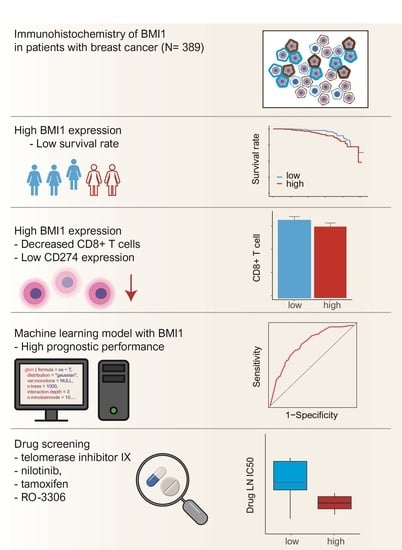
High BMI1 Expression with Low CD8+ and CD4+ T Cell Activity Could Promote Breast Cancer Cell Survival: A Machine Learning Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Tissue Microarray Construction and Immunohistochemistry
2.3. Gene Sets, In Silico Flow Cytometry, and Network-Based Analysis
2.4. Machine Learning Algorithm
2.5. Data Extraction from the GDSC and COSMIC Databases
2.6. Statistical Analysis
3. Results
3.1. Clinicopathologic Characteristics and Survival Rate of Patients with Breast Cancer Stratified by BMI1 Expression
3.2. Gene Set Enrichment Analysis, Anticancer Immune Response, and Network-Based Analysis of BMI1
3.3. Pathway-Based Network Analysis, Machine Learning and Drug Screening
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [Green Version]
- Magro, G.; Salvatorelli, L.; Puzzo, L.; Piombino, E.; Bartoloni, G.; Broggi, G.; Vecchio, G. Practical approach to diagnosis of bland-looking spindle cell lesions of the breast. Pathologica 2019, 111, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Ascierto, P.A.; Darcy, P.; Demaria, S.; Eggermont, A.M.; Redmond, W.L.; Seliger, B.; Marincola, F.M. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur. J. Cancer 2017, 81, 116–129. [Google Scholar] [CrossRef]
- Lipson, E.J.; Forde, P.M.; Hammers, H.-J.; Emens, L.A.; Taube, J.M.; Topalian, S.L. Antagonists of PD-1 and PD-L1 in Cancer Treatment. Semin. Oncol. 2015, 42, 587–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukumo, S.-I.; Yasutomo, K. Regulation of CD8+ T Cells and Antitumor Immunity by Notch Signaling. Front. Immunol. 2018, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Emens, L.A. Breast Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savas, P.P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.K.; Loi, S. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241. [Google Scholar] [CrossRef]
- Cimino-Mathews, A.; Thompson, E.; Taube, J.M.; Ye, X.; Lu, Y.; Meeker, A.; Xu, H.; Sharma, R.; Lecksell, K.; Cornish, T.; et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum. Pathol. 2016, 47, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Lai, J.; Dai, D.; Chen, R.; Li, X.; Liao, N. JAK1 as a prognostic marker and its correlation with immune infiltrates in breast cancer. Aging 2019, 11, 11124–11135. [Google Scholar] [CrossRef]
- Broggi, G.; Filetti, V.; Ieni, A.; Rapisarda, V.; Ledda, C.; Vitale, E.; Varricchio, S.; Russo, D.; Lombardo, C.; Tuccari, G.; et al. MacroH2A1 Immunoexpression in Breast Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Bai, X.; Li, Y.; Ni, C.; Zhao, X.; Zhang, D. ITGA3 Is Associated With Immune Cell Infiltration and Serves as a Favorable Prognostic Biomarker for Breast Cancer. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Valk-Lingbeek, M.E.; Bruggeman, S.W.; van Lohuizen, M. Stem Cells and Cancer: The Polycomb Connection. Cell 2004, 118, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, E.; Waghray, M.; Lee, C.; Heidt, D.G.; Yalamanchili, M.; Li, C.; Bednar, F.; Simeone, D.M. Bmi1 Enhances Tumorigenicity and Cancer Stem Cell Function in Pancreatic Adenocarcinoma. PLoS ONE 2013, 8, e55820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, T.; Miyagi, S.; Saraya, A.; Aoki, R.; Seki, A.; Morita, Y.; Yonemitsu, Y.; Yokosuka, O.; Taniguchi, H.; Nakauchi, H.; et al. The Polycomb Gene Product BMI1 Contributes to the Maintenance of Tumor-Initiating Side Population Cells in Hepatocellular Carcinoma. Cancer Res. 2008, 68, 7742–7749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Xu, L.-B.; Zeng, H.; Yu, X.-H.; Wang, J.; Liu, C. Elevated expression of Bmi1 in hepatocellular carcinoma with bile duct tumor thrombi. Hepatogastroenterology 2013, 60, 2042–2047. [Google Scholar] [PubMed]
- Zhang, R.; Xu, L.-B.; Yue, X.-J.; Yu, X.-H.; Wang, J.; Liu, C. Bmi1 gene silencing inhibits the proliferation and invasiveness of human hepatocellular carcinoma cells and increases their sensitivity to 5-fluorouracil. Oncol. Rep. 2013, 29, 967–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leenders, G.J.; Dukers, D.; Hessels, D.; Kieboom, S.W.V.D.; Hulsbergen, C.A.; Witjes, J.A.; Otte, A.P.; Meijer, C.J.; Raaphorst, F.M. Polycomb-Group Oncogenes EZH2, BMI1, and RING1 Are Overexpressed in Prostate Cancer With Adverse Pathologic and Clinical Features. Eur. Urol. 2007, 52, 455–463. [Google Scholar] [CrossRef]
- Yu, J.; Chen, L.; Bao, Z.; Liu, Y.; Liu, G.; Li, F.; Li, L. BMI-1 promotes invasion and metastasis in endometrial adenocarcinoma and is a poor prognostic factor. Oncol. Rep. 2020, 43, 1630–1640. [Google Scholar] [CrossRef]
- Saudy, N.S.; Fawzy, I.M.; Azmy, E.; Goda, E.F.; Eneen, A.; Salam, E.M.A. BMI1 gene expression in myeloid leukemias and its impact on prognosis. Blood Cells Mol. Dis. 2014, 53, 194–198. [Google Scholar] [CrossRef]
- Song, L.-B.; Zeng, M.; Liao, W.-T.; Zhang, L.; Mo, H.-Y.; Liu, W.-L.; Shao, J.-Y.; Wu, Q.-L.; Li-Bing, S.; Xia, Y.-F.; et al. Bmi-1 Is a Novel Molecular Marker of Nasopharyngeal Carcinoma Progression and Immortalizes Primary Human Nasopharyngeal Epithelial Cells. Cancer Res. 2006, 66, 6225–6232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Geng, Y.; Gu, W.; Ning, Z.; Jiang, J.; Pei, H. Prognostic role of high Bmi-1 expression in Asian and Caucasian patients with solid tumors: A meta-analysis. Biomed. Pharmacother. 2014, 68, 969–977. [Google Scholar] [CrossRef]
- Paranjape, A.N.; Balaji, S.A.; Mandal, T.; Krushik, E.V.; Nagaraj, P.; Mukherjee, G.; Rangarajan, A. Bmi1 regulates self-renewal and epithelial to mesenchymal transition in breast cancer cells through Nanog. BMC Cancer 2014, 14, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojo, D.; Lin, X.; Wu, Y.; Cockburn, J.; Bane, A.; Tang, D. Polycomb complex protein BMI1 confers resistance to tamoxifen in estrogen receptor positive breast cancer. Cancer Lett. 2018, 426, 4–13. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, W.; Wang, C.-Y. BMI1 Inhibition Eliminates Residual Cancer Stem Cells after PD1 Blockade and Activates Antitumor Immunity to Prevent Metastasis and Relapse. Cell Stem Cell 2020, 27, 238–253.e6. [Google Scholar] [CrossRef]
- Basile, T.M.A.; Fanizzi, A.; Losurdo, L.; Bellotti, R.; Tangaro, S.; La Forgia, D.; Didonna, V.; Massafra, R.; Tamborra, P.; Moschetta, M.; et al. Hough transform for clustered microcalcifications detection in full-field digital mammograms. Appl. Digit. Image Process. XL 2017, 41. [Google Scholar] [CrossRef]
- Fanizzi, A.; Basile, T.M.; Losurdo, L.; Bellotti, R.; Bottigli, U.; Campobasso, F.; Didonna, V.; Fausto, A.; Massafra, R.; Tagliafico, A.; et al. Ensemble Discrete Wavelet Transform and Gray-Level Co-Occurrence Matrix for Microcalcification Cluster Classification in Digital Mammography. Appl. Sci. 2019, 9, 5388. [Google Scholar] [CrossRef]
- Losurdo, L.; Fanizzi, A.; Basile, T.M.A.; Bellotti, R.; Bottigli, U.; Dentamaro, R.; Didonna, V.; Lorusso, V.; Massafra, R.; Tamborra, P.; et al. Radiomics Analysis on Contrast-Enhanced Spectral Mammography Images for Breast Cancer Diagnosis: A Pilot Study. Entropy 2019, 21, 1110. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.-W.; Wang, K.; Xia, Y.-X.; Wang, J.-S.; Wang, X.-H.; Li, X.-C. Integrating Machine Learning and Tumor Immune Signature to Predict Oncologic Outcomes in Resected Biliary Tract Cancer. Ann. Surg. Oncol. 2020, 28, 4018–4029. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, A.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Garnett, M.J.; Edelman, E.J.; Heidorn, S.J.; Greenman, C.D.; Dastur, A.; Lau, K.W.; Greninger, P.; Thompson, I.R.; Luo, X.; Soares, J.; et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 2012, 483, 570–575. [Google Scholar] [CrossRef] [Green Version]
- Iorio, F.; Knijnenburg, T.A.; Vis, D.J.; Bignell, G.R.; Menden, M.P.; Schubert, M.; Aben, N.; Gonçalves, E.; Barthorpe, S.; Lightfoot, H.; et al. A Landscape of Pharmacogenomic Interactions in Cancer. Cell 2016, 166, 740–754. [Google Scholar] [CrossRef] [Green Version]
- Sauerbrei, W.; Taube, S.E.; McShane, L.M.; Cavenagh, M.M.; Altman, D.G. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J. Natl. Cancer Inst. 2018, 110, 803–811. [Google Scholar] [CrossRef]
- Remmele, W.; Hildebrand, U.; Hienz, H.A.; Klein, P.-J.; Vierbuchen, M.; Heicke, B.; Scheidt, E. Comparative histological, histochemical, immunohistochemical and biochemical studies on oestrogen receptors, lectin receptors, and Barr bodies in human breast cancer. Virchows Arch. 1986, 409, 127–147. [Google Scholar] [CrossRef]
- Peng, X.; Chen, Z.; Farshidfar, F.; Xu, X.; Lorenzi, P.L.; Wang, Y.; Cheng, F.; Tan, L.; Mojumdar, K.; Du, D.; et al. Molecular Characterization and Clinical Relevance of Metabolic Expression Subtypes in Human Cancers. Cell Rep. 2018, 23, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Althobiti, M.; Muftah, A.A.; Aleskandarany, M.A.; Joseph, C.; Toss, M.S.; Green, A.; Rakha, E. The prognostic significance of BMI1 expression in invasive breast cancer is dependent on its molecular subtypes. Breast Cancer Res. Treat. 2020, 182, 581–589. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, T.; Sun, W.; Liu, C.; Fang, X. Bmi-1 overexpression as an efficient prognostic marker in patients with nonsmall cell lung cancer. Medicine 2017, 96, e7346. [Google Scholar] [CrossRef]
- Koren, A.; Rijavec, M.; Sodja, E.; Kern, I.; Sadikov, A.; Kovac, V.; Korosec, P.; Cufer, T. High BMI1 mRNA expression in peripheral whole blood is associated with favorable prognosis in advanced non-small cell lung cancer patients. Oncotarget 2017, 8, 25384–25394. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Takenobu, H.; Akita, N.; Nakazawa, A.; Ochiai, H.; Shimozato, O.; Fujimura, Y.-I.; Koseki, H.; Yoshino, I.; Kimura, H.; et al. Bmi1 regulates cell fate via tumor suppressor WWOX repression in small-cell lung cancer cells. Cancer Sci. 2011, 102, 983–990. [Google Scholar] [CrossRef]
- Rhyu, M.S. Telomeres, Telomerase, and Immortality. J. Natl. Cancer Inst. 1995, 87, 884–894. [Google Scholar] [CrossRef]
- Morin, G.B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 1989, 59, 521–529. [Google Scholar] [CrossRef]
- di Fagagna, F.D.A.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA Damage Checkpoint Response in Telomere-Initiated Senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Weinrich, S.L.; Pruzan, R.; Ma, L.; Ouellette, M.; Tesmer, V.M.; Holt, S.E.; Bodnar, A.G.; Lichtsteiner, S.; Kim, N.W.; Trager, J.B.; et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat. Genet. 1997, 17, 498–502. [Google Scholar] [CrossRef]
- Elkak, A.; Mokbel, R.; Wilson, C.; Jiang, W.; Newbold, R.F.; Mokbel, K. hTERT mRNA expression is associated with a poor clinical outcome in human breast cancer. Anticancer. Res. 2006, 26, 4901–4904. [Google Scholar]
- Hiyama, E.; Yokoyama, T.; Tatsumoto, N.; Hiyama, K.; Imamura, Y.; Murakami, Y.; Kodama, T.; Piatyszek, M.A.; Shay, J.W.; Matsuura, Y. Telomerase activity in gastric cancer. Cancer Res. 1995, 55, 3258–3262. [Google Scholar]
- Haber, D.A. Telomeres, Cancer, and Immortality. N. Engl. J. Med. 1995, 332, 955–956. [Google Scholar] [CrossRef]
- Chadéneau, C.; Hay, K.; Hirte, H.W.; Gallinger, S.; Bacchetti, S. Telomerase activity associated with acquisition of malignancy in human colorectal cancer. Cancer Res. 1995, 55, 2533–2536. [Google Scholar]
- Dimri, G.P.; Martinez, J.-L.; Jacobs, J.J.L.; Keblusek, P.; Itahana, K.; Van Lohuizen, M.; Campisi, J.; Wazer, D.E.; Band, V. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 2002, 62, 4736–4745. [Google Scholar]
- Fan, C.; He, L.; Kapoor, A.; Gillis, A.; Rybak, A.; Cutz, J.-C.; Tang, D. Bmi1 promotes prostate tumorigenesis via inhibiting p16INK4A and p14ARF expression. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2008, 1782, 642–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wazir, U.; Orakzai, M.; Martin, T.A.; Jiang, W.G.; Mokbel, K. Correlation of TERT and Stem Cell Markers in the Context of Human Breast Cancer. Cancer Genom. Proteom. 2019, 16, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, C.; Zhang, X.; Hua, R.; Gan, L.; Huang, M.; Zhao, L.; Ni, S.; Guo, W. Bmi-1 regulates stem cell-like properties of gastric cancer cells via modulating miRNAs. J. Hematol. Oncol. 2016, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelsen, I.B.; Mannelqvist, M.; Stefansson, I.M.; Carter, S.L.; Beroukhim, R.; Øyan, A.M.; Otte, A.P.; Kalland, K.H.; Akslen, L.A.; Salvesen, H.B. Low BMI-1 expression is associated with an activated BMI-1-driven signature, vascular invasion, and hormone receptor loss in endometrial carcinoma. Br. J. Cancer 2008, 98, 1662–1669. [Google Scholar] [CrossRef] [PubMed]





| Parameter | BMI1 Expression | p Value | |
|---|---|---|---|
| Low (n = 225), n (%) | High (n = 164), n (%) | ||
| Age | |||
| ≤55 y | 161 (71.6) | 121 (73.8) | 0.627 |
| >55 y | 64 (28.4) | 43 (26.2) | |
| T stage | |||
| 1 | 102 (45.3) | 84 (51.2) | 0.189 a |
| 2 | 105 (46.7) | 62 (37.8) | |
| 3 | 18 (8%) | 18 (11) | |
| N stage | |||
| 0 | 154 (68.4%) | 107 (65.2%) | 0.758 a |
| 1 | 37 (16.4%) | 34 (20.7%) | |
| 2 | 25 (11.1%) | 17 (10.4%) | |
| 3 | 9 (4.0%) | 6 (3.7%) | |
| Histological grade | |||
| 1 | 39 (17.3) | 27 (16.5) | 0.675 a |
| 2 | 113 (50.2) | 90 (54.9) | |
| 3 | 73 (32.4) | 47 (28.7) | |
| Lymphatic invasion | |||
| Negative | 178 (79.1%) | 131 (79.9%) | 0.954 |
| Positive | 47 (20.9%) | 33 (20.1%) | |
| Vascular invasion | |||
| Negative | 191 (85.3) | 135 (82.3) | 0.433 |
| Positive | 33 (14.7) | 29 (17.7) | |
| ER status | |||
| Negative | 119 (52.9%) | 48 (29.3%) | <0.001 |
| Positive | 106 (47.1%) | 116 (70.7%) | |
| PR status | |||
| Negative | 152 (67.6) | 91 (55.5) | 0.015 |
| Positive | 73 (32.4) | 73 (44.5) | |
| HER2 status | |||
| Negative | 147 (65.3%) | 106 (64.6%) | 0.972 |
| Positive | 78 (34.7%) | 58 (35.4%) | |
| Survival | Univariate a | Multivariate b | HR | 95% CI | |
|---|---|---|---|---|---|
| Disease-free survival | |||||
| BMI1 (low vs. high) | 0.029 | 0.028 | 1.934 | 1.076 | 3.475 |
| T stage (1 or 2 vs. 3) | 0.015 | 0.358 | 1.418 | 0.673 | 2.987 |
| N stage (0, 1 or 2 vs. 3) | <0.001 | 0.002 | 3.006 | 1.500 | 6.023 |
| Histologic grade (1 or 2 vs. 3) | 0.052 | 0.806 | 0.924 | 0.492 | 1.734 |
| Lymphatic invasion (negative vs. positive) | <0.001 | 0.026 | 2.027 | 1.089 | 3.774 |
| ER/PR (positive vs. negative) | 0.310 | 0.033 | 0.509 | 0.273 | 0.948 |
| HER2 (negative vs. positive) | 0.634 | 0.632 | 0.868 | 0.487 | 1.547 |
| Disease-specific survival | |||||
| BMI1 (low vs. high) | 0.015 | 0.019 | 2.139 | 1.132 | 4.039 |
| T stage (1 or 2 vs. 3) | <0.001 | 0.106 | 1.894 | 0.873 | 4.109 |
| N stage (0, 1 or 2 vs. 3) | <0.001 | 0.006 | 2.819 | 1.341 | 5.922 |
| Histologic grade (1 or 2 vs. 3) | 0.049 | 0.639 | 1.174 | 0.601 | 2.291 |
| Lymphatic invasion (negative vs. positive) | <0.001 | 0.008 | 2.485 | 1.264 | 4.886 |
| ER/PR status (positive vs. negative) | 0.938 | 0.645 | 0.851 | 0.429 | 1.688 |
| HER2 status (negative vs. positive) | 0.845 | 0.924 | 0.970 | 0.515 | 1.825 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.; Min, K.-W.; Kim, D.-H.; Son, B.K.; Do, S.-I.; Chae, S.W.; Kwon, M.J. High BMI1 Expression with Low CD8+ and CD4+ T Cell Activity Could Promote Breast Cancer Cell Survival: A Machine Learning Approach. J. Pers. Med. 2021, 11, 739. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm11080739
Chung Y, Min K-W, Kim D-H, Son BK, Do S-I, Chae SW, Kwon MJ. High BMI1 Expression with Low CD8+ and CD4+ T Cell Activity Could Promote Breast Cancer Cell Survival: A Machine Learning Approach. Journal of Personalized Medicine. 2021; 11(8):739. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm11080739
Chicago/Turabian StyleChung, Yumin, Kyueng-Whan Min, Dong-Hoon Kim, Byoung Kwan Son, Sung-Im Do, Seoung Wan Chae, and Mi Jung Kwon. 2021. "High BMI1 Expression with Low CD8+ and CD4+ T Cell Activity Could Promote Breast Cancer Cell Survival: A Machine Learning Approach" Journal of Personalized Medicine 11, no. 8: 739. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm11080739