Directly Acting Antiviral-Based Treatment for HCV-Infected Persons Who Inject Drugs: A Multicenter Real-Life Study
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Outcomes
2.3. Laboratory Methods
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Baselines Characteristics of Patients
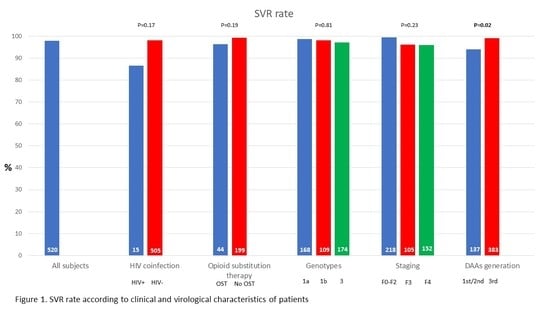
3.2. Virological Response and Associated Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Hepatitis C virus | HCV |
| interferon alpha | IFN |
| ribavirin | RBV |
| sustained virological response | SVR |
| directly acting antiviral agents | DAAs |
| people who inject drugs | PWID |
| World Health Organization | WHO |
| Service for Dependence | SerD |
References
- World Health Organization. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar] [CrossRef]
- Degenhardt, L.; Peacock, A.; Colledge, S.; Leung, J.; Grebely, J.; Vickerman, P.; Stone, J.; Cunningham, E.B.; Trickey, A.; Dumchev, K.; et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. Lancet Glob. Health 2017, 5, e1192–e1207. [Google Scholar] [CrossRef] [Green Version]
- Cousien, A.; Tran, V.C.; Deuffic-Burban, S.; Jauffret-Roustide, M.; Dhersin, J.S.; Yazdanpanah, Y. Hepatitis C treatment as prevention of viral transmission and liver-related morbidity in persons who inject drugs. Hepatology 2016. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.; Martin, N.K.; Brummer-Korvenkontio, H.; Carrieri, P.; Dalgard, O.; Dillon, J.; Goldberg, D.J.; Hutchinson, S.J.; Jauffret-Roustide, M.; Kåberg, M.; et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J. Hepatol. 2018, 68, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Iversen, J.; Dore, G.J.; Catlett, B.; Cunningham, P.; Grebely, J.; Maher, L. Association between rapid utilisation of direct hepatitis C antivirals and decline in the prevalence of viremia among people who inject drugs in Australia. J. Hepatol. 2019, 70, 33–39. [Google Scholar] [CrossRef]
- Iversen, J.; Grebely, J.; Topp, L.; Wand, H.; Dore, G.; Maher, L. Uptake of hepatitis C treatment among people who inject drugs attending Needle and Syringe Programs in Australia, 1999–2011. J. Viral Hepat. 2013, 21, 198–207. [Google Scholar] [CrossRef]
- Spearman, C.W.; Dusheiko, G.M.; Hellard, M.; Sonderup, M. Hepatitis C. Lancet 2019, 394, 1451–1466. [Google Scholar] [CrossRef]
- Lalezari, J.; Sullivan, J.G.; Varunok, P.; Galen, E.; Kowdley, K.V.; Rustgi, V.; Aguilar, H.; Felizarta, F.; McGovern, B.; King, M.; et al. Ombitasvir/paritaprevir/r and dasabuvir plus ribavirin in HCV genotype 1-infected patients on methadone or buprenorphine. J. Hepatol. 2015, 63, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Dore, G.J.; Altice, F.; Litwin, A.H.; Dalgard, O.; Gane, E.J.; Shibolet, O.; Luetkemeyer, A.; Nahass, R.; Peng, C.-Y.; Conway, B.; et al. Elbasvir–Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy. Ann. Intern. Med. 2016, 165, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Grebely, J.; Dalgard, O.; Conway, B.; Cunningham, E.B.; Bruggmann, P.; Hajarizadeh, B.; Amin, J.; Bruneau, J.E.; Hellard, M.; Litwin, A.H.; et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): An open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Grebely, J.; Hajarizadeh, B.; Dore, G.J. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 641–651. [Google Scholar] [CrossRef]
- Hajarizadeh, B.; Cunningham, E.B.; Reid, H.; Law, M.; Dore, G.J.; Grebely, J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2018, 3, 754–767. [Google Scholar] [CrossRef]
- Asher, A.K.; Portillo, C.J.; Cooper, B.A.; Dawson-Rose, C.; Vlahov, D.; Page, K.A. Clinicians’ Views of Hepatitis C Virus Treatment Candidacy With Direct-Acting Antiviral Regimens for People Who Inject Drugs. Subst. Use Misuse 2016, 51, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Starace, M.; Minichini, C.; De Pascalis, S.; Macera, M.; Occhiello, L.; Messina, V.; SanGiovanni, V.; Adinolfi, L.E.; Claar, E.; Precone, D.; et al. Virological patterns of HCV patients with failure to interferon-free regimens. J. Med. Virol. 2018, 90, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Minichini, C.; Starace, M.; De Pascalis, S.; Macera, M.; Occhiello, L.; Caroprese, M.; Vitrone, M.; Iovinella, V.; Guerrera, B.; Masarone, M.; et al. HCV-genotype 3h, a difficult-to-diagnose sub-genotype in the DAA era. Antivir. Ther. 2018, 23, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Persico, M.; Aglitti, A.; Milella, M.; Coppola, C.; Messina, V.; Claar, E.; Gentile, I.; Sogari, F.; Pierri, P.; Surace, L.A.; et al. Real-life glecaprevir/pibrentasvir in a large cohort of patients with hepatitis C virus infection: The MISTRAL study. Liver Int. 2019, 39, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef]
- Pawlotsky, J.-M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Agarwal, K.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [Green Version]
- Chung, R.T.; Ghany, M.G.; Kim, A.Y.; Marks, K.M.; Naggie, S.; Vargas, H.E.I.; Aronsohn, A.; Bhattacharya, D.; Broder, T. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin. Infect. Dis. 2018, 67, 1477–1492. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021; Global Hepatitis Programme, Department of the HIV/AIDS; Pan American Health Organization: Washington, DC, USA, 2016. [Google Scholar]
- Messina, V.; Russo, A.; Parente, E.; Russo, G.; Raimondo, T.; Salzillo, A.; Simeone, F.; Onorato, L.; Di Caprio, G.; Pisaturo, M.; et al. Innovative procedures for micro-elimination of HCV infection in persons who use drugs. J. Viral Hepat. 2020, 27, 1437–1443. [Google Scholar] [CrossRef]
- 2Barua, S.; Greenwald, R.; Grebely, J.; Dore, G.J.; Swan, T.; Taylor, L.E. Restrictions for Medicaid Reimbursement of Sofosbuvir for the Treatment of Hepatitis C Virus Infection in the United States. Ann. Intern. Med. 2015, 163, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Marshall, A.D.; Cunningham, E.B.; Nielsen, S.; Aghemo, A.; Alho, H.; Backmund, M.; Bruggmann, P.; Dalgard, O.; Seguin-Devaux, C.; Flisiak, R.; et al. Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol. Hepatol. 2018, 3, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, E.B.; Hajarizadeh, B.; Amin, J.; Litwin, A.H.; Gane, E.; Cooper, C.; Lacombe, K.; Hellard, M.; Read, P.; Powis, J.; et al. Adherence to Once-daily and Twice-daily Direct-acting Antiviral Therapy for Hepatitis C Infection Among People With Recent Injection Drug Use or Current Opioid Agonist Therapy. Clin. Infect. Dis. 2020, 71, e115–e124. [Google Scholar] [CrossRef] [PubMed]
- Grebely, J.; Conway, B.; Cunningham, E.B.; Fraser, C.; Moriggia, A.; Gane, E.; Stedman, C.; Cooper, C.; Castro, E.; Schmid, P.; et al. Paritaprevir, ritonavir, ombitasvir, and dasabuvir with and without ribavirin in people with HCV genotype 1 and recent injecting drug use or receiving opioid substitution therapy. Int. J. Drug Policy 2018, 62, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Scherz, N.; Bruggmann, P.; Brunner, N. Direct-acting antiviral therapy for hepatitis C infection among people receiving opioid agonist treatment or heroin assisted treatment. Int. J. Drug Policy 2018, 62, 74–77. [Google Scholar] [CrossRef]
- Macías, J.; Morano, L.E.; Téllez, F.; Granados, R.; Román, A.R.; Palacios, R.; Ríos, M.; Merino, D.; Pérez-Pérez, M.; Collado, A.; et al. Response to direct-acting antiviral therapy among ongoing drug users and people receiving opioid substitution therapy. J. Hepatol. 2019, 71, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P. Treatment of hepatitis C in difficult-to-treat patients. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 284–292. [Google Scholar] [CrossRef]
- Grebely, J.; Dore, G.J.; Alami, N.N.; Conway, B.; Dillon, J.F.; Gschwantler, M.; Felizarta, F.; Hézode, C.; Tomasiewicz, K.; Fredrick, L.M.; et al. Safety and efficacy of glecaprevir/pibrentasvir in patients with chronic hepatitis C genotypes 1–6 receiving opioid substitution therapy. Int. J. Drug Policy 2019, 66, 73–79. [Google Scholar] [CrossRef]
- Grebely, J.; Puoti, M.; Wedemeyer, H.; Cooper, C.; Sulkowski, M.S.; Foster, G.R.; Berg, T.; Villa, E.; Rodriguez-Perez, F.; Wyles, D.L.; et al. Efficacy and Safety of Ombitasvir/Paritaprevir/Ritonavir and Dasabuvir With or Without Ribavirin in Patients With Chronic Hepatitis C Virus Genotype 1 Infection Receiving Opioid Substitution Therapy: A Post Hoc Analysis of 12 Clinical Trials. Open Forum Infect. Dis. 2018, 5. [Google Scholar] [CrossRef]
- Christensen, S.; Buggisch, P.; Mauss, S.; Böker, K.H.W.; Schott, E.; Klinker, H.; Zimmermann, T.; Weber, B.; Reimer, J.; Serfert, Y.; et al. Direct-acting antiviral treatment of chronic HCV-infected patients on opioid substitution therapy: Still a concern in clinical practice? Addiction 2018, 113, 868–882. [Google Scholar] [CrossRef]
| No Patients | 520 |
|---|---|
| Mean age (SD), years | 47.7 (9.3) |
| Males,n(%) | 456 (87.5) |
| HCV-RNA, median (IQR), UI/mL | 1.2 × 106 (IQR 3.64 × 105–3.5 × 106) |
| HCV genotype,n(%) | |
| - Genotype 1a | 168 (32.3) |
| - Genotype 1b | 109 (21.0) |
| - Genotype 2 | 29 (5.6) |
| - Genotype 3 | 174 (33.5) |
| - Genotype 4 | 24 (4.6) |
| - Mixed genotypes | 8 (1.5) |
| - Unknown | 8 (1.5) |
| HIV coinfection,n(%) | 16 (3.1) |
| Staging of fibrosis (Metavir),n(%) | |
| - F0/F1 | 130 (25.0) |
| - F2 | 88 (16.9) |
| - F3 | 105 (20.2) |
| - F4 | 152 (29.2) |
| - Unknown | 45 (8.7) |
| AST (xULN), mean (SD) | 1.48 (1.3) |
| ALT (xULN), mean (SD) | 1.95 (1.8) |
| Creatinine (mean, SD), mg/dl | 0.87 (0.96) |
| Patients treated with OST,n(%) * | 169 (33.6) |
| N(%) pts treated with DAAs of | |
| - First generation | 9 (1.7) |
| - Second generation | 128 (24.6) |
| - Third generation | 383 (73.7) |
| Length of treatment, (median, IQR), weeks | 12 (8–12) |
| SVR | no-SVR | p Value | |
|---|---|---|---|
| No patients | 509 | 11 | |
| Mean age (SD), years | 47.8 (9.3) | 46.5 (8.4) | 0.62 |
| Males,n(%) | 446 (87.6) | 10 (90.9) | 0.37 * |
| HCV RNA, median (IQR), UI/mL | 1.2 × 106 (3.68 × 105–3.43 × 106) | 1.36 × 106 (3.0 × 105–3.45 × 106) | 0.41 |
| HCV genotype,n(%) | |||
| - Genotype 1a | 166 (32.6) | 2 (18.2) | 0.81 |
| - Genotype 1b | 107 (21.0) | 2 (18.2) | |
| - Genotype 2 | 28 (5.5) | 1 (9.1) | |
| - Genotype 3 | 169 (33.2) | 5 (45.4) | |
| - Genotype 4 | 24 (4.7) | 0 (0.0) | |
| - Mixed genotypes | 8 (1.6) | 0 (0.0) | |
| - Unknown | 7 (1.4) | 1 (9.1) | |
| Staging of fibrosis (Metavir),n(%) | |||
| - F0/F1 | 129 (25.3) | 1 (9.1) | 0.09 |
| - F2 | 88 (17.3) | 0 (0.0) | |
| - F3 | 101 (19.8) | 4 (36.4) | |
| - F4 | 146 (28.7) | 6 (54.5) | |
| - Unknown | 45 (8.8) | 0 (0.0) | |
| HIV coinfection,n(%) | 13 (2.6) | 2 (18.2) | 0.03 * |
| AST (xULN), mean (SD) | 1.48 (1.3) | 1.47 (0.3) | 0.95 |
| ALT (xULN), mean (SD) | 1.95 (1.8) | 2 (0.5) | 0.88 |
| Creatinine (mean, SD), mg/dL | 0.87 (0.97) | 0.73 (0.2) | 0.16 |
| Opioid substitution therapy,n(%) ** | |||
| - Patients receiving OST | 163 (31.3) | 6 (54.5) | <0.001 |
| - Patients not receiving OST | 332 (63.8) | 2 (18.2) | |
| - Unknown | 14 (2.7) | 3 (27.3) | |
| N(%) pts treated with DAA of | |||
| - First generation | 9 (1.7) | 0 (0.0) | 0.0015 |
| - Second generation | 120 (23.6) | 8 (72.7) | |
| - Third generation- | 380 (74.7) | 3 (27.3) | |
| Length of treatment, (median, IQR), weeks | 12 (8–12) | 12 (12–12) | 0.48 |
| Variables | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Gender (M vs. F) | 1.59 | 0.10 | 25.2 | 0.74 |
| Age | 1.12 | 0.98 | 1.27 | 0.09 |
| Liver fibrosis (F0–F3 vs. F4) | 0.28 | 0.03 | 2.24 | 0.23 |
| HIV serostatus (negative vs. positive) | 0.20 | 0.02 | 2.02 | 0.17 |
| Treatment received (1st/2nd vs. 3rd generation regimen) | 10.4 | 1.43 | 76.1 | 0.02 |
| Opioid substitution therapy (non-OST vs. OST) | 0.29 | 0.04 | 1.88 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, V.; Onorato, L.; Di Caprio, G.; Claar, E.; Iovinella, V.; Russo, A.; Rosato, V.; Salzillo, A.; Nevola, R.; Simeone, F.; et al. Directly Acting Antiviral-Based Treatment for HCV-Infected Persons Who Inject Drugs: A Multicenter Real-Life Study. Life 2021, 11, 17. https://0-doi-org.brum.beds.ac.uk/10.3390/life11010017
Messina V, Onorato L, Di Caprio G, Claar E, Iovinella V, Russo A, Rosato V, Salzillo A, Nevola R, Simeone F, et al. Directly Acting Antiviral-Based Treatment for HCV-Infected Persons Who Inject Drugs: A Multicenter Real-Life Study. Life. 2021; 11(1):17. https://0-doi-org.brum.beds.ac.uk/10.3390/life11010017
Chicago/Turabian StyleMessina, Vincenzo, Lorenzo Onorato, Giovanni Di Caprio, Ernesto Claar, Vincenzo Iovinella, Antonio Russo, Valerio Rosato, Angela Salzillo, Riccardo Nevola, Filomena Simeone, and et al. 2021. "Directly Acting Antiviral-Based Treatment for HCV-Infected Persons Who Inject Drugs: A Multicenter Real-Life Study" Life 11, no. 1: 17. https://0-doi-org.brum.beds.ac.uk/10.3390/life11010017