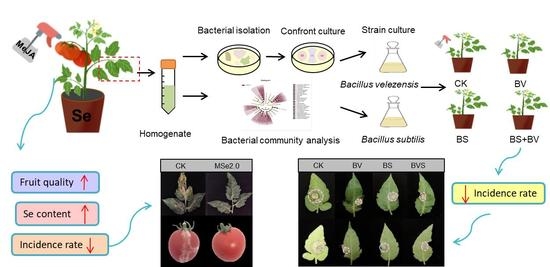
Selenium Combined with Methyl Jasmonate to Control Tomato Gray Mold by Optimizing Microbial Community Structure in Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Disease Incidence
2.2.1. Determination of the Disease Incidence of Tomato Fruits
2.2.2. Determination of the Disease Incidence of Tomato Leaves
2.3. Determination of the Contents of Se, Soluble Protein, and Vitamin C
2.4. Isolation of Bacteria from Tomato Leaves
2.5. Determination of the Antagonistic Activities of Bacteria against Botrytis Cinerea In Vitro
2.6. DNA Extraction and Sequencing
2.6.1. Identification of Antagonistic Strains
2.6.2. 16S rRNA Amplicon Sequencing
2.7. Statistical Analysis
3. Results
3.1. Evaluation of Se andMeJA for the Prevention and Inhibition of Botrytis cinerea in Tomato Fruits
3.2. Effects of Se and MeJA on Se Content, Vitamin C Content, and Soluble Protein Content in Tomatoes
3.3. Effect of Se and MeJA on the Bacterial Community Composition and the Diversity-Inhabiting Fruits and Leaf of the Tomato
3.4. Effect of Se and MeJA on Peculiar Clades among the Bacterial Communities of Tomato Leaf
3.5. Isolation of Phyllosphere Bacteria and Their Antagonism with Botrytis cinerea
3.6. Bacillus velezensis and Bacillus subtilis Improved Plant Disease Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soylu, E.M.; Kurt, S.; Soylu, S. In vitro and in vivo antifungal activity of essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Vos, C.M.; Yang, Y.; Coninck, B.D.; Cammue, B. Fungal (-like) biocontrol organisms in tomato disease control. Biol. Control 2014, 74, 65–81. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.; Acosta, A.; Rodriguez, C. Fungicide resistance of Botrytis cinerea in tomato greenhouses in the Canary Islands and effectiveness of non-chemical treatments against gray mold. World J. Microb. Biotechnol. 2014, 30, 2397–2406. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Zhang, L.B.; Li, Y.; Wang, Z.S.; Yu, Y.; Zhang, N.; Yang, C.; Zeng, Q.C.; Wang, Q. Synthetic community with six Pseudomonas strains screened from garlic rhizosphere microbiome promotes plant growth. Microb. Biotechnol. 2020, 14, 488–502. [Google Scholar] [CrossRef]
- Shi, Y.; Delgado-Baquerizo, M.; Li, Y.T.; Yang, Y.F.; Zhu, Y.G.; Penuelas, J.; Chu, H.Y. Abundance of kinless hubs within soil microbial networks are associated with high functional potential in agricultural ecosystems. Environ. Int. 2021, 142, 105869. [Google Scholar] [CrossRef]
- Li, H.; La, S.K.; Zhang, X.; Gao, L.H.; Tian, Y.Q. Salt-induced recruitment of specific root-associated bacterial consortium capable of enhancing plant adaptability to salt stress. ISME J. 2021, 15, 2865–2882. [Google Scholar] [CrossRef]
- Kwak, M.J.; Kong, H.G.; Choi, K.; Kwon, S.K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef]
- Kusstatscher, P.; Cernava, T.; Abdelfattah, A.; Gokul, J.; Korsten, L.; Berg, G. Microbiome approaches provide the key to biologically control postharvest pathogens and storability of fruits and vegetables. FEMS Microbiol. Ecol. 2020, 96, fiaa119. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, Y.G.; Wang, J.T.; Singh, B.; Han, L.L.; Shen, J.P.; Li, P.P.; Wang, G.B.; Wu, C.F.; Ge, A.H.; et al. Host selection shapes crop microbiome assembly and network complexity. New Phytol. 2020, 229, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Veloso, T.G.R.; da Silva, M.D.S.; Cardoso, W.S.; Guarconi, R.C.; Kasuya, M.C.M.; Pereira, L.L. Effects of environmental factors on microbiota of fruits and soil of Coffea arabica in Brazil. Sci. Rep. 2020, 10, 14692. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.M.; Zhou, X.; Zhang, A.M.; Cai, L. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Li, J.Y.; Carvalhais, L.C.; Percy, C.D.; Verma, J.P.; Schenk, P.M.; Singh, B.K. Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 2021, 229, 2873–2885. [Google Scholar] [CrossRef]
- De Tender, C.; Haegeman, A.; Vandecasteele, B.; Clement, L.; Cremelie, P.; Dawyndt, P.; Maes, M.; Debode, J. Dynamics in the Strawberry Rhizosphere Microbiome in Response to Biochar and Botrytis cinerea Leaf Infection. Front. Microbiol. 2016, 7, 2062. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Zhang, Y.B.; Liu, J.; Chen, Y.L.; Zhang, X.J. Exploring the effects of selenium treatment on the nutritional quality of tomato fruit. Food Chem. 2018, 252, 9–15. [Google Scholar] [CrossRef]
- D’Amato, R.; De Feudis, M.; Hasuoka, P.E.; Regni, L.; Pacheco, P.H.; Onofri, A.; Businelli1, D.; Proietti1, P. The selenium supplementation influences olive tree production and oil stability against oxidation and can alleviate the water deficiency effects. Front. Plant Sci. 2018, 9, 1191. [Google Scholar] [CrossRef]
- Dai, H.P.; Jia, G.L. Effects of Se on the growth, tolerance, and antioxidative systems of three alfalfa cultivars. Environ. Sci. Pollut. Res. 2017, 24, 15196–15201. [Google Scholar] [CrossRef]
- Feng, T.; Chen, S.S.; Gao, D.Q.; Liu, G.Q.; Bai, H.X.; Li, A.; Peng, L.X.; Ren, Z.Y. Selenium improves photosynthesis and protects photosystem II in pear (Pyrus bretschneideri), grape (Vitis vinifera), and peach (Prunus persica). Photosynthetica 2015, 53, 609–612. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Hu, C.X.; Wang, X.; Qing, X.J.; Wang, P.; Zhang, Y.; Zhang, X.; Zhao, X.H. Selenium alleviated chromium stress in Chinese cabbage (brassica campestris L. ssp. pekinensis) by regulating root morphology and metal element uptake. Ecotox. Environ. Safe 2019, 173, 314–321. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Bioch. 2019, 137, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.P.; Qin, N.N.; Sun, L.; Yu, M.Y.; Hu, W.Z.; Qi, Z.Y. Selenium improves physiological parameters and alleviates oxidative stress in strawberry seedlings under low-temperature stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawrylak-Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek-Gawron, R.; Woch, W.; Hasanuzzaman, M. Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol. Biochem. 2018, 127, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Bocchini, M.; D’Amato, R.; Ciancaleoni, S.; Fontanella, M.C.; Palmerini, C.A.; Beone, G.M.; Onofri1, A.; Negri, V.; Marconi, G.; Albertini, E.; et al. Soil selenium (Se) biofortification changes the physiological, biochemical and epigenetic responses to water stress in zea mays l. by inducing a higher drought tolerance. Front. Plant Sci. 2018, 9, 389. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Hu, C.X.; Jia, W.; Cai, M.M.; Zhao, Y.Y.; Tang, Y.N.; Yang, D.D.; Zhou, Y.J.; Sun, X.C.; Zhao, X.H. Selenium reduces the pathogenicity of sclerotinia sclerotiorum by inhibiting sclerotial formation and germination. Ecotox. Environ. Safe 2019, 183, 109503. [Google Scholar] [CrossRef]
- Wu, Y.X.; Zhou, Z.S.; Wang, X.D.; Chi, F.M.; Wang, H.B.; Xu, C.N.; Ji, Z.R. Study on the effect of spraying amino acid selenium foliar fertilizer during the growth period on apple tree rot. China Fruits 2012, 5, 51–53. [Google Scholar]
- Companioni, B.; Medrano, J.; Torres, J.A.; Flores, A.; Benavides-Mendoza, A. Protective action of sodium selenite against Fusarium wilt in tomato: Total protein contents, levels of phenolic compounds and changes in antioxidant potential. Acta Hortic. 2012, 947, 321–328. [Google Scholar] [CrossRef]
- Liu, K.; Cai, M.M.; Hu, C.X.; Sun, X.C.; Cheng, Q.; Jia, W.; Yang, T.N.; Nie, M.; Zhao, X.H. Selenium (Se) reduces sclerotinia stem rot disease incidence of oilseed rape by increasing plant Se concentration and shifting soil microbial community and functional profiles. Environ. Pollut. 2019, 254, 113051. [Google Scholar] [CrossRef]
- Zhu, Z.; Tian, S.P. Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Sci. Hortic. 2012, 142, 38–43. [Google Scholar] [CrossRef]
- Wang, K.T.; Jin, P.; Han, L.; Shang, H.T.; Tang, S.S.; Rui, H.J.; Duan, Y.F.; Kong, F.Y.; Kai, X.; Zheng, Y.H. Methyl jasmonate induces resistance against Penicillium citrinum in Chinese bayberry by priming of defense responses. Postharvest Biol. Technol. 2014, 98, 90–97. [Google Scholar] [CrossRef]
- Guo, J.; Fang, W.W.; Lu, H.P.; Zhu, R.Y.; Lu, L.F.; Zheng, X.D.; Yu, T. Inhibition of green mold disease in mandarins by preventive applications of methyl jasmonate and antagonistic yeast Cryptococcus laurentii. Postharvest Biol. Technol. 2014, 88, 72–78. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.Y.; Chen, T.; Xu, Y.; Tian, S.P. Antifungal effects of hinokitiol on development of Botrytis cinerea in vitro and in vivo. Postharvest Biol. Technol. 2020, 159, 111038. [Google Scholar] [CrossRef]
- Fan, X.Y.; Matsumoto, H.; Wang, Y.; Hu, Y.; Liu, Y.F.; Fang, H.D.; Nitkiewicz, B.; Lau, S.R.Y.L.; Wang, Q.W.; Fang, H.; et al. Microenvironmental Interplay Predominated by Beneficial Aspergillus Abates Fungal Pathogen Incidence in Paddy Environment. Environ. Sci. Technol. 2019, 53, 13042–13052. [Google Scholar] [CrossRef]
- Yin, C.; Hulbert, S.H.; Schroeder, K.L.; Mavrodi, O.; Mavrodi, D.; Dhingra, A.; Schillinger, W.F.; Paulitz, T.C. Role of bacterial communities in the natural suppression of Rhizoctonia solani bare patch disease of wheat (Triticum aestivum L.). Appl. Environ. Microbiol. 2013, 79, 7428–7438. [Google Scholar] [CrossRef] [Green Version]
- Sun, A.Q.; Jiao, X.Y.; Chen, Q.L.; Wu, A.L.; Zheng, Y.; Lin, Y.X.; He, J.Z.; Hu, H.W. Microbial communities in crop phyllosphere and root endosphere are more resistant than soil microbiota to fertilization. Soil Biol. Biochem. 2021, 153, 108113. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Zhang, X.J.; Li, M. Effect of foliar treatment of sodium selenate on postharvest decay and quality of tomato fruits. Sci. Hortic. 2016, 198, 304–310. [Google Scholar] [CrossRef]
- Durán, P.; Acuña, J.J.; Jorquera, M.A.; Azcón, R.; Paredes, C.; Rengel, Z.; Mora, M.D. Endophytic bacteria from selenium-supplemented wheat plants could be useful for plant-growth promotion, biofortification and gaeumannomyces graminis biocontrol in wheat production. Biol. Fert. Soils 2014, 50, 983–990. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Remorini, D.; Gentile, M.L.; Massai, R. Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. J. Sci Food Agric. 2012, 92, 781–786. [Google Scholar] [CrossRef]
- Angeli, D.; Sare, A.R.; Jijakli, M.H.; Pertot, I.; Massart, S. Insights gained from metagenomic shotgun sequencing of apple fruit epiphytic microbiota. Postharvest Biol. Technol. 2019, 153, 96–106. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Wisniewski, M.; Droby, S.; Schena, L. Spatial and compositional variation in the fungal communities of organic and conventionally grown apple fruit at the consumer point-of-purchase. Hortic. Res. 2016, 3, 16047. [Google Scholar] [CrossRef] [Green Version]
- Vitulo, N.; Lemos, W.J.F.; Calgaro, M.; Confalone, M.; Felis, G.E.; Zapparoli, G.; Nardi, T. Bark and grape microbiome of Vitis vinifera: Influence of geographic patterns and agronomic management on bacterial diversity. Front. Microbiol. 2019, 9, 3203. [Google Scholar] [CrossRef] [Green Version]
- Diskin, S.; Feygenberg, O.; Maurer, D.; Droby, S.; Prusky, D.; Alkan, N. Microbiome alterations are correlated with occurrence of postharvest stem-end rot in mango fruit. Phytobiomes J. 2017, 1, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.W.; Lei, J.Q.; Hussain, M.; Cao, S.; Dui, B.; Wang, R. Structure and function of the fruit microbiome in healthy and diseased kiwifruit. Pak. J. Agric. Sci. 2019, 56, 577–585. [Google Scholar] [CrossRef]
- Kusstatscher, P.; Zachow, C.; Harms, K.; Maier, J.; Eigner, H.; Berg, G.; Cernava, T. Microbiome-driven identification of microbial indicators for postharvest diseases of sugar beets. Microbiome 2019, 7, 112. [Google Scholar] [CrossRef]
- Yin, C.; Vargas, J.C.; Schlatter, D.C.; Hagerty, C.; Hulbert, S.H.; Paulitz, T.C. Rhizosphere community selection reveals bacteria associated with reduced root disease. Microbiome 2021, 9, 86. [Google Scholar] [CrossRef]
- Matsumoto, H.; Fan, X.Y.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.L.; Chen, S.L.; Qiao, K.; Wang, Y.L.; Ma, B.; et al. Bacterial seed endophyte shapes disease resistance in rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Brettell, L.E. Plant defense by VOC-induced microbial priming. Trends Plant Sci. 2019, 24, 187–189. [Google Scholar] [CrossRef]
- Sun, X.L.; Xu, Z.H.; Xie, J.Y.; Hesselberg-Thomsen, V.; Tan, T.M.; Zheng, D.Y.; Strube, M.L.; Dragoš, A.; Shen, Q.R.; Zhang, R.F.; et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Li, Y.; Zhuang, L.B.; Yu, Y.; Liu, J.; Zhang, L.X.; Gao, Z.J.; Wu, Y.F.; Gao, W.; Ding, G.C.; et al. A rhizosphere-derived consortium of Bacillus subtilis and Trichoderma harzianum suppresses common scab of potato and increases yield. Comput. Struct. Biotechnol. 2019, 17, 645–653. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Hu, C.; Xie, J.; Shi, G.; Wang, X.; Yuan, X.; Li, K.; Chen, S.; Zhao, X.; Fan, G. Selenium Combined with Methyl Jasmonate to Control Tomato Gray Mold by Optimizing Microbial Community Structure in Plants. J. Fungi 2022, 8, 731. https://0-doi-org.brum.beds.ac.uk/10.3390/jof8070731
Li C, Hu C, Xie J, Shi G, Wang X, Yuan X, Li K, Chen S, Zhao X, Fan G. Selenium Combined with Methyl Jasmonate to Control Tomato Gray Mold by Optimizing Microbial Community Structure in Plants. Journal of Fungi. 2022; 8(7):731. https://0-doi-org.brum.beds.ac.uk/10.3390/jof8070731
Chicago/Turabian StyleLi, Changyin, Chengxiao Hu, Jiatao Xie, Guangyu Shi, Xu Wang, Xiang Yuan, Keyi Li, Siqi Chen, Xiaohu Zhao, and Guocheng Fan. 2022. "Selenium Combined with Methyl Jasmonate to Control Tomato Gray Mold by Optimizing Microbial Community Structure in Plants" Journal of Fungi 8, no. 7: 731. https://0-doi-org.brum.beds.ac.uk/10.3390/jof8070731