mellitus. Mycoses 2005, 48, 339–342. [Google Scholar] [CrossRef]
- Dogra, S.; Kumar, B.; Bhansali, A.; Chakrabarty, A. Epidemiology of onychomycosis in patients with diabetes mellitus in India. Int. J. Dermatol. 2002, 41, 647–651. [Google Scholar] [CrossRef]
- Eckhard, M.; Lengler, A.; Liersch, J.; Bretzel, R.G.; Mayser, P. Fungal foot infections in patients with diabetes mellitus-results of two independent investigations. Mycoses 2007, 50, 14–19. [Google Scholar] [CrossRef]
- Wijesuriya, T.M.; Weerasekera, M.M.; Kottahachchi, J.; Ranasinghe, K.N.P.; Dissanayake, M.S.S.; Prathapan, S.; Gunasekara, T.D.C.P.; Nagahawatte, A.; Guruge, L.D.; Bulugahapitiya, U.; et al. Proportion of lower limb fungal foot infections in patients with type 2 diabetes at a tertiary care hospital in Sri Lanka. Indian J. Endocrinol. Metab. 2014, 18, 63–69. [Google Scholar]
- Boyko, E.J.; Ahroni, J.H.; Cohen, V.; Nelson, K.M.; Heagerty, P.J. Prediction of Diabetic Foot Ulcer Occurrence Using Commonly Available Clinical Information: The Seattle Diabetic Foot Study. Diabetes Care 2006, 29, 1202–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johargy, A.K. Antimicrobial susceptibility of bacterial and fungal infections among infected diabetic patients. J. Pak. Med. Assoc. 2016, 66, 1291–1295. [Google Scholar] [PubMed]
- Gupta, A.K.; Konnikov, N.; Macdonald, P.; Rich, P.; Rodger, N.W.; Edmonds, M.W.; Mcmanus, R.; Summerbell, R.C. Prevalence and epidemiology of toenail onychomycosis in diabetic subjects: A multicentre survey. Br. J. Dermatol. 1998, 139, 665–671. [Google Scholar] [CrossRef]
- Chi, C.-C.; Wang, S.-H.; Chou, M.-C. The causative pathogens of onychomycosis in southern Taiwan. Mycoses 2005, 48, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, Y.; Li, Z.; Xue, L.; Li, X.; Liu, Y.; Li, C.; Wang, H. The Effect of Candida albicans on the Expression Levels of Toll-like Receptor 2 and Interleukin-8 in HaCaT Cells Under High- and Low-glucose Conditions. Indian J. Dermatol. 2018, 63, 201–207. [Google Scholar] [PubMed]
- Delamaire, M.; Maugendre, D.; Moreno, M.; Le Goff, M.-C.; Allannic, H.; Genetet, B. Impaired Leucocyte Functions in Diabetic Patients. Diabet. Med. 1997, 14, 29–34. [Google Scholar] [CrossRef]
- Llorente, L.; De La Fuente, H.; Richaud-Patin, Y.; Alvarado-De La Barrera, C.; Diaz-Borjón, A.; López-Ponce, A.; Lerman-Garber, I.; Jakez-Ocampo, J. Innate immune response mechanisms in non-insulin dependent diabetes mellitus patients assessed by flow cytoenzymology. Immunol. Lett. 2000, 74, 239–244. [Google Scholar] [CrossRef]
- De Souza Ferreira, C.; Pennacchi, P.C.; Araújo, T.H.; Taniwaki, N.N.; De Araújo Paula, F.B.; Da Silveira Duarte, S.M.; Rodrigues, M.R. Aminoguanidine treatment increased NOX2 response in diabetic rats: Improved phagocytosis and killing of Candida albicans by neutrophils. Eur. J. Pharmacol. 2016, 772, 83–91. [Google Scholar] [CrossRef]
- Pupim, A.C.E.; Campois, T.G.; Araújo, E.J.A.; Svidizinski, T.I.E.; Felipe, I. Infection and tissue repair of experimental cutaneous candidiasis in diabetic mice. J. Med. Microbiol. 2017, 66, 808–815. [Google Scholar] [CrossRef]
- Rubin, B.G.; German, M.L.; Louis, S. Candida infection with aneurysm formation in the juxtarenal aorta. J. Vasc. Surg. 1994, 20, 311–314. [Google Scholar] [CrossRef]
- King, A.J.F. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosius, F.C.; Alpers, C.E.; Bottinger, E.P.; Breyer, M.D.; Coffman, T.M.; Gurley, S.B.; Harris, R.C.; Kakoki, M.; Kretzler, M.; Leiter, E.H.; et al. Mouse Models of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2009, 20, 2503–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, K.A.; Hayes, J.M.; Wiggin, T.D.; Backus, C.; Su Oh, S.; Lentz, S.I.; Brosius, F.; Feldman, E.L. Mouse models of diabetic neuropathy. Neurobiol. Dis. 2007, 28, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, K.A.; Lentz, S.I.; Roberts, J.L., Jr.; Feldman, E.L. Criteria for Creating and Assessing Mouse Models of Diabetic Neuropathy. Curr. Drug Targets 2008, 9, 3. [Google Scholar] [PubMed]
- Franconi, F.; Seghieri, G.; Canu, S.; Straface, E.; Campesi, I.; Malorni, W. Are the available experimental models of type 2 diabetes appropriate for a gender perspective? Pharmacol. Res. 2008, 57, 6–18. [Google Scholar] [CrossRef]
- Inada, A.; Arai, H.; Nagai, K.; Miyazaki, J.; Yamada, Y.; Seino, Y.; Fukatsu, A. Gender Difference in ICER Iγ Transgenic Diabetic Mouse. Biosci. Biotechnol. Biochem. 2007, 71, 1920–1926. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.; Poon, W.; Baker, M.; Linton, C.; Mühlschlegel, F.A. Confirmed Candida albicans endogenous fungal endophthalmitis in a patient with chronic candidiasis. Med. Mycol. Case Rep. 2012, 1, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Woodrum, D.T.; Welke, K.F.; Fillinger, M.F. Candida infection associated with a solitary mycotic common iliac artery aneurysm. J. Vasc. Surg. 2001, 34, 166–168. [Google Scholar] [CrossRef] [Green Version]
- Dowling, R.D.; Baladi, N.; Zenati, M.; Dummer, J.S.; Kormos, R.L.; Armitage, J.M.; Yousem, S.A.; Hardesty, R.L.; Griffith, B.P. Disruption of the aortic anastomosis after heart-lung transplantation. Ann. Thorac. Surg. 1990, 49, 118–122. [Google Scholar] [CrossRef]
- Laouad, I.; Buchler, M.; Noel, C.; Sadek, T.; Maazouz, H.; Westeel, P.F.; Lebranchu, Y. Renal Artery Aneurysm Secondary to Candida albicans in Four Kidney Allograft Recipients. Transplant. Proc. 2005, 37, 2834–2836. [Google Scholar] [CrossRef]
- Mai, H.; Champion, L.; Ouali, N.; Hertig, A.; Peraldi, M.-N.; Glotz, D.; Rondeau, E.; Costa, M.-A.; Snanoudj, R.; Benoit, G.; et al. Candida albicans Arteritis Transmitted by Conservative Liquid After Renal Transplantation: A Report of Four Cases and Review of the Literature. Transplantation 2006, 82, 1163–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentine, R.J.; Chung, J. Primary Vascular Infection. Curr. Probl. Surg. 2012, 49, 128–182. [Google Scholar] [CrossRef] [PubMed]
- Chapuis-Taillard, C.; Manuel, O.; Bille, J.; Calandra, T.; Rotman, S.; Tarr, P.E. Candida Arteritis in Patients Who Have Not Received Organ Transplants: Case Report and Review of the Literature. Clin. Infect. Dis. 2008, 46, e106–e111. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; Busuttil, R.W.; Baker, J.D.; Machleder, H.I.; Moore, W.S.; Barker, W.F. Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J. Vasc. Surg. 1984, 1, 541–547. [Google Scholar] [CrossRef]
- Potti, A.; Danielson, B.; Sen, K. “True” mycotic aneurysm of a renal artery allograft. Am. J. Kidney Dis. 1998, 31, E3. [Google Scholar] [CrossRef] [PubMed]
- Oderich, G.S.; Panneton, J.M.; Bower, T.C.; Cherry, K.J., Jr.; Rowland, C.M.; Noel, A.A.; Hallett, J.W., Jr.; Gloviczk, P. Infected aortic aneurysms: Aggressive presentation, complicated early outcome, but durable results. J. Vasc. Surg. 2001, 34, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Sergio, P.; De Araujo, R.; Medeiros, Z.; Melo, F.L. De Case Report Candida famata- induced fulminating cholecystitis. Rev Soc Bras Med Trop. 2013, 46, 795–796. [Google Scholar]
- Kakeya, H.; Izumikawa, K.; Yamada, K.; Narita, Y.; Nishino, T.; Obata, Y.; Takazono, T.; Kurihara, S.; Kosai, K.; Morinaga, Y.; et al. Concurrent subcutaneous candidal abscesses and pulmonary cryptococcosis in a patient with diabetes mellitus and a history of corticosteroid therapy. Intern Med. 2014, 53, 1385–1390. [Google Scholar] [CrossRef]
- Florescu, D.F.; Brostrom, S.E.; Dumitru, I.; Kalil, A.C. Candida albicans Skin Abscess in a Heart Transplant Recipient. Infect. Dis. Clin. Pract. 2010, 18, 243–246. [Google Scholar] [CrossRef]
- Neves, N.; Santos, L.; Reis, C.; Sarmento, A. Candida albicans brain abscesses in an injection drug user patient: A case report. BMC Res. Notes 2014, 7, 837. [Google Scholar] [CrossRef]
- Honda, H.; Warren, D.K. Central Nervous System Infections: Meningitis and Brain Abscess. Infect. Dis. Clin. N. Am. 2009, 23, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Fennelly, A.M.; Slenker, A.K.; Murphy, L.C.; Moussouttas, M.; DeSimone, J.A. Candida cerebral abscesses: A case report and review of the literature. Med. Mycol. 2013, 51, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; He, X.-S.; Chen, J.; Ouyang, B.; Zhu, X.-F.; Chen, M.-Y.; Xie, W.-F.; Chen, L.; Zheng, D.-H.; Zhong, Y.; et al. Fungal infection in patients after liver transplantation in years 2003 to 2012. Ann. Transplant. 2012, 17, 59–63. [Google Scholar] [PubMed]
- Abraham, G.; Kumar, V.; Nayak, K.S.; Ravichandran, R.; Srinivasan, G.; Krishnamurthy, M.; Prasath, A.K.; Kumar, S.; Thiagarajan, T.; Mathew, M.; et al. Predictors of long-term survival on peritoneal dialysis in south india: A multicenter study. Perit. Dial. Int. 2010, 30, 29–34. [Google Scholar] [CrossRef]
- Yuvaraj, A.; Rohit, A.; Koshy, P.J.; Nagarajan, P.; Nair, S.; Abraham, G. Rare occurrence of fatal Candida haemulonii peritonitis in a diabetic CAPD patient. Ren. Fail. 2014, 36, 1466–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zappella, N.; Desmard, M.; Chochillon, C.; Ribeiro-Parenti, L.; Houze, S.; Marmuse, J.P.; Montravers, P. Positive peritoneal fluid fungal cultures in postoperative peritonitis after bariatric surgery. Clin. Microbiol. Infect. 2015, 21, 853.e1–853.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulcahy, J.J. Long-term experience with salvage of infected penile implants. J. Urol. 2000, 163, 481–482. [Google Scholar] [CrossRef]
- Peppas, D.S.; Moul, J.W.; McLeod, D.G. Candida albicans corpora abscess following penile prosthesis placement. J. Urol. 1988, 140, 1541–1542. [Google Scholar] [CrossRef]
- Mulcahy, J.J.; Carson, C.C. Long-Term Infection Rates in Diabetic Patients Implanted With Antibiotic-Impregnated Versus Nonimpregnated Inflatable Penile Prostheses: 7-Year Outcomes. Eur. Urol. 2011, 60, 167–172. [Google Scholar] [CrossRef]
- Cotta, B.H.; Butcher, M.; Welliver, C.; Mcvary, K.; Köhler, T. Two Fungal Infections of Inflatable Penile Prostheses in Diabetics. Sex. Med. 2015, 3, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Maatouk, I.; Hajjar, M.; Moutran, R. Candida albicans and Streptococcus pyogenes balanitis: Diabetes or STI? Int. J. Std Aids 2015, 26, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, M.; Kuzaka, B.; Borkowski, T.; Kuzaka, P.; Kawecki, D.; Radziszewski, P. Fournier’s Gangrene—Current Concepts. Polish J. Microbiol. 2014, 63, 267–273. [Google Scholar]
- Saha, K.; Sit, N.K.; Maji, A.; Jash, D. Recovery of fluconazole sensitive Candida ciferrii in a diabetic chronic obstructive pulmonary disease patient presenting with pneumonia. Lung India 2013, 30, 338–340. [Google Scholar] [CrossRef] [PubMed]



| Physiopathology | Reference (s) | |
|---|---|---|
| Uncontrolled hyperglycemia (high HbA1c) and high glucose levels in saliva | -Uncontrolled hyperglycemia may cause intensification in salivary glucose levels because in diabetics the basement membrane of the parotid salivary gland is more permeable -High glucose levels allow Candida sp. to multiply, even in the presence of normal bacterial flora -During hyperglycemic episodes, the chemically reversible glycosylation products with proteins in tissues and the accumulation of glycosylation products on buccal epithelial cells may sequentially increase the number of available receptors for Candida sp. -Glucose suppression of the killing capacity of neutrophils, emphasizing colonization (immunosuppression) -Glucose, maltose, and sucrose boost the adhesion of Candida to buccal epithelial cells | [15,29,31,52,134,140,141,142,143,144,145,146,147] |
| Lower salivary pH | -The growth of Candida in saliva is accompanied by a rapid decline in pH, which favors their growth and triggers the extracellular phospholipase (PL) and acid proteases, increasing the yeast adhesion to oral mucosal surfaces | [31,128,148] |
| Tissue response to injury is diminished | -Diabetes mellitus (DM) is known to diminish the host resistance and modify the tissue response to injury. This can result in severe colonization, even in the absence of any clinically evident oral candidiasis and possibly with further dissemination via the blood. | [130,149] |
| Oral epithelium | -It is most probable that the host oral epithelium of patients with diabetes favors the adhesion of colonization and subsequent infection. | [150,151] |
| Poor oral hygiene | The lack of control of the oral environment, especially concerning the prevention of dental caries (coronary, root, and periodontal), leads to a higher rate of oral candidiasis, especially in DM older patients | [152,153,154] |
| Aging | Diabetic women, orally colonized with Candida sp. have higher oral glucose levels than diabetics without oral Candida sp. | [134] |
| Gender | ||
| Prostheses | Inadequate use of prostheses, together with inadequate hygienization, favours the growth of Candida sp. | [155,156,157] |
| Drugs | Xerostomia (abnomal lack if saliva): Candida sp. stagnation and growth on oral tissues | [30,109,110,111,136,158,159] |
| Condition | Physiopathology | Reference (s) |
|---|---|---|
| Uncontrolled hyperglycemia (high HbA1c) and high glucose levels in vaginal mucosae | - The increased serum glucose level is thought to lead to impaired monocyte, granulocyte, and neutrophil adherence, as well as reduced chemotaxis, phagocytosis, and pathogen killing - Diabetics secretions contain glucose, which can be used as a nutrient by yeasts - pH, nutritional substance, temperature, and adherence capacity in the vulvovaginal tissue may induce Candida sp. virulence. The vaginal epithelial cell receptor of fucose supports the adhesion of Candida sp. to vaginal epithelial cells -The rise in vaginal glucose and secretions and activities of hydrolytic enzymes [e.g., secreted aspartyl proteinases (SAPs), PL] increases the pathogenicity of Candida sp. -Increased levels of glycogen increase colonization and infection by Candida sp. by lowering the vaginal pH, facilitating the development of vulvovaginal candidiasis (VVC) | [55,56,59,219,222,224,225,231,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253] |
| Pregnancy | -The increased circulation of estrogen levels and the deposition of glycogen and other substrates in the vagina leads to a 10–50% higher incidence of vaginal colonization by Candida sp. -Variability of constitutive defensins (e.g., lactoferrins, peptides) and lysozyme, leading to a poor innate immune response -Hyperglycemia can rise the anaerobic glycolysis in vaginal epithelial cells, increasing lactic acid and acetone production, decreasing the vaginal pH, thus enabling fungal colonization and proliferation | [241,254,255,256,257,258,259,260] |
| Diabetes type | - The incidence of VVC related to the type 1 DM or type 2 DM and is variable among studies | [218,221,222,223] |
| Aging | - The older one is, the higher VVC prevalence is | [222,223] |
| Condition | Physiopathology | Reference (s) |
|---|---|---|
| Uncontrolled hyperglycemia (high HbA1c) and high glucose levels in urinary tract (UT) mucosae or blood | - Favorable microenvironment for the gas-forming organisms, such as Candida sp., to grow | [282,283] |
| Gender | -An association between candiduria and being female | [27,273,279] |
| Drugs | -SGLT2 inhibitors (e.g., dapagliflozin, canagliflozin, tofogliflozin) administration leads to a greater susceptibility to urinary tract infection (UTI) -Association with a persistent increase in urine glucose concentration | [287] |
| Condition | Physiopathology | Reference (s) |
|---|---|---|
| Uncontrolled hyperglycemia (high HbA1c) and high glucose levels in vaginal mucosae | -Circulatory disorders affecting the lower extremities (peripheral circulation), peripheral neuropathy, and retinopathy - Nail thickness is associated with an elevated HbA1c value - Diabetics using hemodialysis exhibit a higher probability of onychomycosis | [329,330,334,335,336,337,338] |
| Duration of DM | -More time leads to a higher probability of onychomycosis | [336] |
| Gender Aging | -Being male and being older are directly associated with onychomycosis in diabetics | [331,336] |
| Condition | Physiopathology | Candida sp. Found in the Study | Reference (s) |
|---|---|---|---|
| Arterial infection | -Direct invasion or haematogenous spread of Candida sp. to the vessels wall (less common): a. infection of a pre-existing aneurysm (most possible from atherosclerotic) with fungi after an episode of candidemia b. contained fungal infection of the arterial wall (linked mostly to intravenous drug abuse). | C. albicans C. tropicalis C. parapsilosis Other Candida sp. (not specified) | [348,349,350,351,352,353,354,355,356,357] |
| Endophthalmitis | - Uncontrolled DM concomitant with other co-morbidities | C. albicans | [358] |
| Cholecystitis | - Uncontrolled DM concomitant with other co-morbidities | C. famata | [359] |
| Skin abscesses | - Uncontrolled DM concomitant with other co-morbidities | C. albicans | [360,361] |
| Brain abscesses | -Immunocompromised states and poorly-controlled diabetes (the role of DM as an immunosuppressive condition in this particular case is uncertain) | C. albicans C. parapsilosis C. tropicalis C. guilliermondi Other Candida sp. (not specified) | [362,363,364] |
| Fungal infections after liver transplantation | - Chronically high blood sugar may be a predisposing factor (among other factors) | C. albicans | [365] |
| Peritonitis | - Uncontrolled DM concomitant with other co-morbidities | C. haemulonii C. glabrata Other Candida sp. (not specified) | [366,367,368] |
| Penile prosthesis Infections | Other Candida sp. (not specified) | [369,370,371,372] | |
| Balanitis | C. albicans | [373] | |
| Fournier’s gangrene | Candida sp. (not specified) | [374] | |
| Chronic obstructive pulmonary disease | C. ciferrii | [375] |
| Diabetes Mellitus | Model/Induction | Studies | Features |
|---|---|---|---|
| Type 1 | Chemical induction | ||
| High dose streptozotocin | New formulations of insulin | Hyperglicaemia model | |
| Alloxan | Transplantation | Induced insulitis | |
| Multiple low dose streptozotocin | Treatment to prevent β-cell death | ||
| Spontaneous autoimmune | |||
| NOD mice | Understanding genetic mechanisms | Autoimmune β-cell destruction | |
| BB rats | |||
| LEW.1AR1/-iddm rats | Treatment to prevent β-cell death or to manipulate autoimmune processes | ||
| Genetically Induced | |||
| AKITA rats* | New formulations of insulin Transplantation Treatment to prevent ER stress | β-cell destruction due to ER + Insulin dependent | |
| Virally induction | |||
| Coxsackie B virus | Study of potencial role of viruses in DM type 1 | Viral β-cell destruction | |
| Encephalomyocarditis virus | |||
| Kilham rat virus | |||
| Lymphocytic choriomeningitis virus (LCMV) under insulin promoter | |||
| Type 2 | Monogenic—obese models | ||
| Obese-induced hyperglycemia | |||
| Lepob/ob mice | Treatment to improve insulin resistance | ||
| Leprdb/db mice | Treatment to improve β-cell resistance | ||
| KDF rats | |||
| Polygenic—obese models | |||
| KK mice | Treatment to insulin resistance | ||
| OLEFT rat | Treatment to improve β-cell function | ||
| NZO mice | Some models show diabetic complications | ||
| TallyHO/Jng mice | |||
| NoncNZO10/|LtJ mice | |||
| Induced obesity | |||
| High fat feeding (mice or rats) | Treatment to insulin resistance | ||
| Nile grass rat | Treatment to prevent diet-induced obesity | ||
| Desert gerbil | Treatment to improve β-cell function | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Candida sp. Infections in Patients with Diabetes Mellitus. J. Clin. Med. 2019, 8, 76. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8010076
Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. Infections in Patients with Diabetes Mellitus. Journal of Clinical Medicine. 2019; 8(1):76. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8010076
Chicago/Turabian StyleRodrigues, Célia F., Maria Elisa Rodrigues, and Mariana Henriques. 2019. "Candida sp. Infections in Patients with Diabetes Mellitus" Journal of Clinical Medicine 8, no. 1: 76. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8010076