Microporous Hydroxyapatite-Based Ceramics Alter the Physiology of Endothelial Cells through Physical and Chemical Cues
Abstract
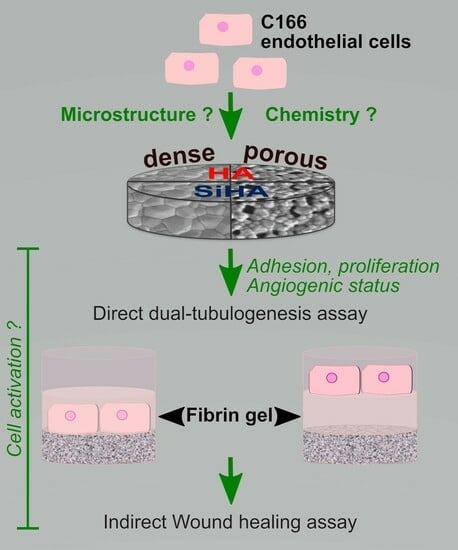
:1. Introduction
2. Materials and Methods
2.1. Ceramic Processing and Characterization
2.2. In Vitro Assays
2.2.1. Cell Culture
2.2.2. Tubulogenesis
2.2.3. Viability, Cell Density and Proliferation
2.2.4. Dosage of VEGF-A Secretion by ELISA
2.2.5. Western Blot
2.2.6. In Situ Expression of Angiogenesis Markers
2.2.7. Dosage of Chemical Elements in the Culture Medium
2.2.8. Wound Healing Assay in Ceramics Dissolution Media
2.3. Statistical Analysis
3. Results
3.1. Characterization of Hydroxyapatite-Based Ceramics
3.2. Ceramics’ Biocompatibility towards C166 Endothelial Cells
3.3. Expression of Angiogenesis Markers by C166 Cells Cultured on Ceramics
3.4. Tubulogenesis
3.5. Dissolution of HA-Based Ceramics
3.6. Effect of Ceramic Extracts on C166 Activation
3.7. Impact of Calcium Deficiency in the Culture Medium on C166 Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blokhuis, T.J.; Arts, J.J.C. Bioactive and Osteoinductive Bone Graft Substitutes: Definitions, Facts and Myths. Injury 2011, 42 (Suppl. 2), S26–S29. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Saran, U.; Gemini Piperni, S.; Chatterjee, S. Role of Angiogenesis in Bone Repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef]
- Malhotra, A.; Habibovic, P. Calcium Phosphates and Angiogenesis: Implications and Advances for Bone Regeneration. Trends Biotechnol. 2016, 34, 983–992. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of Dopant-Induced Osteogenesis and Angiogenesis in Calcium Phosphate Ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xue, K.; Kong, N.; Liu, K.; Chang, J. Silicate Bioceramics Enhanced Vascularization and Osteogenesis through Stimulating Interactions between Endothelia Cells and Bone Marrow Stromal Cells. Biomaterials 2014, 35, 3803–3818. [Google Scholar] [CrossRef]
- Li, H.; Chang, J. Stimulation of Proangiogenesis by Calcium Silicate Bioactive Ceramic. Acta Biomater. 2013, 9, 5379–5389. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, J. Bioactive Silicate Materials Stimulate Angiogenesis in Fibroblast and Endothelial Cell Co-Culture System through Paracrine Effect. Acta Biomater. 2013, 9, 6981–6991. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, H.; Chen, L.; Lin, X.; Huang, Y.; Dai, K.; Naoki, K.; Chen, G.; Chang, J. Silicate Bioceramics Induce Angiogenesis during Bone Regeneration. Acta Biomater. 2012, 8, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Szurkowska, K.; Kolmas, J. Hydroxyapatites Enriched in Silicon–Bioceramic Materials for Biomedical and Pharmaceutical Applications. Prog. Nat. Sci. Mater. Int. 2017, 27, 401–409. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Sánchez-Salcedo, S.; Feito, M.J.; Serrano, M.C.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B.; et al. Silicon Substituted Hydroxyapatite/VEGF Scaffolds Stimulate Bone Regeneration in Osteoporotic Sheep. Acta Biomater. 2020, 101, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted Hydroxyapatites for Bone Regeneration: A Review of Current Trends: Substituted HA for Bone Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef]
- Šupová, M. Substituted Hydroxyapatites for Biomedical Applications: A Review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Bohner, M. Silicon-Substituted Calcium Phosphates—A Critical View. Biomaterials 2009, 30, 6403–6406. [Google Scholar] [CrossRef]
- Dashnyam, K.; El-Fiqi, A.; Buitrago, J.O.; Perez, R.A.; Knowles, J.C.; Kim, H.-W. A Mini Review Focused on the Proangiogenic Role of Silicate Ions Released from Silicon-Containing Biomaterials. J. Tissue Eng. 2017, 8, 204173141770733. [Google Scholar] [CrossRef]
- Gallo, M.; Tadier, S.; Meille, S.; Chevalier, J. Resorption of Calcium Phosphate Materials: Considerations on the in Vitro Evaluation. J. Eur. Ceram. Soc. 2018, 38, 899–914. [Google Scholar] [CrossRef]
- Rouahi, M.; Gallet, O.; Champion, E.; Dentzer, J.; Hardouin, P.; Anselme, K. Influence of Hydroxyapatite Microstructure on Human Bone Cell Response. J. Biomed. Mater. Res. A 2006, 78A, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A.; Annaz, B.; Saeed, S.; Revell, P.A.; Buckland, T. Microporosity Enhances Bioactivity of Synthetic Bone Graft Substitutes. J. Mater. Sci. Mater. Med. 2005, 16, 467–475. [Google Scholar] [CrossRef]
- Faia-Torres, A.B.; Guimond-Lischer, S.; Rottmar, M.; Charnley, M.; Goren, T.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Differential Regulation of Osteogenic Differentiation of Stem Cells on Surface Roughness Gradients. Biomaterials 2014, 35, 9023–9032. [Google Scholar] [CrossRef]
- Gariboldi, M.I.; Best, S.M. Effect of Ceramic Scaffold Architectural Parameters on Biological Response. Front. Bioeng. Biotechnol. 2015, 3, 151. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, X.; Barbieri, D.; Barradas, A.M.C.; de Bruijn, J.D.; van Blitterswijk, C.A.; Yuan, H. The Size of Surface Microstructures as an Osteogenic Factor in Calcium Phosphate Ceramics. Acta Biomater. 2014, 10, 3254–3263. [Google Scholar] [CrossRef]
- Magnaudeix, A. Calcium Phosphate Bioceramics: From Cell Behavior to Chemical-Physical Properties. Front. Biomater. Sci. 2022, 1, 942104. [Google Scholar] [CrossRef]
- Ziebart, T.; Schnell, A.; Walter, C.; Kämmerer, P.W.; Pabst, A.; Lehmann, K.M.; Ziebart, J.; Klein, M.O.; Al-Nawas, B. Interactions between Endothelial Progenitor Cells (EPC) and Titanium Implant Surfaces. Clin. Oral Investig. 2013, 17, 301–309. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Schedle, A.; Wieland, M.; Andrukhov, O.; Matejka, M.; Rausch-Fan, X. Proliferation, Behavior, and Cytokine Gene Expression of Human Umbilical Vascular Endothelial Cells in Response to Different Titanium Surfaces. J. Biomed. Mater. Res. A 2009, 93A, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Peng, M.; Yang, Z.; Zhou, X.; Deng, Y.; Jiang, C.; Xiao, M.; Wang, J. 3D-Printed IFN-γ-Loading Calcium Silicate-β-Tricalcium Phosphate Scaffold Sequentially Activates M1 and M2 Polarization of Macrophages to Promote Vascularization of Tissue Engineering Bone. Acta Biomater. 2018, 71, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Magnaudeix, A.; Usseglio, J.; Lasgorceix, M.; Lalloue, F.; Damia, C.; Brie, J.; Pascaud-Mathieu, P.; Champion, E. Quantitative Analysis of Vascular Colonisation and Angio-Conduction in Porous Silicon-Substituted Hydroxyapatite with Various Pore Shapes in a Chick Chorioallantoic Membrane (CAM) Model. Acta Biomater. 2016, 38, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Rytlewski, J.A.; Alejandra Aldon, M.; Lewis, E.W.; Suggs, L.J. Mechanisms of Tubulogenesis and Endothelial Phenotype Expression by MSCs. Microvasc. Res. 2015, 99, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Iruela-Arispe, M.L.; Beitel, G.J. Tubulogenesis. Dev. Camb. Engl. 2013, 140, 2851–2855. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Greer, P.; Auerbach, R. Isolation and Propagation of Yolk-Sac-Derived Endothelial Cells from a Hypervascular Transgenic Mouse Expressing a Gain-of-FunctionFPS/FES Proto-Oncogene. Vitr. Cell. Dev. Biol.-Animal 1996, 32, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium Phosphate Apatites with Variable Ca/P Atomic Ratio I. Synthesis, Characterisation and Thermal Stability of Powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef]
- Palard, M.; Combes, J.; Champion, E.; Foucaud, S.; Rattner, A.; Bernache-Assollant, D. Effect of Silicon Content on the Sintering and Biological Behaviour of Ca10(PO4)6−x(SiO4)x(OH)2−x Ceramics. Acta Biomater. 2009, 5, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Palard, M.; Champion, E.; Foucaud, S. Synthesis of Silicated Hydroxyapatite Ca10(PO4)6−x(SiO4)x(OH)2−x. J. Solid State Chem. 2008, 181, 1950–1960. [Google Scholar] [CrossRef]
- Marchat, D.; Zymelka, M.; Coelho, C.; Gremillard, L.; Joly-Pottuz, L.; Babonneau, F.; Esnouf, C.; Chevalier, J.; Bernache-Assollant, D. Accurate Characterization of Pure Silicon-Substituted Hydroxyapatite Powders Synthesized by a New Precipitation Route. Acta Biomater. 2013, 9, 6992–7004. [Google Scholar] [CrossRef]
- Drouet, C.; Aufray, M.; Rollin-Martinet, S.; Vandecandelaère, N.; Grossin, D.; Rossignol, F.; Champion, E.; Navrotsky, A.; Rey, C. Nanocrystalline Apatites: The Fundamental Role of Water. Am. Miner. 2018, 103, 550–564. [Google Scholar] [CrossRef]
- Fernández, E.; Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Calcium Phosphate Bone Cements for Clinical Applications. Part I: Solution Chemistry. J. Mater. Sci. Mater. Med. 1999, 10, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Marchat, D.; Champion, E. Ceramic Devices for Bone Regeneration. In Advances in Ceramic Biomaterials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 279–311. ISBN 978-0-08-100881-2. [Google Scholar]
- Costa, D.O.; Prowse, P.D.H.; Chrones, T.; Sims, S.M.; Hamilton, D.W.; Rizkalla, A.S.; Dixon, S.J. The Differential Regulation of Osteoblast and Osteoclast Activity by Surface Topography of Hydroxyapatite Coatings. Biomaterials 2013, 34, 7215–7226. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, H.; Wu, C.; Chen, L.; Lin, X.; Naoki, K.; Chen, G.; Chang, J. Stimulatory Effects of the Ionic Products from Ca–Mg–Si Bioceramics on Both Osteogenesis and Angiogenesis in Vitro. Acta Biomater. 2013, 9, 8004–8014. [Google Scholar] [CrossRef]
- Wang, X.; Gao, L.; Han, Y.; Xing, M.; Zhao, C.; Peng, J.; Chang, J. Silicon-Enhanced Adipogenesis and Angiogenesis for Vascularized Adipose Tissue Engineering. Adv. Sci. 2018, 5, 1800776. [Google Scholar] [CrossRef]
- Mao, L.; Xia, L.; Chang, J.; Liu, J.; Jiang, L.; Wu, C.; Fang, B. The Synergistic Effects of Sr and Si Bioactive Ions on Osteogenesis, Osteoclastogenesis and Angiogenesis for Osteoporotic Bone Regeneration. Acta Biomater. 2017, 61, 217–232. [Google Scholar] [CrossRef]
- Šalandová, M.; Hengel, I.A.J.; Apachitei, I.; Zadpoor, A.A.; Eerden, B.C.J.; Fratila-Apachitei, L.E. Inorganic Agents for Enhanced Angiogenesis of Orthopedic Biomaterials. Adv. Healthc. Mater. 2021, 10, 2002254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, X.; Gao, L.; Jing, L.; Zhou, Q.; Chang, J. The Role of the Micro-Pattern and Nano-Topography of Hydroxyapatite Bioceramics on Stimulating Osteogenic Differentiation of Mesenchymal Stem Cells. Acta Biomater. 2018, 73, 509–521. [Google Scholar] [CrossRef]
- Skoog, S.A.; Kumar, G.; Narayan, R.J.; Goering, P.L. Biological Responses to Immobilized Microscale and Nanoscale Surface Topographies. Pharmacol. Ther. 2018, 182, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Khang, D.; Choi, J.; Im, Y.-M.; Kim, Y.-J.; Jang, J.-H.; Kang, S.S.; Nam, T.-H.; Song, J.; Park, J.-W. Role of Subnano-, Nano- and Submicron-Surface Features on Osteoblast Differentiation of Bone Marrow Mesenchymal Stem Cells. Biomaterials 2012, 33, 5997–6007. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Boroviak, T.E. Origin and Function of the Yolk Sac in Primate Embryogenesis. Nat. Commun. 2020, 11, 3760. [Google Scholar] [CrossRef]
- Lu, L.S.; Wang, S.J.; Auerbach, R. In Vitro and in vivo Differentiation into B Cells, T Cells, and Myeloid Cells of Primitive Yolk Sac Hematopoietic Precursor Cells Expanded > 100-Fold by Coculture with a Clonal Yolk Sac Endothelial Cell Line. Proc. Natl. Acad. Sci. USA 1996, 93, 14782–14787. [Google Scholar] [CrossRef]
- Maes, C.; Goossens, S.; Bartunkova, S.; Drogat, B.; Coenegrachts, L.; Stockmans, I.; Moermans, K.; Nyabi, O.; Haigh, K.; Naessens, M.; et al. Increased Skeletal VEGF Enhances β-Catenin Activity and Results in Excessively Ossified Bones. EMBO J. 2010, 29, 424–441. [Google Scholar] [CrossRef] [PubMed]
- Lan Levengood, S.K.; Poellmann, M.J.; Clark, S.G.; Ingram, D.A.; Yoder, M.C.; Wagoner Johnson, A.J. Human Endothelial Colony Forming Cells Undergo Vasculogenesis within Biphasic Calcium Phosphate Bone Tissue Engineering Constructs. Acta Biomater. 2011, 7, 4222–4228. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.B. Endothelial Progenitor Cells for the Vascularization of Engineered Tissues. Tissue Eng. Part B Rev. 2018, 24, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, P.; Väänänen, M.-A.; Kolari, I.-L.; Mäkinen, P.I.; Kaikkonen, M.U.; Weinberg, M.S.; Morris, K.V.; Korhonen, P.; Malm, T.; Ylä-Herttuala, S.; et al. Nuclear MicroRNA-466c Regulates Vegfa Expression in Response to Hypoxia. PLoS ONE 2022, 17, e0265948. [Google Scholar] [CrossRef]
- Yoon, K.-A.; Cho, H.-S.; Shin, H.-I.; Cho, J.-Y. Differential Regulation of CXCL5 by FGF2 in Osteoblastic and Endothelial Niche Cells Supports Hematopoietic Stem Cell Migration. Stem Cells Dev. 2012, 21, 3391–3402. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Q.; Wang, Y.; Lin, M.; Tong, Y.; Huang, H.; Yang, C.; Wu, J.; Tang, B.; Bai, J.; et al. Metallic Scaffold with Micron-Scale Geometrical Cues Promotes Osteogenesis and Angiogenesis via the ROCK/Myosin/YAP Pathway. ACS Biomater. Sci. Eng. 2022, 8, 3498–3514. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Zhu, X.D.; Tang, Z.R.; Yang, X.; Tan, Y.F.; Fan, Y.J.; Zhang, X.D. Enhanced Effect of β-Tricalcium Phosphate Phase on Neovascularization of Porous Calcium Phosphate Ceramics: In Vitro and in vivo Evidence. Acta Biomater. 2015, 11, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Götz, H.; Baranowski, A.; Heid, F.; Rommens, P.M.; Hofmann, A. Influence of Different Calcium Phosphate Ceramics on Growth and Differentiation of Cells in Osteoblast-Endothelial Co-Cultures: Influence of Different Calcium Phosphate Ceramics on Cells. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1950–1962. [Google Scholar] [CrossRef]
- Chappell, H.F.; Jugdaohsingh, R.; Powell, J.J. Physiological Silicon Incorporation into Bone Mineral Requires Orthosilicic Acid Metabolism to SiO44−. J. R. Soc. Interface 2020, 17, 20200145. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R.; Tucker, K.L.; Qiao, N.; Cupples, L.A.; Kiel, D.P.; Powell, J.J. Dietary Silicon Intake Is Positively Associated with Bone Mineral Density in Men and Premenopausal Women of the Framingham Offspring Cohort. J. Bone Miner. Res. 2003, 19, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Update on the Possible Nutritional Importance of Silicon. J. Trace Elem. Med. Biol. 2014, 28, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R. The Chemistry of Silica and Its Potential Health Benefits. J. Nutr. Health Aging 2007, 11, 94–97. [Google Scholar]
- Ratcliffe, S.; Jugdaohsingh, R.; Vivancos, J.; Marron, A.; Deshmukh, R.; Ma, J.F.; Mitani-Ueno, N.; Robertson, J.; Wills, J.; Boekschoten, M.V.; et al. Identification of a Mammalian Silicon Transporter. Am. J. Physiol.-Cell Physiol. 2017, 312, C550–C561. [Google Scholar] [CrossRef] [PubMed]
- Garneau, A.P.; Carpentier, G.A.; Marcoux, A.-A.; Frenette-Cotton, R.; Simard, C.F.; Rémus-Borel, W.; Caron, L.; Jacob-Wagner, M.; Noël, M.; Powell, J.J.; et al. Aquaporins Mediate Silicon Transport in Humans. PLoS ONE 2015, 10, e0136149. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, S.; Chen, S.; Ganesan, S.; Hanagata, N. Silicon Quantum Dots for Biological Applications. Adv. Healthc. Mater. 2014, 3, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Lerner, T.; Liljenqvist, U. Silicate-Substituted Calcium Phosphate as a Bone Graft Substitute in Surgery for Adolescent Idiopathic Scoliosis. Eur. Spine J. 2013, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Bolger, C.; Jones, D.; Czop, S. Evaluation of an Increased Strut Porosity Silicate-Substituted Calcium Phosphate, SiCaP EP, as a Synthetic Bone Graft Substitute in Spinal Fusion Surgery: A Prospective, Open-Label Study. Eur. Spine J. 2019, 28, 1733–1742. [Google Scholar] [CrossRef]
- Morin, K.T.; Tranquillo, R.T. In Vitro Models of Angiogenesis and Vasculogenesis in Fibrin Gel. Exp. Cell Res. 2013, 319, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Negri, S.; Shekha, M.; Faris, P.; Guerra, G. Endothelial Ca2+ Signaling, Angiogenesis and Vasculogenesis: Just What It Takes to Make a Blood Vessel. Int. J. Mol. Sci. 2019, 20, 3962. [Google Scholar] [CrossRef]
- Fiorio Pla, A.; Munaron, L. Functional Properties of Ion Channels and Transporters in Tumour Vascularization. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130103. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, P.; Eccles, S.A.; Yaqoob, M.M. Coupling between the TRPC3 Ion Channel and the NCX1 Transporter Contributed to VEGF-Induced ERK1/2 Activation and Angiogenesis in Human Primary Endothelial Cells. Cell. Signal. 2017, 37, 12–30. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usseglio, J.; Dumur, A.; Pagès, E.; Renaudie, É.; Abélanet, A.; Brie, J.; Champion, É.; Magnaudeix, A. Microporous Hydroxyapatite-Based Ceramics Alter the Physiology of Endothelial Cells through Physical and Chemical Cues. J. Funct. Biomater. 2023, 14, 460. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb14090460
Usseglio J, Dumur A, Pagès E, Renaudie É, Abélanet A, Brie J, Champion É, Magnaudeix A. Microporous Hydroxyapatite-Based Ceramics Alter the Physiology of Endothelial Cells through Physical and Chemical Cues. Journal of Functional Biomaterials. 2023; 14(9):460. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb14090460
Chicago/Turabian StyleUsseglio, Julie, Adeline Dumur, Esther Pagès, Émeline Renaudie, Alice Abélanet, Joël Brie, Éric Champion, and Amandine Magnaudeix. 2023. "Microporous Hydroxyapatite-Based Ceramics Alter the Physiology of Endothelial Cells through Physical and Chemical Cues" Journal of Functional Biomaterials 14, no. 9: 460. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb14090460