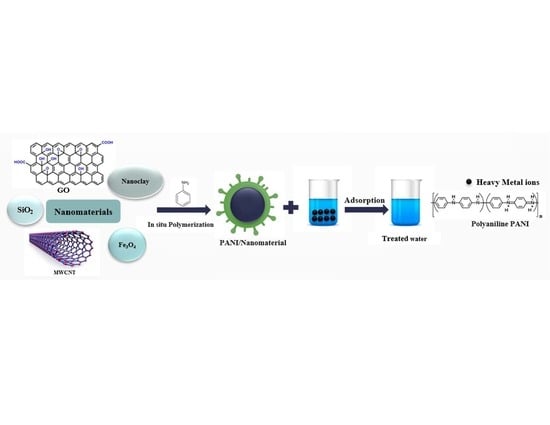
Polyaniline/Nanomaterial Composites for the Removal of Heavy Metals by Adsorption: A Review
Abstract
:1. Introduction
2. Polyaniline Polymer
3. Polyaniline Nanocomposites Synthesis
- (a)
- In situ polymerization of aniline monomer on the surface of the nanomaterials;
- (b)
- One-step redox reactions where the polymerization and the formation of the nanomaterial occur simultaneously;
- (c)
- Physical mixing of the pre-synthesized PANI and nanomaterials.
3.1. In Situ Polymerization
3.1.1. Microemulsion Polymerization
3.1.2. Inverse Emulsion Polymerization
3.2. One-Pot Synthesis Method
3.3. Physical Mixing
4. Polyaniline/Nanomaterial Composites for Adsorption of Heavy Metals
4.1. Metal Oxide or Hydroxide/Polyaniline Composites
4.2. Polyaniline/Magnetic Nanoparticles
4.3. Multifunctional Magnetic Polyaniline Nanocomposites
4.4. Carbon-Based Nanomaterial/PANI Nanocomposites
4.4.1. PANI/Graphene Derivatives
4.4.2. Carbon Nanotubes/PANI
4.5. Silica/Polyaniline Nanocomposite
4.6. Nanoclay/PANI Nanocomposites
4.7. Other Nanomaterial-Based PANI Composites
5. Regeneration of the Adsorbents
6. Conclusions and Perspectives
- Searching for more low-cost and easily available nanomaterials to combine with polyaniline in order to decrease the production costs and sustain the adsorbent-based market;
- Functionalizing nanocomposites by increasing the number of functional groups that are able to enhance the adsorption efficiency and improve the selectivity of the adsorbents;
- Conducting large-scale studies and evaluating the operational costs for real wastewater treatment.
Author Contributions
Funding
Conflicts of Interest
References
- Hong, Y.S.; Song, K.H.; Chung, J.Y. Health effects of chronic arsenic exposure. J. Prev. Med. Public Health 2014, 47, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.Y.U.; Keen, C.L. Copper, oxidative stress and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Kumari, S.; Jamwal, A.R.; Mishra, N.; Singh, D.K. Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100283. [Google Scholar] [CrossRef]
- Brochin, R.; Leone, S.; Philips, D.; Shepard, N.; Zisa, D.; Angerio, A. The Cellular Effect of Lead Poisoning and Its Clinical Picture. Georg. Undergrad. J. Health Sci. 2008, 5, 1–19. [Google Scholar]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Gunten, U.V.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 100809–125342. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Shao, X.; Ma, J.; Tian, G. Properties of magnetic carbon nanomaterials and application in removal organic dyes. Chemosphere 2018, 207, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Machrouhi, A.; Farnane, M.; Elhalil, A.; Abdennouri, M.; Tounsadi, H.; Barka, N. Heavy metals biosorption by Thapsia transtagana stems powder: Kinetics, equilibrium and thermodynamics. Moroc. J. Chem. 2019, 7, 98–110. [Google Scholar]
- Farnane, M.; Machrouhi, A.; Khnifira, M.; Barour, M.; Elmoubaraki, R.; Abdennouri, M.; Tounsadi, H.; Qoursal, S.; Barka, N. Facile nitric acid activation of carob seeds for efficient recovery of heavy metals from water. Desalin. Water Treat. 2020, 204, 174–188. [Google Scholar] [CrossRef]
- Seid, A.; Chouder, D.; Maouche, N.; Bakas, I.; Barka, N. Removal of Cd(II) and Co(II) ions from aqueous solutions by polypyrrole particles: Kinetics, equilibrium and thermodynamics. J. Taiwan Inst. Chem. Eng. 2014, 45, 2969–2974. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Gharibi, F.; McKay, G.; Gupta, V.K.; Puttaiah, S.H.; Marzban, N. Heavy metal adsorption using PAMAM/CNT nanocomposite from aqueous solution in batch and continuous fixed bed systems. Chem. Eng. J. 2018, 346, 258–270. [Google Scholar] [CrossRef]
- Repo, E.; Warchol, J.K.; Bhatnagar, A.; Sillanpää, M. Heavy metals adsorption by novel EDTA-modified chitosan-silica hybrid materials. J. Colloid Interface Sci. 2011, 358, 261–267. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Barka, N.; Ouzaouit, K.; Abdennouri, M.; El Makhfouk, M.; Qourzal, S.; Assabbane, A.; Ait Ichou, Y.; Nounah, A. Kinetics and equilibrium of cadmium removal from aqueous solutions by sorption onto synthesized hydroxyapatite. Desalin. Water Treat. 2012, 43, 8–16. [Google Scholar] [CrossRef]
- Tounsadi, H.; Khalidi, A.; Farnane, M.; Abdennouri, M.; Barka, N. Experimental design for the optimization of preparation conditions of highly efficient activated carbon from Glebionis Coronaria L. and heavy metals removal ability. Process Saf. Environ. Prot. 2016, 102, 710–723. [Google Scholar] [CrossRef]
- Verma, M.; Tyagi, I.; Chandra, R.; Gupta, V.K. Adsorptive removal of Pb (II) ions from aqueous solution using CuO nanoparticles synthesized by sputtering method. J. Mol. Liq. 2016, 225, 936–944. [Google Scholar] [CrossRef]
- Van der Merwe, D.; Pickrell, J.A. Toxicity of Nanomaterials. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 319–326. [Google Scholar]
- Roy, R.; Roy, R.A.; Roy, D.M. Alternative perspectives on “Quasi-crystallinity”: Non-uniformity and nanocomposites. Mater. Lett. 1986, 4, 323–328. [Google Scholar] [CrossRef]
- Venkatachalam, S. Ultraviolet and visible spectroscopy studies of nanofillers and their polymer nanocomposites. In Spectroscopy of Polymer Nanocomposites; Thomas, S., Rouxel, D., Ponnamma, D., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 130–157. [Google Scholar]
- Zare, E.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Sahayam, A.C. Determination of Cd, Cu, Pb and Sb in environmental samples by ICP-AES using polyaniline for separation. Fresenius J. Anal. Chem. 1998, 362, 285–288. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Q.; Fei, G.T.; Xu, S.H.; Guo, X.; Zhang, L.D. Preparation of Hollow Polyaniline Micro/Nanospheres and Their Removal Capacity of Cr (VI) from Wastewater. Nanoscale Res. Lett. 2018, 13, 401. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Qin, Z.; Liang, B.; Tian, F.; Zhao, J.; Liu, N.; Zhu, M. Morphology-dependent capacitive properties of three nanostructured polyanilines through interfacial polymerization in various acidic media. Electrochim. Acta 2015, 177, 343–351. [Google Scholar] [CrossRef]
- Shen, Y.; Qin, Z.; Li, T.; Zeng, F.; Chen, Y.; Liu, N. Boosting the supercapacitor performance of polyaniline nanofibers through sulfonic acid assisted oligomer assembly during seeding polymerization process. Electrochim. Acta 2020, 356, 136841. [Google Scholar] [CrossRef]
- Rahman, S.U.; Röse, P.; Shah, A.U.H.A.; Krewer, U.; Bilal, S. An amazingly simple, fast and green synthesis route to polyaniline nanofibers for efficient energy storage. Polymers 2020, 12, 2212. [Google Scholar] [CrossRef] [PubMed]
- Daka, J.J.; Munyati, O.M.; Nyirenda, J. Iron chlorophyll-a as biomimic catalyst for the green synthesis of polyaniline nanostructures: Evaluation, characterization and optimization. Sustain. Chem. Pharm. 2019, 15, 100194. [Google Scholar] [CrossRef]
- Wang, J.; Deng, B.; Chen, H.; Wang, X.; Zheng, J. Removal of Aqueous Hg(II) by Polyaniline: Sorption Characteristics and Mechanisms. Environ. Sci. Technol. 2009, 43, 5223–5228. [Google Scholar] [CrossRef]
- Lyu, W.; Yu, M.; Fen, J.; Yan, W. Facile synthesis of coral-like hierarchical polyaniline micro/nanostructures with enhanced supercapacitance and adsorption performance. Polymer 2019, 162, 130–138. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.W. Self-calibration of a polyaniline nanowire-based chemiresistive pH sensor. Microelectron. Eng. 2014, 116, 26–32. [Google Scholar] [CrossRef]
- Noby, H.; El-Shazly, A.H.; Elkady, M.F.; Ohshima, M. Strong acid doping for the preparation of conductive polyaniline nanoflowers, nanotubes, and nanofibers. Polymer 2019, 182, 121848. [Google Scholar] [CrossRef]
- Akbar, A.; Das, M.; Sarkar, D. Room temperature ammonia sensing by CdS nanoparticle decorated polyaniline (PANI) nanorods. Sens. Actuators A. 2020, 310, 112071. [Google Scholar] [CrossRef]
- Lei, Y.; Zhou, J.; Yao, Z.; Li, L. Self-assembly of porous polyaniline microspheres via template-free interfacial method for high-performance electromagnetic absorption property. Mater. Lett. 2020, 265, 127389. [Google Scholar] [CrossRef]
- Noby, H.; El-Shazly, A.A.; Elkady, M.F.; Ohshima, M. Novel preparation of self-assembled HCl-doped polyaniline nanotubes using compressed CO2-assisted polymerization. Polymer 2018, 156, 71–75. [Google Scholar] [CrossRef]
- Sun, L.; Zhan, L.; Shi, Y.; Chu, L.; Ge, G.; He, Z. Microemulsion synthesis and electromagnetic wave absorption properties of monodispersed Fe3O4/polyaniline core-shell nanocomposites. Synth. Met. 2014, 187, 102–107. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, H.; Lin, H.; Zhang, J. Synthesis of polyaniline/multi-walled carbon nanotube nanocomposites in water/oil microemulsion. Mater. Lett. 2008, 62, 3919–3921. [Google Scholar] [CrossRef]
- Karim, M.R.; Woo, H.W.; Cheong, I.W.; Park, S.M.; Oh, W.; Yeum, J.H. Conducting Polyaniline-Titanium Dioxide Nanocomposites Prepared by Inverted Emulsion Polymerization. Polym. Polym. Compos. 2008, 31, 83–88. [Google Scholar] [CrossRef]
- Regueira, R.; Suckeveriene, R.Y.; Brook, I.; Mechrez, G.; Tchoudakov, R.; Narkis, M. Investigation of the Electro-Mechanical Behavior of Hybrid Polyaniline/Graphene Nanocomposites Fabricated by Dynamic Interfacial Inverse Emulsion Polymerization. Sci. Res. Publ. 2015, 4, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Chen, J.; Wang, J.; Feng, J.; Yan, W. Removal of methylene blue by Polyaniline/TiO2 hydrate: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2019, 347, 93–102. [Google Scholar] [CrossRef]
- Pina, C.D.; Rossi, M.; Ferretti, A.M.; Ponti, A.; Lo Faro, M.; Falletta, E. One-pot synthesis of polyaniline/Fe3O4 nanocomposites with magnetic and conductive behaviour. Catalytic effect of Fe3O4 nanoparticles. Synth. Met. 2012, 162, 2250–2258. [Google Scholar] [CrossRef]
- Zong, P.; Cheng, Y.; Wang, S.; Wang, L. Simultaneous removal of Cd(II) and phenol pollutions through magnetic graphene oxide nanocomposites coated polyaniline using low temperature plasma technique. Int. J. Hydrog. Energy 2020, 45, 20106–20119. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Ohashi, F.; Ohnishi, Y.; Nonami, T. Synthesis of polyaniline-montmorillonite nanocomposites by the mechanochemical intercalation method. Synth. Met. 2004, 145, 265–270. [Google Scholar] [CrossRef]
- Rajakumar, K.; Kirupha, S.D.; Sivanesan, S.; Sai, R.L. Effective removal of heavy metal ions using Mn2O3 doped polyaniline nanocomposite. J. Nanosci. Nanotechnol. 2014, 14, 2937–2946. [Google Scholar] [CrossRef]
- Khalili, R.; Eisazadeh, H. Preparation and Characterization of Polyaniline/Sb2O3 Nanocomposite and its Application for Removal of Pb(II) from Aqueous Media. Int. J. Eng. 2013, 27, 239–246. [Google Scholar]
- Piri, S.; Piri, F.; Rajabi, B.; Ebrahimi, S.; Zamani, A.; Yaftian, M.R. In situ One-pot Electrochemical Synthesis of Aluminum Oxide/polyaniline Nanocomposite; Characterization and Its Adsorption Properties towards Some Heavy Metal Ions. J. Chin. Chem. Soc. 2015, 62, 1045–1052. [Google Scholar] [CrossRef]
- Nath, B.K.; Chaliha, C.; Kalita, E.; Kalita, M.C. Synthesis and characterization of ZnO:CeO2:nanocellulose:PANI bionanocomposite. A bimodal agent for arsenic adsorption and antibacterial action. Carbohydr. Polym. 2016, 148, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Moosavian, M.A.; Moazezi, N. Removal of cadmium and zinc ions from industrial wastewater using nanocomposites of PANI/ZnO and PANI/CoHCF: A comparative study. Desalin. Water Treat. 2015, 57, 20817–20836. [Google Scholar] [CrossRef]
- Ahmad, R. Polyaniline/ZnO Nanocomposite: A Novel Adsorbent for the Removal of Cr(VI) from Aqueous Solution. In Advances in Composite Materials Development; Lucan, D., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Ahmad, R.; Hasan, I. Efficient Remediation of an Aquatic Environment Contaminated by Cr (VI) and 2,4-Dinitrophenol by XG g-Polyaniline@ZnO Nanocomposite. J. Chem. Eng. Data 2017, 62, 1594–1607. [Google Scholar] [CrossRef]
- Brungesh, K.V.; Nagabhushana, B.M.; Harish, M.N.K.; Hari Krishna, R. An Efficient Removal of Toxic Cr(VI) from Aqueous Solution by MnO2 Coated Polyaniline Nanofibers: Kinetic and Thermodynamic Study. J. Environ. Anal. Toxicol. 2017, 7, 442. [Google Scholar]
- Chen, J.; Wang, N.; Liu, Y.; Zhu, J.; Feng, J.; Yan, W. Synergetic effect in a self-doping polyaniline/TiO2 composite for selective adsorption of heavy metal ions. Synth. Met. 2018, 245, 32–41. [Google Scholar] [CrossRef]
- Khong, C.H.; Teh, G.B.; Phang, S.W. Effect of Titanium Dioxide and Carbon Nanotubes on Polyaniline Nanocomposites for Heavy Metals Removal. Macromol. Symp. 2018, 382, 1800087. [Google Scholar] [CrossRef]
- Sahu, S.; Sahu, U.K.; Patel, R. Modified Thorium Oxide Polyaniline Core−Shell Nanocomposite and Its Application for the Efficient Removal of Cr(VI). J. Chem. Eng. Data 2019, 64, 1294–1304. [Google Scholar] [CrossRef]
- Bhaumik, M.; Gupta, V.K.; Maity, A. Synergetic enhancement of Cr(VI) removal from aqueous solutions using polyaniline@Ni(OH)2 nanocomposites adsorbent. J. Environ. Chem. Eng. 2018, 6, 2514–2527. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Q.; Zhang, H.; Liu, J.; Chen, R.; Li, R.; Li, Z.; Liu, P.; Wang, J. Rapid and efficient uranium(VI) capture by phytic acid/polyaniline/FeOOH composites. J. Colloid Interface Sci. 2018, 511, 1–11. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhao, Z.S.; Jiang, G.B. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef]
- Gu, H.; Rapole, S.B.; Sharma, J.; Huang, Y.; Cao, D.; Colorado, H.A.; Luo, Z.; Haldolaarachchige, N.; Young, D.P.; Walters, B.; et al. Magnetic polyaniline nanocomposites toward toxic hexavalent chromium removal. RSC Adv. 2012, 2, 11007–11018. [Google Scholar] [CrossRef]
- Han, X.; Gai, L.; Jiang, H.; Zhao, L.; Liu, H.; Zhang, W. Core-shell structured Fe3O4/PANI microspheres and their Cr(VI) ion removal properties. Synth. Met. 2013, 171, 1–6. [Google Scholar] [CrossRef]
- Chávez-Guajardo, A.E.; Llamas, J.C.M.; Maqueira, L.; Andrade, C.A.S.; Alves, K.G.B.; Melo, C.P. Efficient removal of Cr(VI) and Cu(II) ions from aqueous media by use of polypyrrole/maghemite and polyaniline/maghemite magnetic nanocomposites. Chem. Eng. J. 2015, 281, 826–836. [Google Scholar] [CrossRef]
- Ebrahim, S.; Shokry, A.; Ibrahim, H.; Soliman, M. Polyaniline/akaganéite nanocomposite for detoxification of noxious Cr(VI) from aquatic environment. J. Polym. Res. 2016, 23, 79. [Google Scholar] [CrossRef]
- Sadeghi, M.M.; Rad, A.S.; Ardjmand, M.; Mirabi, A. Preparation of magnetic nanocomposite based on polyaniline/Fe3O4 towards removal of lead (II) ions from real samples. Synth. Met. 2018, 245, 1–9. [Google Scholar] [CrossRef]
- Lyu, W.; Wu, J.; Zhang, W.; Liu, Y.; Yu, M.; Zhao, Y.; Feng, J.; Yan, W. Easy separated 3D hierarchical coral-like magnetic polyaniline adsorbent with enhanced performance in adsorption and reduction of Cr(VI) and immobilization of Cr(III). Chem. Eng. J. 2019, 363, 107–119. [Google Scholar] [CrossRef]
- Agrawal, S.; Singh, N.B. Removal of toxic hexavalent chromium from aqueous solution by nickel ferrite-polyaniline nanocomposite. Desalin. Water Treat. 2015, 57, 17757–17766. [Google Scholar] [CrossRef]
- Bhaumik, M.; Choi, H.J.; Mccrindle, R.I.; Maity, A. Composite nanofibers prepared from metallic iron nanoparticles and polyaniline: High performance for water treatment applications. J. Colloid Interface Sci. 2014, 425, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Rachna, K. Copper ferrite-Polyaniline nanocomposite and its application for Cr (VI) ion removal from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2019, 14, 100301. [Google Scholar]
- Yang, G.; Tang, L.; Cai, Y.; Zeng, G.; Guo, P.; Chen, G.; Zhou, Y.; Tang, J.; Chen, J.; Xiong, W. Effective removal of Cr(VI) through adsorption and reduction by magnetic mesoporous carbon incorporated with polyaniline. RSC Adv. 2014, 4, 58362–58371. [Google Scholar] [CrossRef]
- Tang, L.; Fang, Y.; Pang, Y.; Zeng, G.; Wang, J.; Zhou, Y.; Deng, Y.; Yang, G.; Cai, Y.; Chen, J. Synergistic adsorption and reduction of hexavalent chromium using highly uniform polyaniline-magnetic mesoporous silica composite. Chem. Eng. J. 2014, 254, 302–312. [Google Scholar] [CrossRef]
- Norouzian, R.S.; Lakouraj, M.M. Preparation and heavy metal ion adsorption behavior of novel supermagnetic nanocomposite based on thiacalix[4]arene and polyaniline: Conductivity, isotherm and kinetic study. Adv. Polym. Technol. 2015, 36, 107–119. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, J.; Ye, S.; Wang, L.; Yang, C.; Sun, P.; Wang, C. Facile fabrication of PS/Fe3O4@PANi nanocomposite particles and their application for the effective removal of Cu2+. N. J. Chem. 2017, 41, 14137–14144. [Google Scholar] [CrossRef]
- Zhang, J.; Han, J.; Wang, M.; Guo, R. Fe3O4/PANI/MnO2 Core-Shell Hybrids as Advanced Adsorbents for Heavy Metal Ions. J. Mater. Chem. A 2017, 5, 4058–4406. [Google Scholar] [CrossRef]
- Lei, C.; Wang, C.; Chen, W.; He, M.; Huang, B. Polyaniline@magnetic chitosan nanomaterials for highly efficient simultaneous adsorption and in-situ chemical reduction of hexavalent chromium: Removal efficacy and mechanisms. Sci. Total Environ. 2020, 733, 139316. [Google Scholar] [CrossRef]
- Kumar, R.; Barakat, M.A.; Taleb, M.A.; Seliem, M.K. A recyclable multifunctional graphene oxide/SiO2@polyaniline microspheres composite for Cu(II) and Cr(VI) decontamination from wastewater. J. Clean Prod. 2020, 268, 122290. [Google Scholar] [CrossRef]
- Taleb, M.A.; Kumar, R.; Al-Rashdi, A.A.; Seliem, M.K.; Barakat, M.A. Fabrication of SiO2/CuFe2O4/polyaniline composite: A highly efficient adsorbent for heavy metals removal from aquatic environment. Arab. J. Chem. 2020, 13, 7533–7543. [Google Scholar] [CrossRef]
- Vargas, L.R.; Poli, A.K.; Dutra, R.C.L.; Souza, C.B.; Baldan, M.R.; Gonçalves, E.S. Formation of composite polyaniline and graphene oxide by physical mixture method. J. Aerosp. Technol. Manag. 2017, 9, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zeng, M.; Xu, W.; Li, J.; Li, J.; Xu, J.; Wang, X. Polyaniline nanorods dotted on graphene oxide nanosheets as a novel super adsorbent for Cr(VI). Dalton Trans. 2013, 42, 7854–7858. [Google Scholar] [CrossRef]
- Fan, Q.; Yang, Y.; Hao, Y.; Zhao, X.; Feng, Y. Preparation of three-dimensional PANI/GO for the separation of Hg(II) from aqueous solution. J. Mol. Liq. 2015, 212, 557–562. [Google Scholar] [CrossRef]
- Harijan, D.K.L.; Chandra, V. Polyaniline functionalized graphene sheets for treatment of toxic hexavalent chromium. J. Environ. Chem. Eng. 2016, 4, 3006–3012. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Asghari, M.; Ramezanzadeh, B.; Bahlakeh, G. Fabrication of an efficient system for Zn ions removal from industrial wastewater based on graphene oxide nanosheets decorated with highly crystalline polyaniline nanofibers (GO- PANI): Experimental and ab initio quantum mechanics approaches. Chem. Eng. J. 2017, 337, 385–397. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.X.; Lü, Q.F.; Lin, T.T. Facile preparation of lignosulfonate-graphene oxide-polyaniline ternary nanocomposite as an effective adsorbent for Pb(II) ions. ACS Sustain. Chem. Eng. 2014, 2, 1203–1211. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Li, Y.; Wang, P.; Dong, Y. Synthesis of magnetic polyaniline/graphene oxide composites and their application in the efficient removal of Cu(II) from aqueous solutions. J. Environ. Chem. Eng. 2015, 4, 825–834. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Yang, F. Polyaniline/reduced graphene oxide/Fe3O4 nano-composite for aqueous Hg(II) removal. Water Sci. Technol. 2015, 72, 2062–2070. [Google Scholar] [CrossRef]
- Shao, D.; Chen, C.; Wang, X. Application of polyaniline and multiwalled carbon nanotube magnetic composites for removal of Pb(II). Chem. Eng. J. 2011, 185–186, 144–150. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, M.O.; Barakat, M.A. DBSA doped polyaniline/multi-walled carbon nanotubes composite for high efficiency removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2013, 228, 748–755. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, M.O.; Alshahrie, A.; Darwesh, R.; Parveen, N.; Yadav, S.K.; Barakat, M.A.; Cho, M.H. Adsorption modeling and mechanistic insight of hazardous chromium on para toluene sulfonic acid immobilized-polyaniline@CNTs nanocomposites. J. Saudi Chem. Soc. 2018, 23, 188–197. [Google Scholar] [CrossRef]
- Ansari, M.O.; Kumar, R.; Ansari, S.A.; Ansari, S.P.; Barakat, M.A.; Alshahrie, A.; Cho, M.H. Anion selective pTSA doped polyaniline@graphene oxide-multiwalled carbon nanotube composites for Cr(VI) and Congo red adsorption. J. Colloid Interface Sci. 2017, 496, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Meenakshi, S. Removal of hexavalent chromium ions using polyaniline/silica gel composite. J. Water Process Eng. 2014, 1, 37–45. [Google Scholar] [CrossRef]
- Javadian, H.; Vahedian, P.; Toosi, M. Adsorption characteristics of Ni(II) from aqueous solution and industrial wastewater onto Polyaniline/HMS nanocomposite powder. Appl. Surf. Sci. 2013, 284, 13–22. [Google Scholar] [CrossRef]
- Javadian, H.; Sorkhrodi, F.Z.; Koutenaei, B.B.; Naushad, M.; Desoky, G.E.B.E. Experimental investigation on enhancing aqueous cadmium removal via nanostructure composite of modified hexagonal type mesoporous silica with polyaniline/polypyrrole nanoparticles. J. Ind. Eng. Chem. 2014, 20, 3678–3688. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Fekry, N.A.; El-latif, M.M.A. Nanocomposites of nanosilica-immobilized-nanopolyaniline and crosslinked nanopolyaniline for removal of heavy metals. Chem. Eng. J. 2016, 304, 679–691. [Google Scholar] [CrossRef]
- Chen, J.; Hong, X.; Zhao, Y.; Xia, Y.; Li, D.; Zhang, Q. Preparation of flake-like polyaniline/montmorillonite nanocomposites and their application for removal of Cr(VI) ions in aqueous solution. J. Mater. Sci. 2013, 48, 7708–7717. [Google Scholar] [CrossRef]
- Olad, A.; Bastanian, M.; Bakht, H.; Hagh, K. Thermodynamic and Kinetic Studies of Removal Process of Hexavalent Chromium Ions from Water by Using Bio conducting Starch–Montmorillonite/Polyaniline Nanocomposite. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1916–1926. [Google Scholar] [CrossRef]
- Ali, M.B.; Wang, F.; Boukherroub, R.; Lei, W.; Xia, M. Phytic acid-doped polyaniline nanofibers-clay mineral for efficient adsorption of copper (II) ions. J. Colloid Interface Sci. 2019, 553, 688–698. [Google Scholar] [PubMed]
- Piri, S.; Zanjani, Z.A.; Piri, F.; Zamani, A.; Yaftian, M.; Davari, M. Potential of polyaniline modified clay nanocomposite as a selective decontamination adsorbent for Pb(II) ions from contaminated waters; kinetics and thermodynamic study. J. Environ. Health Sci. Eng. 2016, 14, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.; Gao, Y.; Tan, X.; Chen, C. Polyaniline-Modified Mg/Al Layered Double Hydroxide Composites and Their Application in Efficient Removal of Cr(VI). ACS Sustain. Chem. Eng. 2016, 4, 4361–4369. [Google Scholar] [CrossRef]
- Dinari, M.; Neamati, S. Surface modified layered double hydroxide/polyaniline nanocomposites: Synthesis, characterization and Pb2+ removal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124438. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Elanchezhiyan, S.S.D.; Preethi, J.; Meenakshi, S.; Park, C.M. Mechanistic performance of polyaniline-substituted hexagonal boron nitride composite as a highly efficient adsorbent for the removal of phosphate, nitrate, and hexavalent chromium ions from an aqueous environment. Appl. Surf. Sci. 2020, 511, 145543. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, F.; Cheng, S.; Zong, L.; Zhu, C.; Ling, C.; Li, A. Recyclable Nanocomposite of Flowerlike MoS2@Hybrid Acid-Doped PANI Immobilized on Porous PAN Nanofibers for the Efficient Removal of Cr(VI). ACS Sustain. Chem. Eng. 2017, 6, 447–456. [Google Scholar] [CrossRef]







| Composite | Adsorbate | Desorbing Agent | Number of Cycles | Desorption (%) | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Xanthan gum/ZnO/PANI | Cr(VI) | 0.2 M NaOH | 5 | 46 | 51.67 | [48] |
| ThO2/PANI | Cr(VI) | 1 M NaOH | 5 | - | 85 | [52] |
| Fe3O4/PANI | Cr(VI) | 1 M HCl/sonication | - | - | 100 | [56] |
| Fe3O4/PANI | Cr(VI) | 0.5 M NaOH | 5 | 90 | - | [57] |
| yFe3O4/PANI | Cu(II) | 0.1 M HCl | 4 | 50 | 98 | [58] |
| yFe3O4/PANI | Cr(VI) | 0.1 M NaOH | 4 | 40 | 80 | [58] |
| CL–PANI/Fe3O4 | Cr(VI) | 0.1 M NaOH | 10 | - | 80 | [61] |
| Magnetic SiO2/PANI | Cr(VI) | 0.01 M NaOH | 5 | - | 66 | [66] |
| Fe3O4@PANI–AmAzoTCA[4] | Cu Cd Co Cr(III) | 0.1 M HCl 0.1 M HCl 0.1 M HCl 0.1 M HCl | 3 3 3 3 | - - - - | 95.9 93.3 92 94 | [67] |
| PS/Fe3O4@PANI | Cu(II) | 0.1 M HCl | 8 | - | 83 | [68] |
| GO/PANI | Hg(II) | 0.1 M HNO3 | 7 | - | 70 | [75] |
| GO/PANI | Zn | pH = 7 | 4 | - | 50 | [77] |
| Fe3O4/GO/PANI | Cu(II) | pH = 5.8 | 6 | - | 90 | [79] |
| rGO/PANI | Hg(II) | pH = 1 pH = 13 | - | 50.4 73.4 | - - | [80] |
| DP/MWCNT/PANI | Cr(VI) | 0.1 M NaCl 0.1 M NaOH 1 M NaOH 1 M NaOH | - - - - | 2.9 10.5 20.77 24 | - - - - | [82] |
| pTSA–Pani@CNT | Cr(VI) | 0.1 M NaOH 0.1 M HCl Acetone | 3 | - | ≃98 | [83] |
| h-BN/PANI | Cr(VI) | 0.1 M NaOH | 5 | - | - | [95] |
| MoS2/PANI/PAN | Cr(VI) | 0.5 M NaOH | 6 | - | ≃88 | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajjaoui, H.; Soufi, A.; Boumya, W.; Abdennouri, M.; Barka, N. Polyaniline/Nanomaterial Composites for the Removal of Heavy Metals by Adsorption: A Review. J. Compos. Sci. 2021, 5, 233. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs5090233
Hajjaoui H, Soufi A, Boumya W, Abdennouri M, Barka N. Polyaniline/Nanomaterial Composites for the Removal of Heavy Metals by Adsorption: A Review. Journal of Composites Science. 2021; 5(9):233. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs5090233
Chicago/Turabian StyleHajjaoui, Hind, Amal Soufi, Wafaa Boumya, Mohamed Abdennouri, and Noureddine Barka. 2021. "Polyaniline/Nanomaterial Composites for the Removal of Heavy Metals by Adsorption: A Review" Journal of Composites Science 5, no. 9: 233. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs5090233