pH-Triggered Interfacial Interaction of Kaolinite/Chitosan Nanocomposites with Anionic Azo Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of NCPs
2.2.2. Thermogravimetric Analysis (TGA)
2.2.3. Adsorption of RR
2.2.4. Desorption of RR
3. Results and Discussions
3.1. Effect of Operating Conditions
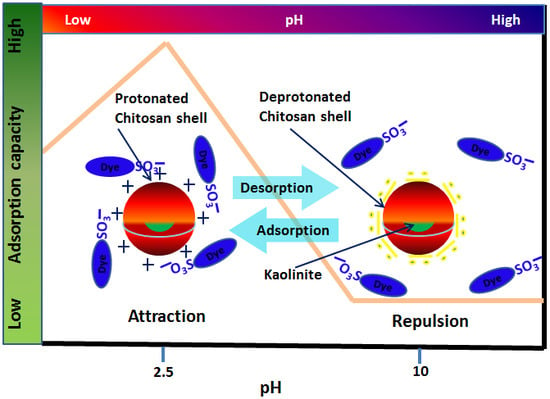
3.1.1. Effect of pH
3.1.2. Effect of Contact Time
3.1.3. Effect of Initial RR Concentration
3.2. Mechanistic View of Adsorption Performance
3.3. Thermogravimetric Analysis (TGA)
3.4. Surface Charge Calculation
3.5. Demonstration of a Reversible Interaction
3.6. Comparison of Adsorption Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhattacharya, M.; Chen, B. Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef]
- Bréchet, Y.; Cavaillé, J.Y.; Chabert, E.; Chazeau, L.; Dendievel, R.; Flandin, L.; Gauthier, C. Polymer based nanocomposites: Effect of filler-filler and filler-matrix interactions. Adv. Eng. Mater. 2001, 3, 571–577. [Google Scholar] [CrossRef]
- Mittal, V. Polymer Layered Silicate Nanocomposites: A Review. Materials 2009, 2, 992–1057. [Google Scholar] [CrossRef] [Green Version]
- Paul, D.; Robeson, L. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Kanchana, V.; Gomathi, T.; Geetha, V.; Sudha, P. Adsorption analysis of Pb (II) by nanocomposites of chitosan with methyl cellulose and clay. Der Pharm. Lett. 2012, 4, 1071–1079. [Google Scholar]
- Zhu, H.-Y.; Jiang, R.; Xiao, L. Adsorption of an anionic azo dye by chitosan/kaolin/γ-Fe2O3 composites. Appl. Clay Sci. 2010, 48, 522–526. [Google Scholar] [CrossRef]
- Chen, I.P.; Kan, C.C.; Futalan, C.M.; Calagui, M.J.C.; Lin, S.S.; Tsai, W.C.; Wan, M.W. Batch and fixed bed studies: Removal of copper (II) using chitosan-coated kaolinite beads from aqueous solution. Sustain. Environ. Res. 2015, 25, 73–81. [Google Scholar]
- Puvaneswari, N.; Muthukrishnan, J.; Gunasekaran, P. Toxicity assessment and microbial degradation of azo dyes. J. Exp. Boil. 2006, 44, 618–626. [Google Scholar]
- Joshi, M.; Bansal, R.; Purwar, R. Colour removal from textile effluents. Indian J. Fibre Text. Res. 2004, 29, 239–259. [Google Scholar]
- Teong, L.; Hanafiah, M.A.K.M.; Ngah, W.W. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Enhanced abilities of highly swollen chitosan beads for color removal and tyrosinase immobilization. J. Hazard. Mater. 2001, 81, 167–177. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Popuri, S.R.; Vijaya, Y.; Boddu, V.M.; Abburi, K. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour. Technol. 2009, 100, 194–199. [Google Scholar] [CrossRef]
- Hasan, S.; Ghosh, T.K.; Viswanath, D.S.; Boddu, V.M. Dispersion of chitosan on perlite for enhancement of copper(II) adsorption capacity. J. Hazard. Mater. 2008, 152, 826–837. [Google Scholar] [CrossRef]
- Chiou, M.-S.; Li, H.-Y. Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J. Hazard. Mater. 2002, 93, 233–248. [Google Scholar] [CrossRef]
- Wan, M.-W.; Kan, C.-C.; Rogel, B.D.; Dalida, M.L.P. Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr. Polym. 2010, 80, 891–899. [Google Scholar] [CrossRef]
- Kalyani, S.; Priya, J.A.; Rao, P.S.; Krishnaiah, A. Removal of Copper and Nickel from Aqueous Solutions Using Chitosan Coated on Perlite as Biosorbent. Sep. Sci. Technol. 2005, 40, 1483–1495. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Randolph, A.J.; Smith, E.D. Removal of copper (II) and nickel (II) ions from aqueous solutions by a composite chitosan biosorbent. Sep. Sci. Technol. 2008, 43, 1365–1381. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad, A.L.; Hameed, B. Adsorption of reactive dye onto cross-linked chitosan/oil palm ash composite beads. Chem. Eng. J. 2008, 136, 164–172. [Google Scholar] [CrossRef]
- Vijaya, Y.; Popuri, S.R.; Boddu, V.M.; Krishnaiah, A. Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr. Polym. 2008, 72, 261–271. [Google Scholar] [CrossRef]
- Tran, H.V.; Tran, L.D.; Nguyen, T.N. Preparation of chitosan/magnetite composite beads and their application for removal of Pb(II) and Ni(II) from aqueous solution. Mater. Sci. Eng. C 2010, 30, 304–310. [Google Scholar] [CrossRef]
- Lee, H.C.; Jeong, Y.G.; Gil Min, B.; Lyoo, W.S.; Lee, S.C. Preparation and acid dye adsorption behavior of polyurethane/chitosan composite foams. Fibers Polym. 2009, 10, 636–642. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, R.; Sun, C.; Ji, C.; Chen, H.; Wang, C.; Niu, Y. Adsorption for metal ions of chitosan coated cotton fiber. J. Appl. Polym. Sci. 2008, 110, 2321–2327. [Google Scholar] [CrossRef]
- Sun, X.; Peng, B.; Ji, Y.; Chen, J.; Li, D. Chitosan(chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AIChE J. 2009, 55, 2062–2069. [Google Scholar] [CrossRef]
- Kumar, M.; Tripathi, B.P.; Shahi, V.K. Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater. J. Hazard. Mater. 2009, 172, 1041–1048. [Google Scholar] [CrossRef]
- Rekik, S.B.; Gassara, S.; Bouaziz, J.; Deratani, A.; Baklouti, S. Development and characterization of porous membranes based on kaolin/chitosan composite. Appl. Clay Sci. 2017, 143, 1–9. [Google Scholar] [CrossRef]
- Rekik, S.B.; Gassara, S.; Bouaziz, J.; Deratani, A.; Baklouti, S. Enhancing hydrophilicity and permeation flux of chitosan/kaolin composite membranes by using polyethylene glycol as porogen. Appl. Clay Sci. 2019, 168, 312–323. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Halloysite nanotubes sandwiched between chitosan layers: Novel bionanocomposites with multilayer structures. New J. Chem. 2018, 42, 8384–8390. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Parisi, F.; Lazzara, G. Thermal Properties of Multilayer Nanocomposites Based on Halloysite Nanotubes and Biopolymers. J. Compos. Sci. 2018, 2, 41. [Google Scholar] [CrossRef]
- dos Santos, B.F.F.; Maciel, M.A.; Tavares, A.A.; de Araújo Fernandes, C.Q.B.; de Sousa, W.J.B.; Lia Fook, M.V.; Leite, I.F.; De Lima Silva, S.M. Synthesis and preparation of chitosan/clay microspheres: Effect of process parameters and clay type. Materials 2018, 11, 2523. [Google Scholar] [CrossRef]
- Ben Dhieb, F.; Dil, E.J.; Tabatabaei, S.H.; Mighri, F.; Ajji, A. Effect of nanoclay orientation on oxygen barrier properties of LbL nanocomposite coated films. RSC Adv. 2019, 9, 1632–1641. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.-C.; Chen, M.-J.; Shi, Q.; Sun, R.; Wang, X. Chitosan/rectorite nanocomposite with injectable functionality for skin hemostasis. J. Mater. Chem. B 2018, 6, 6544–6549. [Google Scholar] [CrossRef]
- Wu, P.; Wu, W.; Li, S.; Xing, N.; Zhu, N.; Li, P.; Wu, J.; Yang, C.; Dang, Z. Removal of Cd2+ from aqueous solution by adsorption using Fe-montmorillonite. J. Hazard. Mater. 2009, 169, 824–830. [Google Scholar] [CrossRef]
- Monvisade, P.; Siriphannon, P. Chitosan intercalated montmorillonite: Preparation, characterization and cationic dye adsorption. Appl. Clay Sci. 2009, 42, 427–431. [Google Scholar] [CrossRef]
- Alkan, M.; Kalay, B.; Doğan, M.; Demirbaş, Ö. Removal of copper ions from aqueous solutions by kaolinite and batch design. J. Hazard. Mater. 2008, 153, 867–876. [Google Scholar] [CrossRef]
- Nandi, B.; Goswami, A.; Purkait, M. Adsorption characteristics of brilliant green dye on kaolin. J. Hazard. Mater. 2009, 161, 387–395. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, J.; Sun, C. Adsorption of Anionic Polyacrylamide onto Coal and Kaolinite Calculated from the Extended DLVO Theory Using the van Oss-Chaudhury-Good Theory. Polymers 2018, 10, 113. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Li, J.; Xue, H.; Zhao, X.; Xie, L. Adsorption Properties of Hydrocarbons (n-Decane, Methyl Cyclohexane and Toluene) on Clay Minerals: An Experimental Study. Energies 2017, 10, 1586. [Google Scholar] [CrossRef]
- Quiroz-Estrada, K.; Hernández-Espinosa, M.; Rojas, F.; Portillo, R.; Rubio, E.; López, L. N2 and CO2 adsorption by soils with high kaolinite content from San Juan Amecac, Puebla, México. Minerals 2016, 6, 73. [Google Scholar] [CrossRef]
- Dey, S.C.; Al-Amin, M.; Rashid, T.U.; Ashaduzzaman, M.; Shamsuddin, S.M. pH Induced Fabrication of Kaolinite-Chitosan Biocomposite. Int. Lett. Chem. Phys. Astron. 2016, 68, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhao, R.; Li, C.; Li, Y.; Zhang, C. Adsorption of basic dyes on activated carbon prepared from Polygonum orientale Linn: Equilibrium, kinetic and thermodynamic studies. Desalination 2010, 254, 68–74. [Google Scholar] [CrossRef]
- Dey, S.C.; Al-Amin, M.; Rashid, T.U.; Sultan, M.Z.; Ashaduzzaman, M.; Sarker, M.; Shamsuddin, S.M. Preparation, characterization and performance evaluation of chitosan as an adsorbent for remazol red. Int. J. Latest Res. Eng. Technol. 2016, 2, 52–62. [Google Scholar]
- Ngah, W.S.W.; Ariff, N.F.M.; Hanafiah, M.A.K.M. Preparation, characterization, and environmental application of crosslinked chitosan-coated bentonite for tartrazine adsorption from aqueous solutions. Water Air Soil Pollut. 2010, 206, 225–236. [Google Scholar] [CrossRef]
- Chen, D.; Chen, J.; Luan, X.; Ji, H.; Xia, Z. Characterization of anion–cationic surfactants modified montmorillonite and its application for the removal of methyl orange. Chem. Eng. J. 2011, 171, 1150–1158. [Google Scholar] [CrossRef]
- Zaker, Y.; Hossain, M.A.; Islam, T.S.A. Adsorption kinetics of methylene blue onto clay fractionated from Bijoypur soil. Bangladesh Res. J. Chem. Sci. 2013, 3, 65–72. [Google Scholar]
- Karima, B.; Mossab, B.L.; A-Hassen, M. Removal of methylene blue from aqueous solutions using an acid activated Algerian bentonite: Equilibrium and kinetic studies. In Proceedings of the International Renewable Energy Congress, Sousse, Tunisia, 5–7 November 2010; pp. 360–367. [Google Scholar]
- Ghribi, A.; Bagane, M.; Chlendi, M. Sorptive removal of congo red from aqueous solutions using raw clay: Batch and dynamic studies. Int. J. Innov. Environ. Stud. Res. 2014, 2, 45–56. [Google Scholar]
- Dunn, N.J.; Humphries, W.H., IV; Offenbacher, A.R.; King, T.L.; Gray, J.A. pH-dependent cis→trans isomerization rates for azobenzene dyes in aqueous solution. J. Phys. Chem. A 2009, 113, 13144–13151. [Google Scholar] [CrossRef]
- Ara, N.J.; Abu Hasan, M.; Rahman, M.A.; Salam, M.A.; Salam, A.; Alam, A.S. Removal of Remazol Red from Textile Waste Water Using Treated Sawdust—An Effective Way of Effluent Treatment. Pharm. J. 2013, 16, 93–98. [Google Scholar] [CrossRef]




| NCP Code | % Chitosan (w/w) | % Kaolinite (w/w) | Chitosan:Kaolinite |
|---|---|---|---|
| NK20C80 | 80 | 20 | 4:1 |
| NK40C60 | 60 | 40 | 3:2 |
| NK50C50 | 50 | 50 | 1:1 |
| NK60C40 | 40 | 60 | 2:3 |
| NK80C20 | 20 | 80 | 1:4 |
| Parameters | Chitosan | NK80C20 |
|---|---|---|
| Adsorption capacity, mg/g | 313.4 (% Error: 6) | 371.8 (% Error: 5) |
| Optimized pH | 3.0 | 2.5 |
| % Removal of RR | 84.1 | 99.6 |
| Equilibrium time, min | 30 | 15 |
| Desorption ratio | 0.967 | 0.999 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, S.C.; Moztahida, M.; Sarker, M.; Ashaduzzaman, M.; Shamsuddin, S.M. pH-Triggered Interfacial Interaction of Kaolinite/Chitosan Nanocomposites with Anionic Azo Dye. J. Compos. Sci. 2019, 3, 39. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs3020039
Dey SC, Moztahida M, Sarker M, Ashaduzzaman M, Shamsuddin SM. pH-Triggered Interfacial Interaction of Kaolinite/Chitosan Nanocomposites with Anionic Azo Dye. Journal of Composites Science. 2019; 3(2):39. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs3020039
Chicago/Turabian StyleDey, Shaikat Chandra, Mokrema Moztahida, Mithun Sarker, Md. Ashaduzzaman, and Sayed Md. Shamsuddin. 2019. "pH-Triggered Interfacial Interaction of Kaolinite/Chitosan Nanocomposites with Anionic Azo Dye" Journal of Composites Science 3, no. 2: 39. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs3020039