The Overlooked Immune State in Candidemia: A Risk Factor for Mortality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects, Setting and Design
2.2. Study Variables
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Population Description
3.2. Impact of Lymphocyte Count on Mortality and Survival
3.3. Univariate and Multivariable Logistic Risk Analysis for Mortality
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sozio, E.; Pieralli, F.; Azzini, A.M.; Tintori, G.; Demma, F.; Furneri, G.; Sbrana, F.; Bertolino, G.; Fortunato, S.; Meini, S.; et al. A prediction rule for early recognition of patients with candidemia in internal medicine: Results from an italian, multicentric, case-control study. Infection 2018, 46, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in us hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Aljeboori, Z.; Gorelik, A.; Jenkins, E.; McFarlane, T.; Darvall, J. Risk factors for candidaemia and their cumulative effect over time in a cohort of critically ill, non-neutropenic patients. Crit. Care Resusc. 2018, 20, 313–319. [Google Scholar]
- Cuervo, G.; Garcia-Vidal, C.; Puig-Asensio, M.; Merino, P.; Vena, A.; Martin-Pena, A.; Montejo, J.M.; Ruiz, A.; Lazaro-Perona, F.; Fortun, J.; et al. Usefulness of guideline recommendations for prognosis in patients with candidemia. Med. Mycol. 2018, 57, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Lausch, K.R.; Sogaard, M.; Rosenvinge, F.S.; Johansen, H.K.; Boysen, T.; Roder, B.; Mortensen, K.L.; Nielsen, L.; Lemming, L.; Olesen, B.; et al. High incidence of candidaemia in a nationwide cohort: Underlying diseases, risk factors and mortality. Int. J. Infect. Dis. 2018, 76, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.A.; Zurko, J.; Camins, B.C.; Griffin, R.L.; Rodriguez, J.M.; McCarty, T.P.; Magadia, J.; Pappas, P.G. Impact of infectious disease consultation on clinical management and mortality in patients with candidemia. Clin. Infect. Dis. 2018, 68, 1585–1587. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.F.; Li, F.Q.; Shi, L.N.; Hu, Y.A.; Wang, Y.; Huang, M.; Kong, Q.Q. Surveillance study of species distribution, antifungal susceptibility and mortality of nosocomial candidemia in a tertiary care hospital in China. BMC Infect. Dis. 2013, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Vaquero-Herrero, M.P.; Ragozzino, S.; Castano-Romero, F.; Siller-Ruiz, M.; Sanchez Gonzalez, R.; Garcia-Sanchez, J.E.; Garcia-Garcia, I.; Marcos, M.; Ternavasio-de la Vega, H.G. The pitt bacteremia score, charlson comorbidity index and chronic disease score are useful tools for the prediction of mortality in patients with candida bloodstream infection. Mycoses 2017, 60, 676–685. [Google Scholar] [CrossRef]
- Poves-Alvarez, R.; Cano-Hernandez, B.; Munoz-Moreno, M.F.; Balbas-Alvarez, S.; Roman-Garcia, P.; Gomez-Sanchez, E.; Martinez-Rafael, B.; Gomez-Pesquera, E.; Lorenzo-Lopez, M.; Alvarez-Fuente, E.; et al. Impact of empirical treatment with antifungal agents on survival of patients with candidemia. Rev. Esp. Quimioter. 2019, 32, 6–14. [Google Scholar]
- Puig-Asensio, M.; Padilla, B.; Garnacho-Montero, J.; Zaragoza, O.; Aguado, J.M.; Zaragoza, R.; Montejo, M.; Munoz, P.; Ruiz-Camps, I.; Cuenca-Estrella, M.; et al. Epidemiology and predictive factors for early and late mortality in candida bloodstream infections: A population-based surveillance in Spain. Clin. Microbiol. Infect. 2014, 20, O245–O254. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou-Olivgeris, M.; Spiliopoulou, A.; Fligou, F.; Spiliopoulou, I.; Tanaseskou, L.; Karpetas, G.; Marangos, M.; Anastassiou, E.D.; Christofidou, M. Risk factors and predictors of mortality of candidaemia among critically ill patients: Role of antifungal prophylaxis in its development and in selection of non-albicans species. Infection 2017, 45, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Stein, B.; Hollick, R.; Lockhart, S.R.; Magill, S.S.; Derado, G.; Park, B.J.; Chiller, T.M. Changes in incidence and antifungal drug resistance in candidemia: Results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin. Infect. Dis. 2012, 55, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Delaloye, J.; Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Tascini, C.; Russo, A.; Sozio, E.; Raponi, G.; Rosin, C.; Pignatelli, P.; Carfagna, P.; Farcomeni, A.; et al. Assessment of risk factors for candidemia in non-neutropenic patients hospitalized in internal medicine wards: A multicenter study. Eur. J. Intern. Med. 2017, 41, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Puig-Asensio, M.; Ruiz-Camps, I.; Fernandez-Ruiz, M.; Aguado, J.M.; Munoz, P.; Valerio, M.; Delgado-Iribarren, A.; Merino, P.; Bereciartua, E.; Fortun, J.; et al. Epidemiology and outcome of candidaemia in patients with oncological and haematological malignancies: Results from a population-based surveillance in Spain. Clin. Microbiol. Infect. 2015, 21, 491.e1-10. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K., Jr.; Calandra, T.F.; Edwards, J.E., Jr.; Filler, S.G.; Fisher, J.F.; Kullberg, B.J.; Ostrosky-Zeichner, L.; et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the infectious diseases society of america. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef]
- Shahin, J.; Allen, E.J.; Patel, K.; Muskett, H.; Harvey, S.E.; Edgeworth, J.; Kibbler, C.C.; Barnes, R.A.; Biswas, S.; Soni, N.; et al. Predicting invasive fungal disease due to candida species in non-neutropenic, critically ill, adult patients in United Kingdom critical care units. BMC Infect. Dis. 2016, 16, 480. [Google Scholar] [CrossRef]
- Giri, S.; Kindo, A.J. A review of candida species causing blood stream infection. Indian J. Med. Microbiol. 2012, 30, 270–278. [Google Scholar]
- Adrie, C.; Lugosi, M.; Sonneville, R.; Souweine, B.; Ruckly, S.; Cartier, J.C.; Garrouste-Orgeas, M.; Schwebel, C.; Timsit, J.F.; OUTCOMEREA study group. Persistent lymphopenia is a risk factor for icu-acquired infections and for death in icu patients with sustained hypotension at admission. Ann. Intensive. Care 2017, 7, 30. [Google Scholar] [CrossRef]
- Drewry, A.M.; Samra, N.; Skrupky, L.P.; Fuller, B.M.; Compton, S.M.; Hotchkiss, R.S. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014, 42, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Vulliamy, P.E.; Perkins, Z.B.; Brohi, K.; Manson, J. Persistent lymphopenia is an independent predictor of mortality in critically ill emergency general surgical patients. Eur. J. Trauma Emerg. Surg. 2016, 42, 755–760. [Google Scholar] [CrossRef] [PubMed]
- King, E.G.; Bauza, G.J.; Mella, J.R.; Remick, D.G. Pathophysiologic mechanisms in septic shock. Lab. Investig. 2014, 94, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Leon, C.; Ruiz-Santana, S.; Saavedra, P.; Almirante, B.; Nolla-Salas, J.; Alvarez-Lerma, F.; Garnacho-Montero, J.; Leon, M.A.; Group, E.S. A bedside scoring system (“candida score”) for early antifungal treatment in nonneutropenic critically ill patients with candida colonization. Crit. Care Med. 2006, 34, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; Sable, C.; Sobel, J.; Alexander, B.D.; Donowitz, G.; Kan, V.; Kauffman, C.A.; Kett, D.; Larsen, R.A.; Morrison, V.; et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, E.; Alvarez, F.J.; Martinez-Rafael, B.; Bustamante, J.; Bermejo-Martin, J.F.; Fierro, I.; Eiros, J.M.; Castrodeza, J.; Heredia, M.; Gomez-Herreras, J.I.; et al. Ventilator-associated pneumonia is an important risk factor for mortality after major cardiac surgery. J. Crit. Care 2012, 27, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Loubon, C.; Fernandez-Molina, M.; Carrascal-Hinojal, Y.; Fulquet-Carreras, E. Cardiac surgery-associated acute kidney injury. Ann. Card. Anaesth. 2016, 19, 687–698. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Adams, K.F., Jr.; Abraham, W.T.; Yancy, C.W.; Boscardin, W.J.; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA 2005, 293, 572–580. [Google Scholar] [CrossRef]
- Guzman, J.A.; Tchokonte, R.; Sobel, J.D. Septic shock due to candidemia: Outcomes and predictors of shock development. J. Clin. Med. Res. 2011, 3, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, H.; Yin, M.; Han, H.; Yue, J.F.; Zhang, F.; Shan, T.C.; Guo, H.P.; Wu, D.W. The differences in the epidemiology and predictors of death between candidemia acquired in intensive care units and other hospital settings. Intern. Med. 2015, 54, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Sbrana, F.; Sozio, E.; Bassetti, M.; Ripoli, A.; Pieralli, F.; Azzini, A.M.; Morettini, A.; Nozzoli, C.; Merelli, M.; Rizzardo, S.; et al. Independent risk factors for mortality in critically ill patients with candidemia on italian internal medicine wards. Intern. Emerg. Med. 2018, 13, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, C.; Cao, J.; Wu, X.; Zhang, L. Clinical characteristics and predictors of mortality in patients with candidemia: A six-year retrospective study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Hesstvedt, L.; Gaustad, P.; Muller, F.; Torp Andersen, C.; Brunborg, C.; Mylvaganam, H.; Leiva, R.A.; Erik Berdal, J.; Egil Ranheim, T.; Johnsen, B.O.; et al. The impact of age on risk assessment, therapeutic practice and outcome in candidemia. Infect. Dis. 2019, 51, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martinez, A.; Vicente-Lopez, N.; Sanchez-Romero, I.; Padilla, B.; Merino-Amador, P.; Garnacho-Montero, J.; Ruiz-Camps, I.; Montejo, M.; Salavert, M.; Mensa, J.; et al. Epidemiology and prognosis of candidaemia in elderly patients. Mycoses 2017, 60, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, T.; Nucci, M.; Mendonca, J.S.; Martinez, R.; Brito, L.R.; Silva, N.; Moretti, M.L.; Salomao, R.; Colombo, A.L. Epidemiology and predictors of a poor outcome in elderly patients with candidemia. Int. J. Infect. Dis. 2012, 16, e442–e447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchiesi, F.; Orsetti, E.; Mazzanti, S.; Trave, F.; Salvi, A.; Nitti, C.; Manso, E. Candidemia in the elderly: What does it change? PLoS ONE 2017, 12, e0176576. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Yoshimura, Y.; Suido, Y.; Shimizu, H.; Ide, K.; Sugiyama, Y.; Matsuno, K.; Nakajima, H. Mortality and risk factor analysis for candida blood stream infection: A multicenter study. J. Infect. Chemother. 2019, 25, 341–345. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Diaz-Martin, A.; Canton-Bulnes, L.; Ramirez, P.; Sierra, R.; Arias-Verdu, D.; Rodriguez-Delgado, M.; Loza-Vazquez, A.; Rodriguez-Gomez, J.; Gordon, M.; et al. Initial antifungal strategy reduces mortality in critically ill patients with candidemia: A propensity score-adjusted analysis of a multicenter study. Crit. Care Med. 2018, 46, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Keighley, C.; Chen, S.C.; Marriott, D.; Pope, A.; Chapman, B.; Kennedy, K.; Bak, N.; Underwood, N.; Wilson, H.L.; McDonald, K.; et al. Candidaemia and a risk predictive model for overall mortality: A prospective multicentre study. BMC Infect. Dis. 2019, 19, 445. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Kim, S.E.; Kim, U.J.; Jang, H.C.; Park, K.H.; Shin, J.H.; Jung, S.I. Clinical characteristics and risk factors for mortality in adult patients with persistent candidemia. J. Infect. 2017, 75, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.; Csonka, K.; Jacobs, C.; Vagvolgyi, C.; Nosanchuk, J.D.; Netea, M.G.; Gacser, A. Candida albicans and candida parapsilosis induce different t-cell responses in human peripheral blood mononuclear cells. J. Infect. Dis. 2013, 208, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Hernandez-Santos, N.; Peterson, A.C. Il-17 signaling in host defense against candida albicans. Immunol. Res. 2011, 50, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Unsinger, J.; Burnham, C.A.; McDonough, J.; Morre, M.; Prakash, P.S.; Caldwell, C.C.; Dunne, W.M., Jr.; Hotchkiss, R.S. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J. Infect. Dis. 2012, 206, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Ahmadi, A.; Kheirali, E.; Hossein Yadegari, M.; Bayat, M.; Shajiei, A.; Amini, A.A.; Ashrafi, S.; Abolhassani, M.; Faezi, S.; et al. Systemic infection with candida albicans in breast tumor bearing mice: Cytokines dysregulation and induction of regulatory t cells. J. Mycol. Méd. 2019, 29, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Sutmuller, R.; Hermann, C.; Van der Graaf, C.A.; Van der Meer, J.W.; van Krieken, J.H.; Hartung, T.; Adema, G.; Kullberg, B.J. Toll-like receptor 2 suppresses immunity against candida albicans through induction of il-10 and regulatory t cells. J. Immunol. 2004, 172, 3712–3718. [Google Scholar] [CrossRef]
- Pfaller, M.; Neofytos, D.; Diekema, D.; Azie, N.; Meier-Kriesche, H.U.; Quan, S.P.; Horn, D. Epidemiology and outcomes of candidemia in 3648 patients: Data from the prospective antifungal therapy (path alliance(r)) registry, 2004-2008. Diagn. Microbiol. Infect. Dis. 2012, 74, 323–331. [Google Scholar] [CrossRef]
- Kollef, M.; Micek, S.; Hampton, N.; Doherty, J.A.; Kumar, A. Septic shock attributed to candida infection: Importance of empiric therapy and source control. Clin. Infect. Dis. 2012, 54, 1739–1746. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Ansaldi, F.; Merelli, M.; Trucchi, C.; De Pascale, G.; Diaz-Martin, A.; Luzzati, R.; Rosin, C.; Lagunes, L.; et al. A multicenter study of septic shock due to candidemia: Outcomes and predictors of mortality. Intensive Care Med. 2014, 40, 839–845. [Google Scholar] [CrossRef]
- Ahmed, A.; Azim, A.; Baronia, A.K.; Marak, K.R.; Gurjar, M. Risk prediction for invasive candidiasis. Indian J. Crit. Care Med. 2014, 18, 682–688. [Google Scholar] [PubMed] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of america. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Cornely, O.A.; Donnelly, J.P.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. Escmid* guideline for the diagnosis and management of candida diseases 2012: Developing european guidelines in clinical microbiology and infectious diseases. Clin. Microbiol. Infect. 2012, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]



| Variable | Total (n = 296) | Survivors (n = 181) | Non-Survivors (n = 115) | p-Value |
|---|---|---|---|---|
| Age | 63 ± 17.4 | 60.0 ± 19.0 | 68.0 ± 13.0 | <0.001 |
| Sex (% male) | 177 (59.4) | 102 (56.4) | 74 (64.3) | 0.172 |
| Comorbidities | ||||
| Alcohol intake | 23 (7.8) | 12 (6.6) | 11 (9.6) | 0.358 |
| COPD | 33 (11.2) | 20 (11.0) | 13 (11.3) | 0.972 |
| Diabetes | 61 (20.6) | 32 (17.7) | 29 (25.2) | 0.124 |
| Renal Disease | 49 (16.6) | 28 (15.5) | 20 (17.4) | 0.662 |
| Cirrhosis | 10 (3.4) | 5 (2.8) | 5 (4.3) | 0.518 |
| HF | 56 (18.9) | 29 (16.0) | 27 (23.5) | 0.110 |
| Dementia | 6 (2.0) | 5 (2.8) | 1 (0.9) | 0.260 |
| Hospital Admission | ||||
| Source Infection | ||||
| CVC | 146 (49.3) | 81 (44.8) | 65 (56.5) | 0.004 |
| Abdominal | 30 (10.1) | 15 (8.3) | 15 (13.0) | |
| TPN | 82 (27.7) | 35 (19.3) | 46 (40.0) | <0.001 |
| Other | 120 (40.5) | 85 (47.0) | 35 (30.4) | |
| Surgery | 126 (42.6) | 72 (39.8) | 54 (47.0) | 0.223 |
| PMV | 123 (41.6) | 58 (32.0) | 65 (56.5) | <0.001 |
| RRT | 56 (18.9) | 28 (15.5) | 28 (24.3) | 0.060 |
| Septic Shock | 146 (49.3) | 70 (38.7) | 75 (65.2) | <0.001 |
| Candida Score | 2.0 ± 1.9 | 2.0 ± 1.8 | 3.0 ± 1.9 | <0.001 |
| Ostrosky Score | 2.4 ± 2.2 | 1.9 ± 2.0 | 3.0 ± 2.2 | <0.001 |
| Lymphocyte count at diagnosis, ×109 cells/L, median (IQR) | 0.952 (1.588–0.462) | 0.998 (1.746–0.535) | 0.778 (1.364–0.410) | 0.045 |
| Lymphocyte count by day 2, ×109 cells/L, median (IQR) | 0.900 (1.688–0.480) | 0.979 (1.745–0.543) | 0.680 (1.372–0.404) | 0.038 |
| Lymphocyte count by day 3, ×109 cells/L, median (IQR) | 0.935 (1.567–0.452) | 1.058 (1.614–0.613) | 0.747 (1.450–0.310) | 0.010 |
| Lymphocyte count by day 4, ×109 cells/L, median (IQR) | 0.922 (1.633–0.513) | 1.078 (1.803–0.581) | 0.753 (1.211–0.418) | 0.011 |
| Lymphocyte count by day five, ×109 cells/L, median (IQR) | 0.947 (1.588–0.526) | 1.058 (1.750–0.611) | 0.858 (1.203–0.478) | 0.050 |
| Echinocandins | 122 (41.2) | 76 (42.0) | 46 (40.0) | 0.735 |
| Causative Organism | ||||
| C. albicans | 179 (60.4) | 96 (62.3) | 83 (58.5) | 0.905 |
| C. parapsilosis | 40 (13.5) | 21 (13.6) | 17 (12.0) | 0.525 |
| C. glabrata | 36 (12.5) | 20 (13.0) | 16 (11.3) | 0.621 |
| C. tropicalis | 29 (9.8) | 11 (7.1) | 18 (12.7) | 0.076 |
| C. lusitaniae | 12 (4.1) | 4 (2.6) | 8 (5.6) | 0.254 |
| Univariate Analysis | ||
|---|---|---|
| OR (95% CI) | p-Value | |
| Age | 1.030 (1.01–1.05) | <0.001 |
| PMV | 2.734 (1.69–4.43) | <0.001 |
| Septic shock | 2.946 (1.81–4.80) | <0.001 |
| Candida Score | 1.282 (1.13–1.45) | <0.001 |
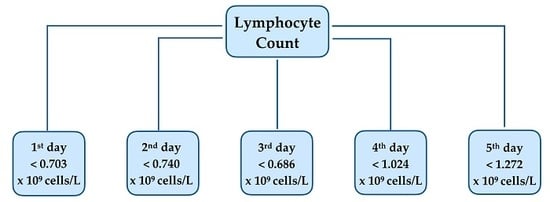
| Lymphocyte count (diagnosis) (<0.703 × 109 cells/L) | 3.11 (1.62–5.98) | 0.001 |
| Lymphocyte count (day 2) (<0.740 × 109 cells/L) | 2.108 (1.16–3.83) | 0.014 |
| Lymphocyte count (day 3) (<0.686 × 109 cells/L) | 2.213 (1.23–3.97) | 0.008 |
| Lymphocyte count (day 4) (<1.024 × 109 cells/L) | 2.737 (1.40–5.36) | 0.003 |
| Lymphocyte count (day 5) (<1.272 × 109 cells/L) | 3.435 (1.60–7.38) | 0.002 |
| Multivariable Analysis at Diagnosis | Multivariable Analysis at Day 5 | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| PMV | 3.07 (1.44–6.51) | 0.004 | 3.98 (1.77–8.95) | 0.001 |
| Age | 1.49 (1.02–1.08) | 0.001 | 1.05 (1.01–1.08) | 0.012 |
| Lymphocyte count (diagnosis) < 0.703 × 109 cells/L | 5.01 (2.29–10.93) | 0.002 | ||
| Lymphocyte count (day 5) < 1.272 × 109 cells/L | 3.27 (1.24–8.62) | 0.016 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Loubon, C.; Cano-Hernández, B.; Poves-Alvarez, R.; Muñoz-Moreno, M.F.; Román-García, P.; Balbás-Alvarez, S.; de la Varga-Martínez, O.; Gómez-Sánchez, E.; Gómez-Pesquera, E.; Lorenzo-López, M.; et al. The Overlooked Immune State in Candidemia: A Risk Factor for Mortality. J. Clin. Med. 2019, 8, 1512. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8101512
Ortega-Loubon C, Cano-Hernández B, Poves-Alvarez R, Muñoz-Moreno MF, Román-García P, Balbás-Alvarez S, de la Varga-Martínez O, Gómez-Sánchez E, Gómez-Pesquera E, Lorenzo-López M, et al. The Overlooked Immune State in Candidemia: A Risk Factor for Mortality. Journal of Clinical Medicine. 2019; 8(10):1512. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8101512
Chicago/Turabian StyleOrtega-Loubon, Christian, Beatriz Cano-Hernández, Rodrigo Poves-Alvarez, María Fe Muñoz-Moreno, Patricia Román-García, Sara Balbás-Alvarez, Olga de la Varga-Martínez, Esther Gómez-Sánchez, Estefanía Gómez-Pesquera, Mario Lorenzo-López, and et al. 2019. "The Overlooked Immune State in Candidemia: A Risk Factor for Mortality" Journal of Clinical Medicine 8, no. 10: 1512. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8101512