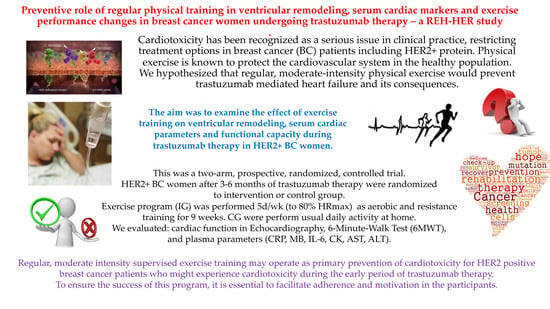
The Preventive Role of Regular Physical Training in Ventricular Remodeling, Serum Cardiac Markers, and Exercise Performance Changes in Breast Cancer in Women Undergoing Trastuzumab Therapy—An REH-HER Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Participants
2.3. Randomization and Blinding Procedures
2.4. Measurement Scheme
2.5. Physical Exercise Program
2.6. Usual Care
2.7. Outcome measures
2.8. Measurements
2.8.1. Anthropometric Measurements
2.8.2. Measurements of Cardiac Function
2.8.3. Physical Capacity Tests
2.8.4. Measurements of Plasma Parameters
2.9. Statistical Analysis
3. Results
3.1. Study Group
3.2. Analysis of the Echocardiography Results
3.3. Results of the Physical Capacity Test
3.4. Analysis of the Blood Parameter Results
3.5. Correlations between Study Parameters
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, N.; Singh, S.V.; Malvi, P.; Chaube, B.; Athavale, D.; Vanuopadath, M.; Nair, B.; Bhat, M.K. Strategy to enhance efficacy of doxorubicin in solid tumor cells by methyl-β-cyclodextrin: Involvement of p53 and Fas receptor ligand complex. Sci. Rep. 2015, 5, 11853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, N.; Bhattacharya, S.; Steele, R.; Ray, R.B. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOC3. Oncotarget 2016, 7, 58595–58605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loung, Ch.Y.; Fernando, W.; Rupasinghe, H.P.V.; Hoskin, D.W. Apple Peel Flavonoid Fraction 4 Suppresses Breast Cancer Cell Growth by Cytostatic and Cytotoxic Mechanisms. Molecules 2019, 24, 3335. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, N.; Steele, R.; Isbell, T.S.; Philips, N.; Ray, R.B. Bitter melon extract inhibits breast cancer growth in preclinical model by inducing autophagic cell death. Oncotarget 2017, 8, 66226–66236. [Google Scholar] [CrossRef] [Green Version]
- Dobovisek, L.; Krstanovic, F.; Borstnar, S.; Debeljak, N. Cannabinoids and Hormone Receptor-Positive Breast Cancer Treatment. Cancers 2020, 12, 525. [Google Scholar] [CrossRef] [Green Version]
- Binkley, J.M.; Harris, S.R.; Levangie, P.K.; Pearl, M.; Guglielmino, J.; Kraus, V.; Rowden, D. Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer 2012, 118, 2207–2216. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in her2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Pillai, U.S.; Kayal, S.; Cyriac, S.; Nisha, Y.; Dharanipragada, K.; Kamalanathan, S.K.; Halanaik, D.; Kumar, N.; Madasamy, P.; Muniswamy, D.K.; et al. Late Effects of Breast Cancer Treatment and Outcome after Corrective Interventions. Asian Pac. J. Cancer Prev. 2019, 20, 2673–2679. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Gernaat, S.A.M.; Ho, P.J.; Rijnberg, N.; Emaus, M.J.; Baak, L.M.; Hartman, M.; Grobbee, D.E.; Verkooijen, H.M. Risk of death from cardiovascular disease following breast cancer: A systematic review. Breast Cancer Res. Treat. 2017, 164, 537–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, B.T.; Varga, Z.V.; Wu, W.J.; Pacher, P. Trastuzumab cardiotoxicity: From clinical trials to experimental studies. Br. J. Pharmacol. 2017, 174, 3727–3748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Zeglinski, M.; Ludke, A.; Jassal, D.S.; Singal, P.K. Trastuzumab-induced cardiac dysfunction: A ‘dual-hit’. Exp. Clin. Cardiol. 2011, 16, 70–74. [Google Scholar] [PubMed]
- Barish, R.; Gates, E.; Barac, A. Trastuzumab-Induced Cardiomyopathy. Cardiol. Clin. 2019, 37, 407–418. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovas. Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef]
- Schwartz, R.G.; Jain, D.; Storozynsky, E. Traditional and novel methods to assess and prevent chemotherapy-related cardiac dysfunction noninvasively. J. Nucl. Cardiol. 2013, 20, 443–464. [Google Scholar] [CrossRef] [Green Version]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Al-Mallah, M.H.; Sakr, S.; Al-Qunaibet, A. Cardiorespiratory fitness and cardiovascular disease prevention: An update. Curr. Atheroscler. Rep. 2018, 20, 1. [Google Scholar] [CrossRef]
- Hardcastle, S.J.; Galliott, M.; Lynch, B.M.; Nguyen, N.H.; Cohen, P.A.; Mohan, G.R.; Johansen, N.J.; Saunders, C. ‘If I Had Someone Looking Over My Shoulder…’: Exploration of Advice Received and Factors Influencing Physical Activity Among Non-metropolitan Cancer Survivors. Int. J. Behav. Med. 2019, 26, 551–561. [Google Scholar] [CrossRef]
- Shallwani, S.M.; King, J.; Thomas, R.; Thevenot, O.; De Angelis, G.; Aburub, A.S.; Brosseau, L. Methodological quality of clinical practice guidelines with physical activity recommendations for people diagnosed with cancer: A systematic critical appraisal using the AGREE II tool. PLoS ONE 2019, 14, e0214846. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Speck, R.M.; Courneya, K.S.; Mâsse, L.C.; Duval, S.; Schmitz, K.H. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2010, 4, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.I.; Scherer, R.W.; Snyder, C.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O.; Gotay, C.C.; Snyder, C. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst. Rev. 2012, 8, CD008465. [Google Scholar] [CrossRef]
- Furmaniak, A.C.; Menig, M.; Markes, M.H. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst. Rev. 2016, 9, CD005001. [Google Scholar] [CrossRef]
- Lee, J. A Meta-analysis of the Association Between Physical Activity and Breast Cancer Mortality. Cancer Nurs. 2019, 42, 271–285. [Google Scholar] [CrossRef]
- Nair, N.; Gongora, E. Heart failure in chemotherapy-related cardiomyopathy: Can exercise make a difference? BBA Clin. 2016, 6, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Kapila, A.K.; Hamdi, M.; Patel, A. Clinicians Should Actively Promote Exercise in Survivors of Breast Cancer. Clin. Breast Cancer 2018, 18, e747–e749. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Doyle, C.; Kushi, L.H.; Byers, T.; Courneya, K.S.; Demark-Wahnefried, W.; Grant, B.; McTiernan, A.; Rock, C.L.; Thompson, C.; Gansler, T.; et al. 2006 Nutrition, Physical Activity and Cancer Survivorship Advisory Committee; American Cancer Society. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J. Clin. 2006, 56, 323–353. [Google Scholar] [CrossRef] [Green Version]
- Amireault, S.; Godin, G.; Lacombe, J.; Sabiston, C.M. The use of the Godin-Shepard Leisure-Time Physical Activity Questionnaire in oncology research: A systematic review. BMC Med. Res. Methodol. 2015, 12, 15–60. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Vogt, L.; Thiel, C.; Jäger, E.; Banzer, W. Validity of the six- minute walk test in cancer patients. Int. J. Sports Med. 2013, 34, 631–636. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. 2019. Available online: https://www.R-project.org/ (accessed on 5 July 2019).
- Hansen, D.; Dendale, P.; Coninx, K.; Vanhees, L.; Piepoli, M.F.; Niebauer, J.; Cornelissen, V.; Pedretti, R.; Geurts, E.; Ruiz, G.R.; et al. The European Association of Preventive Cardiology Exercise Prescription in Everyday Practice and Rehabilitative Training (EXPERT) Tool: A Digital Training and Decision Support System for Optimized Exercise Prescription in Cardiovascular Disease. Concept, Definitions and Construction Methodology. Eur. J. Prev. Cardiol. 2017, 24, 1017–1031. [Google Scholar] [CrossRef]
- Hansen, D.; Niebauer, J.; Cornelissen, V.; Barna, O.; Neunhäuserer, D.; Stettler, C.; Tonoli, C.; Greco, E.; Fagard, R.; Coninx, K.; et al. Exercise Prescription in Patients with Different Combinations of Cardiovascular Disease Risk Factors: A Consensus Statement from the EXPERT Working Group. Sports Med. 2018, 48, 1781–1797. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Mackey, J.R.; Thompson, R.B.; Jones, L.W.; Paterson, D.I. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin. Cancer Res. 2009, 15, 4963–4967. [Google Scholar] [CrossRef] [Green Version]
- Ciani, O.; Piepoli, M.; Smart, N.; Uddin, J.; Walker, S.; Warren, F.C.; Zwisler, A.D.; Davos, C.H.; Taylor, R.S. Validation of Exercise Capacity as a Surrogate Endpoint in Exercise-Based Rehabilitation for Heart Failure: A Meta-Analysis of Randomized Controlled Trials. JACC Heart Fail. 2018, 6, 596–604. [Google Scholar] [CrossRef]
- Waart, H.; Stuiver, M.M.; van Harten, W.H.; Geleijn, E.; Kieffer, J.M.; Buffart, L.M.; de Maaker-Berkhof, M.; Boven, E.; Schrama, J.; Geenen, M.M.; et al. Effect of Low-Intensity Physical Activity and Moderate- To High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J. Clin. Oncol. 2015, 10, 1918–1927. [Google Scholar] [CrossRef] [Green Version]
- Rajappa, M.; Sharma, A. Biomarkers of cardiac injury: An update. Angiology 2005, 56, 677–691. [Google Scholar] [CrossRef]
- Frauenfelder, H.; McMahon, B.H.; Austin, R.H.; Chu, K.; Groves, J.T. The role of structure, energy landscape, dynamics, and allostery in the enzymatic function of myoglobin. Proc. Natl. Acad. Sci. USA 2001, 98, 2370–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassaf, T.; Flogel, U.; Drexhage, C.; Hendgen-Cotta, U.; Kelm, M.; Schrader, J. Nitrite reductase function of deoxymyoglobin: Oxygen sensor and regulator of cardiac energetics and function. Circ. Res. 2007, 100, 1749–1754. [Google Scholar] [CrossRef]
- Kreutzer, U.; Jue, T. Role of myoglobin as a scavenger of cellular NO in myocardium. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H985–H991. [Google Scholar] [CrossRef]
- Wollert, K.C.; Drexler, H. The role of interleukin-6 in the failing heart. Heart Fail. Rev. 2001, 6, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zheng, R.; Hu, S.; Ma, Y.; Choudhry, M.A.; Messina, J.L.; Rue, L.W.; Bland, K.I.; Chaudry, I.H. Mechanism of cardiac depression after trauma-hemorrhage: Increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2183–H2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, N.; Mojet, M.H.; Latchman, D.S.; Marber, M.S.; Duchen, M.R.; Heads, R.J. IL-6 induces PI 3-kinase and nitric oxide-dependent protection and preserves mitochondrial function in cardiomyocytes. Cardiovasc. Res. 2006, 69, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. 2002, 16, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Fischer, C.P. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, M.; Nakaji, S.; Suzuki, K.; Sugawara, K.; Sato, K. Break point of serum creatine kinase release after endurance exercise. J. Appl. Physiol. 2002, 93, 1280–1286. [Google Scholar] [CrossRef]
- Brancaccio, P.; Limongelli, F.M.; Maffulli, N. Monitoring of serum enzymes in sport. Br. J. Sports Med. 2006, 40, 96–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevion, S.; Moran, D.S.; Heled, Y.; Heled, Y.; Shani, Y.; Regev, G.; Abbou, B.; Berenshtein, E.; Stadtman, E.R.; Epstein, Y.; et al. Plasma antioxidant status and cell injury after severe physical exercise. Proc. Natl. Acad. Sci. USA 2003, 100, 5119–5123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, H.K.; Vincent, K.R. The effect of training status on the plasma creatine kinase response, soreness and muscle function following resistance exercise. Int. J. Sports Med. 1997, 18, 431–437. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, W.R.; Poterucha, J.J. Evaluation of elevated liver enzymes. Clin. Liver Dis. 2012, 16, 183–198. [Google Scholar] [CrossRef]
- Chamera, T.; Spieszny, M.; Klocek, T.; Kostrzewa-Nowak, D.; Nowak, R.; Lachowicz, M.; Buryta, R.; Cięszczyk, P. Could biochemical liver profile help to assess metabolic response to aerobic effort in athletes? J. Strength Cond. Res. 2014, 28, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, A.; Kostrzewa-Nowak, D.; Buryta, R.; Nowak, R. Blood Biomarkers of Recovery Efficiency in Soccer Players. Int. J. Environ. Res. Public Health 2019, 16, 3279. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Intervention Group (IG) n = 26 | Control Group (CG) n = 21 | PIG vs. CG |
|---|---|---|---|
| Anthropometrical data (Mean ± SD) | |||
| Age (years) | 54.44 ± 6.29 | 54.64 ± 5.26 | 0.961 |
| Weight (kg) | 65.69 ± 5.25 | 68.73 ± 2.53 | 0.182 |
| Height (m) | 1.65 ± 0.04 | 1.68 ± 0.04 | 0.937 |
| BMI (kg/m2) | 24.35 ± 2.8 | 25.35 ± 1.89 | 0.429 |
| Medical history (n (%)) | |||
| Stage of cancer | |||
| IB | 2 (7.7%) | 0 | - |
| IIA | 9 (34.6%) | 10 (47.6%) | - |
| IIB | 12 (46.1%) | 11 (52.4%) | - |
| IIIA | 3 (11.5%) | 0 | - |
| Surgical treatment | |||
| Side of operated breast - Left | 15 (57.7%) | 11 (52.4%) | - |
| Side of operated breast - Right | 11 (42.3%) | 10 (47.6%) | -- |
| BCT | 13 (50%) | 14 (66.6) | |
| Mastectomy | 5 (19.2%) | 2 (9.5%) | - |
| Mastectomy with reconstruction | 8 (30.7%) | 5 (23.8%) | - |
| Additional oncological treatment | |||
| Previous anthracycline treatment | 20 (76.9%) | 18 (85.7%) | - |
| Previous radiotherapy | 16 (61.5%) | 13 (61.9%) | - |
| Hormonal therapy | 18 (69.2%) | 16 (76.2%) | - |
| Other comorbidities | |||
| Diabetes | 1 (3.8%) | 0 | - |
| Dyslipidemia | 4 (15.4%) | 2 (9.5%) | - |
| Hypertension | 5 (19.2%) | 3 (14.3%) | - |
| Previous heart failure | 1 (3.8%) | 0 | - |
| Additional data | |||
| History of smoking | 6 (23%) | 2 (9.5%) | - |
| NYHA functional class - I | 22 (84.6%) | 20 (95.2%) | - |
| NYHA functional class - II | 4 (15.4%) | 1 (4.7%) | - |
| Parameter | Time | Intervention Group (IG) | Control Group (CG) | PIG vs. UG | ||
|---|---|---|---|---|---|---|
| Mean ± SD | PT1vsT2 | Mean ± SD | PT1vsT2 | |||
| LVEF (%) | T1 | 65.69 ± 5.02 | 0.143 | 63.9 ± 2.72 | 0.009 * | 0.126 |
| T2 | 64.88 ± 5.81 | 59.82 ± 4.02 | 0.003 * | |||
| GLS (%) | T1 | 17.5 ± 2.5 | 0.946 | 17.3 ± 2.5 | 0.782 | 0.746 |
| T2 | 17.6 ± 2.5 | 16.8 ± 2.5 | 0.422 | |||
| LAVI (mL/m) | T1 | 24.6 ± 2.5 | 0.244 | 23.8 ± 2.5 | 0.864 | 0.624 |
| T2 | 26.2 ± 2.5 | 24.2 ± 2.5 | 0.484 | |||
| RVEF (%) | T1 | 53.3 ± 6.5 | 0.488 | 52.8 ± 7.5 | 0.788 | 0.812 |
| T2 | 54.2 ± 5.2 | 52.1 ± 6.6 | 0.575 | |||
| TAPSE (mm) | T1 | 20.3 ± 2.5 | 0.764 | 21 ± 3.1 | 0.824 | 0.686 |
| T2 | 21.2 ± 2.2 | 20.1 ± 3.6 | 0.755 | |||
| E/A | T1 | 1.4 ± 0.5 | 0.75 | 1.4 ± 0.6 | 0.711 | 0.944 |
| T2 | 1.5 ± 0.5 | 1.5 ± 0.4 | 0.998 | |||
| HR (bpm) | T1 | 65.3 ± 5.5 | 0.675 | 65.3 ± 6.4 | 0.712 | 0.997 |
| T2 | 64.6 ± 8.7 | 66.2 ± 7.02 | 0.356 | |||
| SBP (mmHg) | T1 | 127.3 ± 7.8 | 0.334 | 125.2 ± 6.6 | 0.914 | 0.512 |
| T2 | 123.8 ± 10.3 | 126.5 ± 7.3 | 0.588 | |||
| DBP (mmHg) | T1 | 83.2 ± 7.2 | 0.432 | 81.1 ± 6.4 | 0.856 | 0.634 |
| T2 | 81.2 ± 4.1 | 82.7 ± 4.5 | 0.912 | |||
| Parameter | Time | Intervention Group (IG) | Control Group (CG) | PIGvs. UG | ||
|---|---|---|---|---|---|---|
| Mean ± SD | PT1vsT2 | Mean ± SD | PT1vsT2 | |||
| 6MWD (m) | T1 | 448.7 ± 50.06 | 0.312 | 441.6 ± 24.88 | 0.042 * | 0.314 |
| T2 | 449.6 ± 55.33 | 416 ± 31.68 | 0.089 | |||
| MET Unit | T1 | 3.14 ± 0.24 | 0.077 | 3.11 ± 0.12 | 0.081 | 0.211 |
| T2 | 3.19 ± 0.26 | 2.93 ± 0.15 | 0.041 * | |||
| Borg scale (point) | T1 | 1.62 ± 0.72 | 0.773 | 1.82 ± 0.6 | 0.149 | 0.398 |
| T2 | 1.69 ± 0.7 | 2.09 ± 0.3 | 0.069 | |||
| Parameter | Time | Intervention Group (IG) | Control Group (CG) | PIG vs. UG | ||
|---|---|---|---|---|---|---|
| Mean ± SD | PT1vsT2 | Mean ± SD | PT1vsT2 | |||
| hsCRP (mg/L) | T1 | 2.2 ± 2.24 | 0.832 | 3.1 ± 2.4 | 0.788 | 0.565 |
| T2 | 3.5 ± 2.7 | 3.2 ± 3.61 | 0.988 | |||
| MYO (pg/mL) | T1 | 26.04 ± 15.24 | 0.877 | 24.78 ± 5.89 | 0.412 | 0.517 |
| T2 | 28.52 ± 18.67 | 22.54 ± 4.3 | 0.219 | |||
| IL-6 (pg/mL) | T1 | 4.45 ± 11.81 | 0.069 | 3.69 ± 12.92 | 0.241 | 0.133 |
| T2 | 6.29 ± 12.28 | 2.61 ± 11.5 | 0.091 | |||
| CK (U/L) | T1 | 29.36 ± 32.34 | 0.092 | 32.81 ± 15.63 | 0.841 | 0.421 |
| T2 | 43 ± 35.96 | 32.55 ± 17.2 | 0.138 | |||
| CK-MB (U/L) | T1 | 4.61 ± 2.84 | 0.532 | 4.47 ± 5.42 | 0.57 | 0.71 |
| T2 | 5.54 ± 4.13 | 3.75 ± 6.17 | 0.317 | |||
| AST (U/L) | T1 | 23.91 ± 13.18 | 0.7 | 26.02 ± 7.52 | 0.629 | 0.144 |
| T2 | 27.16 ± 9.41 | 24.76 ± 8.07 | 0.691 | |||
| ALT (U/L) | T1 | 10.22 ± 5.8 | 0.997 | 11.28 ± 5.42 | 0.998 | 0.489 |
| T1 | 10.21 ± 5.98 | 11.63 ± 5.62 | 0.374 | |||
| Assessment Time | Group | Parameters | |||||
|---|---|---|---|---|---|---|---|
| BMI | 6MWD | Borg Scale | LVEF | MET Units | |||
| T1 | IG | CK-MB | r = 0.707 * | r = −0.694 * | r = 0.536 * | r = −0.568 * | r = −0.694 * |
| T2 | r = 0.518 * | r = −0.286 | r = 0.286 | r = −0.279 | r = −0.276 | ||
| T1 | CG | CK-MB | - | r = −0.436 | - | - | r = −0.436 |
| T2 | - | r = −0.764 * | - | - | r = −0.764 * | ||
| T1 | IL-6 | - | r = −0.764 * | - | - | r = −0.764 * | |
| T2 | - | r = −0.545 | - | - | r = −0.545 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojan, K.; Procyk, D.; Horyńska-Kęstowicz, D.; Leporowska, E.; Litwiniuk, M. The Preventive Role of Regular Physical Training in Ventricular Remodeling, Serum Cardiac Markers, and Exercise Performance Changes in Breast Cancer in Women Undergoing Trastuzumab Therapy—An REH-HER Study. J. Clin. Med. 2020, 9, 1379. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm9051379
Hojan K, Procyk D, Horyńska-Kęstowicz D, Leporowska E, Litwiniuk M. The Preventive Role of Regular Physical Training in Ventricular Remodeling, Serum Cardiac Markers, and Exercise Performance Changes in Breast Cancer in Women Undergoing Trastuzumab Therapy—An REH-HER Study. Journal of Clinical Medicine. 2020; 9(5):1379. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm9051379
Chicago/Turabian StyleHojan, Katarzyna, Danuta Procyk, Dorota Horyńska-Kęstowicz, Ewa Leporowska, and Maria Litwiniuk. 2020. "The Preventive Role of Regular Physical Training in Ventricular Remodeling, Serum Cardiac Markers, and Exercise Performance Changes in Breast Cancer in Women Undergoing Trastuzumab Therapy—An REH-HER Study" Journal of Clinical Medicine 9, no. 5: 1379. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm9051379