Preparation and Characterization of Moldable Demineralized Bone Matrix/Calcium Sulfate Composite Bone Graft Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. DBM Powder Preparation
2.2. dsDNA Measurement
2.3. Collagen Content Measurement
2.4. Calcium Sulfate Hemihydrate Preparation
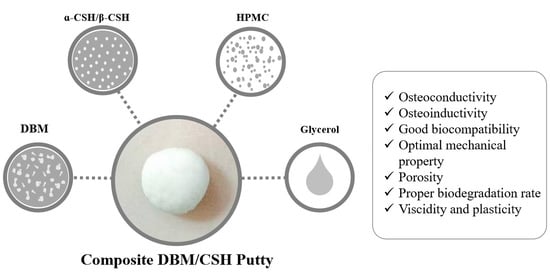
2.5. DBM/CSH Putty Preparation
2.6. Scanning Electron Microscope (SEM) and Energy Dispersive Spectrometer (EDS)
2.7. Infrared Spectroscopy (FTIR)
2.8. X-ray Diffraction (XRD)
2.9. Mechanical Compression Testing
2.10. Washout Resistant Test
2.11. Cell Viability
3. Results
3.1. DBM Preparation
3.1.1. Preparation of DBM Powder
3.1.2. Characterization of DBM Powder
3.2. Preparation of Calcium Sulfate Hemihydrate (CaSO4·0.5H2O, CSH)
3.3. DBM/CaSO4 Composite Bone Graft Materials
3.3.1. Preparation of DBM/CaSO4 Composite Putty
3.3.2. Properties of DBM/CaSO4 Composite Putty
Compressive Strength
Wash out Properties
Biocompatibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlickewei, W.; Schlickewei, C. The Use of Bone Substitutes in the Treatment of Bone Defects—The Clinical View and History. Macromol. Symp. 2007, 253, 10–23. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Bi, Q.; Zhao, C.; Chen, J.-Y.; Cai, M.-H.; Chen, X.-Y. Recent Advances in Biomaterials for the Treatment of Bone Defects. Organogenesis 2020, 16, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N. Bone-graft substitutes: Facts, fictions, and applications. J. Bone Jt. Surg. 2001, 83, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Boyce, T.; Edwards, J.; Scarborough, N. ALLOGRAFT BONE: The Influence of Processing on Safety and Performance. Orthop. Clin. N. Am. 1999, 30, 571–581. [Google Scholar] [CrossRef]
- Urist, M.R.; Dowell, T.A. Inductive substratum for osteogenesis in pellets of particulate bone matrix. Clin. Orthop. Relat. Res. 1968, 61, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, L.; Yang, X.G.; Wang, F.; Feng, J.T.; Hua, K.C.; Li, Q.; Hu, Y.C. Demineralized Bone Matrix Carriers and their Clinical Applications: An Overview. Orthop. Surg. 2019, 11, 725–737. [Google Scholar] [CrossRef]
- Pietrzak, W.S.; Woodell-May, J.; McDonald, N. Assay of bone morphogenetic protein-2, -4, and -7 in human demineralized bone matrix. J. Craniofac. Surg. 2006, 17, 84–90. [Google Scholar] [CrossRef]
- Jang, C.H.; Park, H.; Cho, Y.B.; Song, C.H. Mastoid obliteration using a hyaluronic acid gel to deliver a mesenchymal stem cells-loaded demineralized bone matrix: An experimental study. Int. J. Pediatric Otorhinolaryngol. 2008, 72, 1627–1632. [Google Scholar] [CrossRef]
- Schallenberger, M.A.; Rossmeier, K.; Lovick, H.M.; Meyer, T.R.; Aberman, H.M.; Juda, G.A. Comparison of the osteogenic potential of OsteoSelect demineralized bone matrix putty to NovaBone calcium-phosphosilicate synthetic putty in a cranial defect model. J. Craniofac. Surg. 2014, 25, 657–661. [Google Scholar] [CrossRef] [Green Version]
- Lewis, K.N.; Thomas, M.V.; Puleo, D.A. Mechanical and degradation behavior of polymer-calcium sulfate composites. J. Mater. Sci. Mater. Med. 2006, 17, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.V.; Puleo, D.A. Calcium sulfate: Properties and clinical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88B, 597–610. [Google Scholar] [CrossRef]
- Coetzee, A.S. Regeneration of bone in the presence of calcium sulfate. Arch. Otolaryngol. 1980, 106, 405–409. [Google Scholar] [CrossRef]
- Borrelli, J., Jr.; Prickett, W.D.; Ricci, W.M. Treatment of nonunions and osseous defects with bone graft and calcium sulfate. Clin. Orthop. Relat. Res. 2003, 411, 245–254. [Google Scholar] [CrossRef]
- Evaniew, N.; Tan, V.; Parasu, N.; Jurriaans, E.; Finlay, K.; Deheshi, B.; Ghert, M. Use of a calcium sulfate-calcium phosphate synthetic bone graft composite in the surgical management of primary bone tumors. Orthopedics 2013, 36, e216–e222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Liu, H.; Liu, X.; Lian, X.; Guo, Z.; Jiang, H.J.; Cui, F.Z. Improved workability of injectable calcium sulfate bone cement by regulation of self-setting properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.W.; Andreana, S.; Dziak, R. Current Use of Calcium Sulfate Bone Grafts. Med Res. Arch. 2020, 8. [Google Scholar] [CrossRef]
- Tan, V.; Evaniew, N.; Finlay, K.; Jurriaans, E.; Ghert, M.; Deheshi, B.; Parasu, N. Chronology of the Radiographic Appearances of the Calcium Sulfate-Calcium Phosphate Synthetic Bone Graft Composite Following Resection of Bone Tumors: A Follow-up Study of Postoperative Appearances. Can. Assoc. Radiol. J. J. L’association Can. Des Radiol. 2016, 67, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. Magnesium-enriched hydroxyapatite compared to calcium sulfate in the healing of human extraction sockets: Radiographic and histomorphometric evaluation at 3 months. J. Periodontol. 2009, 80, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Baranes, D.; Kurtzman, G.M. Biphasic Calcium Sulfate as an Alternative Grafting Material in Various Dental Applications. J. Oral Implantol. 2019, 45, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahav, A.; Kurtzman, G.M.; Katzap, M.; Dudek, D.; Baranes, D. Bone Regeneration: Properties and Clinical Applications of Biphasic Calcium Sulfate. Dent. Clin. N. Am. 2020, 64, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lee, E.J.; Park, C.S.; Yoon, B.H.; Shin, D.S.; Kim, H.E.; Koh, Y.H.; Park, S.H. Calcium Sulfate Hemihydrate Powders with a Controlled Morphology for Use as Bone Cement. J. Am. Ceram. Soc. 2008, 91, 2039–2042. [Google Scholar] [CrossRef] [Green Version]
- Chiang, H.-H.; Su, C.-Y.; Hsu, L.-H.; Yang, M.-H.; Fang, H.-W. Improved Anti-Washout Property of Calcium Sulfate/Tri-Calcium Phosphate Premixed Bone Substitute with Glycerin and Hydroxypropyl Methylcellulose. Appl. Sci. 2021, 11, 8136. [Google Scholar] [CrossRef]
- Ma, B.G.; Ru, X.H.; Lu, S.W.; Zou, K.B. Preparation of α-Calcium Sulfate Hemihydrate from Phosphogypsum in CaCl2 Solution under Atmospheric Pressure. Adv. Mater. Res. 2012, 554–556, 570–574. [Google Scholar] [CrossRef]
- Guan, Q.; Sui, Y.; Zhang, F.; Yu, W.; Bo, Y.; Wang, P.; Peng, W.; Jin, J. Preparation of α-calcium sulfate hemihydrate from industrial by-product gypsum: A review. Physicochem. Probl. Miner. Process. 2021, 57, 168–181. [Google Scholar] [CrossRef]
- Hsu, H.-J.; Waris, R.A.; Ruslin, M.; Lin, Y.-H.; Chen, C.-S.; Ou, K.-L. An innovative α-calcium sulfate hemihydrate bioceramic as a potential bone graft substitute. J. Am. Ceram. Soc. 2018, 101, 419–427. [Google Scholar] [CrossRef]
- Park, J.-H.; Suh, S.-J.; Lee, Y.-S.; Lee, J.-H.; Ryu, K.-Y.; Kang, D.-G. Lytic Complications after Skull Reconstruction Using GeneX®. Korean J. Neurotrauma 2015, 11, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, L.; Xiang, H.; Ye, J. Influence of anti-washout agents on the rheological properties and injectability of a calcium phosphate cement. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2007, 81B, 410–418. [Google Scholar] [CrossRef]
- Eagle, M.J.; Rooney, P.; Kearney, J.N. Production of an osteoinductive demineralised bone matrix powder without the use of organic solvents. Cell Tissue Bank. 2015, 16, 433–441. [Google Scholar] [CrossRef]
- Eagle, M.J.; Man, J.; Rooney, P.; Hogg, P.; Kearney, J.N. Assessment of an improved bone washing protocol for deceased donor human bone. Cell Tissue Bank. 2015, 16, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Middendorf, B. Calcium sulphate hemihydrate hydration leading to gypsum crystallization. Prog. Cryst. Growth Charact. Mater. 2007, 53, 57–77. [Google Scholar] [CrossRef]
- Ling, Y.; Demopoulos, G.P. Preparation of α-Calcium Sulfate Hemihydrate by Reaction of Sulfuric Acid with Lime. Ind. Eng. Chem. Res. 2005, 44, 715–724. [Google Scholar] [CrossRef]
- Luo, K.; Li, C.; Xiang, L.; Li, H.; Ning, P. Influence of temperature and solution composition on the formation of calcium sulfates. Particuology 2010, 8, 240–244. [Google Scholar] [CrossRef]
- D’Agostino, P.; Barbier, O. An investigation of the effect of AlloMatrix bone graft in distal radial fracture: A prospective randomised controlled clinical trial. Bone Jt. J. 2013, 95, 1514–1520. [Google Scholar] [CrossRef]
- Fu, T.S.; Wang, I.C.; Lu, M.L.; Hsieh, M.K.; Chen, L.H.; Chen, W.J. The fusion rate of demineralized bone matrix compared with autogenous iliac bone graft for long multi-segment posterolateral spinal fusion. BMC Musculoskelet. Disord. 2016, 17, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapkas, G.S.; Mavrogenis, A.F.; Themistocleous, G.S.; Zachos, V.C.; Kelalis, G.; Papagelopoulos, P.J. Posterior lumbar interbody fusion versus circumferential fusion using the B-Twin expandable spinal system. J. Long-Term Eff. Med. Implant. 2007, 17, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, R.M.; Kelly, C.M. The effect of allomatrix injectable putty on the outcome of long bone applications. Orthopedics 2003, 26, S567–S570. [Google Scholar] [CrossRef] [PubMed]










| Group | Defatting Reagent | Demineralization Reagent |
|---|---|---|
| DBM1: Chl/Met + Acidic AlCl3 | Chloroform/Methanol | 0.5 M AlCl3/3% HCl/5% Formic acid |
| DBM2: Et2O + Acidic AlCl3 | Diethyl ester | 0.5 M AlCl3/3% HCl/5% Formic acid |
| DBM3: Chl/Met + HCl | Chloroform/Methanol | 0.6 N HCl |
| DBM4: Et2O + HCl | Diethyl ester | 0.6 N HCl |
| DBM/α-CSH Composite Bone Graft Materials | |||
|---|---|---|---|
| Powder | 100% DBM/α-CSH [9:1] + 0% HPMC | 98% DBM/α-CSH [9:1] + 2% HPMC | 96% DBM/α-CSH [9:1] + 4% HPMC |
| Liquid | 70% Glycerol + 30% water | 70% Glycerol + 30% water | 70% Glycerol + 30% water |
| L/P (Liquid/Powder) | 0.25 | 0.25 | 0.25 |
| Mixing | Yes | Yes | Yes |
| Self-hardening | – | – | Yes |
| Working time | – | – | >1 h |
| Setting time | – | – | 12 h |
| Moldable | No | No | Yes |
| Appearance | cement | clay | Putty * |
| DBM/β-CSH Composite Bone Graft Materials | |||
|---|---|---|---|
| Powder | 100% DBM/β-CSH [9:1] + 0% HPMC | 98% DBM/β-CSH [9:1] + 2% HPMC | 96% DBM/β-CSH [9:1] + 4% HPMC |
| Liquid | 70% Glycerol + 30% water | 70% Glycerol + 30% water | 70% Glycerol + 30% water |
| L/P (Liquid/Powder) | 0.3 | 0.3 | 0.3 |
| Mixing | Yes | Yes | Yes |
| Self-hardening | – | – | Yes |
| Working time | – | – | >1 h |
| Setting time | – | – | 4 h |
| Moldable | No | No | Yes |
| Appearance | cement | clay | Putty * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, I.-C.; Su, C.-Y.; Lai, C.-C.; Tsou, Y.-S.; Zheng, Y.; Fang, H.-W. Preparation and Characterization of Moldable Demineralized Bone Matrix/Calcium Sulfate Composite Bone Graft Materials. J. Funct. Biomater. 2021, 12, 56. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb12040056
Chen I-C, Su C-Y, Lai C-C, Tsou Y-S, Zheng Y, Fang H-W. Preparation and Characterization of Moldable Demineralized Bone Matrix/Calcium Sulfate Composite Bone Graft Materials. Journal of Functional Biomaterials. 2021; 12(4):56. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb12040056
Chicago/Turabian StyleChen, I-Cheng, Chen-Ying Su, Chun-Cheih Lai, Yi-Syue Tsou, Yudong Zheng, and Hsu-Wei Fang. 2021. "Preparation and Characterization of Moldable Demineralized Bone Matrix/Calcium Sulfate Composite Bone Graft Materials" Journal of Functional Biomaterials 12, no. 4: 56. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb12040056