Nanoporous Polymers Based on Liquid Crystals
Abstract
:1. Introduction
2. Nanoporous LC Networks
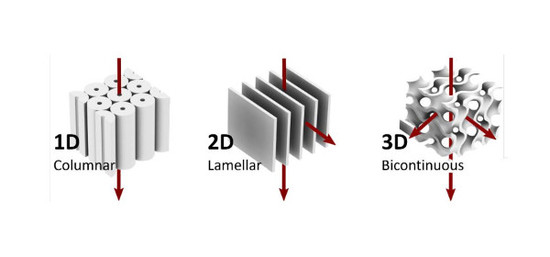
2.1. One-Dimensional Pores
2.2. Two-Dimensional Pores
2.3. Three-Dimensional Pores
3. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four Billion People Facing Severe Water Scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.M. Study Role of Climate Change in Extreme Threats to Water Quality. Nat. News 2016, 535, 349. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Cai, W.; Jiang, Y.; Wang, C. Impacts on Quality-Induced Water Scarcity: Drivers of Nitrogen-Related Water Pollution Transfer under Globalization from 1995 to 2009. Environ. Res. Lett. 2016, 11, 074017. [Google Scholar] [CrossRef]
- Aldaya, M.M. Environmental Science: Eating Ourselves Dry. Nature 2017, 543, 633. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kang, K.H.; Ko, S.H.; Kim, S.J. Direct Seawater Desalination by Ion Concentration Polarization. Nat. Nanotechnol. 2010, 5, 297. [Google Scholar]
- Cheremisinoff, N.P. Chapter 9—Membrane Separation Technologies. In Handbook of Water and Wastewater Treatment Technologies; Butterworth-Heinemann: Woburn, UK, 2002; pp. 335–371. [Google Scholar]
- Košutić, K.; Kaštelan-Kunst, L.; Kunst, B. Porosity of Some Commercial Reverse Osmosis and Nanofiltration Polyamide Thin-Film Composite Membranes. J. Membr. Sci. 2000, 168, 101–108. [Google Scholar] [CrossRef]
- Hwee Foo, Y.; Spahn, C.; Zhang, H.; Heilemann, M.; Kenney, L.J. Single Cell Super-Resolution Imaging of E. coli OmpR during Environmental Stress. Integr. Biol. 2015, 7, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Manfrinato, V.R.; Stein, A.; Zhang, L.; Nam, C.-Y.; Yager, K.G.; Stach, E.A.; Black, C.T. Aberration-Corrected Electron Beam Lithography at the One Nanometer Length Scale. Nano Lett. 2017, 17, 4562–4567. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF Thin Films: Existing and Future Applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Choi, M.; Ryoo, R. Recent Advances in the Synthesis of Hierarchically Nanoporous Zeolites. Microporous Mesoporous Mater. 2013, 166, 3–19. [Google Scholar] [CrossRef]
- Cho, J.; Ishida, Y. Macroscopically Oriented Porous Materials with Periodic Ordered Structures: From Zeolites and Metal—Organic Frameworks to Liquid-Crystal-Templated Mesoporous Materials. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Ordered Porous Materials for Emerging Applications. Nature 2002, 417, 813. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.A.; Hillmyer, M.A. Nanoporous Membranes Derived from Block Copolymers: From Drug Delivery to Water Filtration. ACS Nano 2010, 4, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Sinturel, C.; Bates, F.S.; Hillmyer, M.A. High Χ–Low N Block Polymers: How Far Can We Go? ACS Macro Lett. 2015, 4, 1044–1050. [Google Scholar] [CrossRef]
- Zhang, Y.; Mulvenna, R.A.; Qu, S.; Boudouris, B.W.; Phillip, W.A. Block Polymer Membranes Functionalized with Nanoconfined Polyelectrolyte Brushes Achieve Sub-Nanometer Selectivity. ACS Macro Lett. 2017, 6, 726–732. [Google Scholar] [CrossRef]
- Mulder, D.J.; Schenning, A.P.H.J.; Bastiaansen, C.W.M. Chiral-Nematic Liquid Crystals as One Dimensional Photonic Materials in Optical Sensors. J. Mater. Chem. C 2014, 2, 6695–6705. [Google Scholar] [CrossRef]
- Broer, D.J.; Lub, J.; Mol, G.N. Wide-Band Reflective Polarizers from Cholesteric Polymer Networks with a Pitch Gradient. Nature 1995, 378, 467–469. [Google Scholar] [CrossRef]
- Peeters, E.; Lub, J.; Steenbakkers, J.A.M.; Broer, D.J. High-Contrast Thin-Film Polarizers by Photo-Crosslinking of Smectic Guest—Host Systems. Adv. Mater. 2006, 18, 2412–2417. [Google Scholar] [CrossRef]
- De Haan, L.T.; Sánchez-Somolinos, C.; Bastiaansen, C.M.W.; Schenning, A.P.H.J.; Broer, D.J. Engineering of Complex Order and the Macroscopic Deformation of Liquid Crystal Polymer Networks. Angew. Chem. Int. Ed. 2012, 51, 12469–12472. [Google Scholar] [CrossRef] [PubMed]
- Gelebart, A.H.; Jan Mulder, D.; Varga, M.; Konya, A.; Vantomme, G.; Meijer, E.W.; Selinger, R.L.B.; Broer, D.J. Making Waves in a Photoactive Polymer Film. Nature 2017, 546, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, C.L.; Bastiaansen, C.W.M.; Broer, D.J. Printed Artificial Cilia from Liquid-Crystal Network Actuators Modularly Driven by Light. Nat. Mater. 2009, 8, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-K.; Lee, H.; Ko, Y.H.; Chang, Y.J.; Oh, N.-K.; Zin, W.-C.; Kim, K. Synthesis of a Nanoporous Polymer with Hexagonal Channels from Supramolecular Discotic Liquid Crystals. Angew. Chem. Int. Ed. 2001, 40, 2669–2671. [Google Scholar] [CrossRef]
- Mulder, D.J.; Scheres, L.M.W.; Dong, J.; Portale, G.; Broer, D.J.; Schenning, A.P.H.J. Fabrication and Postmodification of Nanoporous Liquid Crystalline Networks via Dynamic Covalent Chemistry. Chem. Mater. 2017, 29, 6601–6605. [Google Scholar] [CrossRef] [PubMed]
- Villazor, K.R.; Swager, T.M. Chiral Supramolecular Materials from Columnar Liquid Crystals. Mol. Cryst. Liq. Cryst. 2004, 410, 247–253. [Google Scholar] [CrossRef]
- Bögels, G.M.; Lugger, J.A.M.; Goor, O.J.G.M.; Sijbesma, R.P. Size-Selective Binding of Sodium and Potassium Ions in Nanoporous Thin Films of Polymerized Liquid Crystals. Adv. Funct. Mater. 2016, 26, 8023–8030. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Lugger, J.A.M.; Sijbesma, R.P. Tailoring Pore Size and Chemical Interior of near 1 Nm Sized Pores in a Nanoporous Polymer Based on a Discotic Liquid Crystal. Macromolecules 2017, 50, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Gracia, I.; Romero, P.; Serrano, J.L.; Barberá, J.; Omenat, A. Templated Nanoporous Membranes Based on Hierarchically Self-Assembled Materials. J. Mater. Chem. C 2017, 5, 2033–2042. [Google Scholar] [CrossRef]
- Castell, P.; Serra, A.; Galià, M. Liquid-Crystalline Thermosets from Liquid-Crystalline Epoxy Resins Containing Bisazomethinebiphenylene Mesogens in the Central Core: Copolymerization with a Nonmesomorphic Epoxy Resin. J. Polym. Sci. Part Polym. Chem. 2004, 42, 3631–3643. [Google Scholar] [CrossRef]
- Concellón, A.; Marcos, M.; Romero, P.; Serrano, J.L.; Termine, R.; Golemme, A. Not Only Columns: High Hole Mobility in a Discotic Nematic Mesophase Formed by Metal-Containing Porphyrin-Core Dendrimers. Angew. Chem. 2017, 129, 1279–1283. [Google Scholar] [CrossRef]
- Jackson, G.L.; Perroni, D.V.; Mahanthappa, M.K. Roles of Chemical Functionality and Pore Curvature in the Design of Nanoporous Proton Conductors. J. Phys. Chem. B 2017, 121, 9429–9436. [Google Scholar] [CrossRef] [PubMed]
- Küpfer, J.; Finkelmann, H. Nematic Liquid Single Crystal Elastomers. Makromol. Chem. Rapid Commun. 1991, 12, 717–726. [Google Scholar] [CrossRef]
- Ramón-Gimenez, L.; Storz, R.; Haberl, J.; Finkelmann, H.; Hoffmann, A. Anisotropic Ionic Mobility of Lithium Salts in Lamellar Liquid Crystalline Polymer Networks. Macromol. Rapid Commun. 2012, 33, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Beginn, U.; Zipp, G.; Möller, M. Functional Membranes Containing Ion-Selective Matrix-Fixed Supramolecular Channels. Adv. Mater. 2000, 12, 510–513. [Google Scholar] [CrossRef]
- Beginn, U.; Zipp, G.; Mourran, A.; Walther, P.; Möller, M. Membranes Containing Oriented Supramolecular Transport Channels. Adv. Mater. 2000, 12, 513–516. [Google Scholar] [CrossRef]
- Gonzalez, C.L.; Bastiaansen, C.W.M.; Lub, J.; Loos, J.; Lu, K.; Wondergem, H.J.; Broer, D.J. Nanoporous Membranes of Hydrogen-Bridged Smectic Networks with Nanometer Transverse Pore Dimensions. Adv. Mater. 2008, 20, 1246–1252. [Google Scholar] [CrossRef]
- Feng, X.; Nejati, S.; Cowan, M.G.; Tousley, M.E.; Wiesenauer, B.R.; Noble, R.D.; Elimelech, M.; Gin, D.L.; Osuji, C.O. Thin Polymer Films with Continuous Vertically Aligned 1 Nm Pores Fabricated by Soft Confinement. ACS Nano 2016, 10, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.J.; Jho, J.Y. Room Temperature Supramolecular Columnar Liquid Crystals Formed by Hydrogen Bonding of Isoquinoline Derivatives. Phase Transit. 2014, 87, 656–665. [Google Scholar] [CrossRef]
- Lee, J.H. Fabrication of Conjugated Polymeric Nanochannels from Star-Shaped Supramolecular Liquid Crystals Containing Two Different Photoreactive Groups. Liq. Cryst. 2014, 41, 738–746. [Google Scholar] [CrossRef]
- Yoshio, M.; Kagata, T.; Hoshino, K.; Mukai, T.; Ohno, H.; Kato, T. One-Dimensional Ion-Conductive Polymer Films: Alignment and Fixation of Ionic Channels Formed by Self-Organization of Polymerizable Columnar Liquid Crystals. J. Am. Chem. Soc. 2006, 128, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Yoshio, M.; Shimizu, S.; Ichikawa, T.; Ohno, H.; Kato, T. Columnar Nanostructured Polymer Films Containing Ionic Liquids in Supramolecular One-Dimensional Nanochannels. J. Polym. Sci. Part Polym. Chem. 2015, 53, 366–371. [Google Scholar] [CrossRef]
- Li, C.; Cho, J.; Yamada, K.; Hashizume, D.; Araoka, F.; Takezoe, H.; Aida, T.; Ishida, Y. Macroscopic Ordering of Helical Pores for Arraying Guest Molecules Noncentrosymmetrically. Nat. Commun. 2015, 6, 8418. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Tousley, M.E.; Cowan, M.G.; Wiesenauer, B.R.; Nejati, S.; Choo, Y.; Noble, R.D.; Elimelech, M.; Gin, D.L.; Osuji, C.O. Scalable Fabrication of Polymer Membranes with Vertically Aligned 1 Nm Pores by Magnetic Field Directed Self-Assembly. ACS Nano 2014, 8, 11977–11986. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Garvey, C.J.; Zhao, H.; Huang, K.; Kong, L. Toward the Fabrication of Advanced Nanofiltration Membranes by Controlling Morphologies and Mesochannel Orientations of Hexagonal Lyotropic Liquid Crystals. Membranes 2017, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yasuda, T.; Kamikawa, Y.; Yoshio, M. Self-Assembly of Functional Columnar Liquid Crystals. Chem. Commun. 2009, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y. Organic Zeolite Analogues Based on Multi-Component Liquid Crystals: Recognition and Transformation of Molecules within Constrained Environments. Materials 2011, 4, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Sanders, J.K.M. The Nature of .Pi.-.Pi. Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Wöhrle, T.; Wurzbach, I.; Kirres, J.; Kostidou, A.; Kapernaum, N.; Litterscheidt, J.; Haenle, J.C.; Staffeld, P.; Baro, A.; Giesselmann, F.; et al. Discotic Liquid Crystals. Chem. Rev. 2016, 116, 1139–1241. [Google Scholar]
- Zhou, M.; Kidd, T.J.; Noble, R.D.; Gin, D.L. Supported Lyotropic Liquid-Crystal Polymer Membranes: Promising Materials for Molecular-Size-Selective Aqueous Nanofiltration. Adv. Mater. 2005, 17, 1850–1853. [Google Scholar] [CrossRef]
- Smith, R.C.; Fischer, W.M.; Gin, D.L. Ordered Poly(p-Phenylenevinylene) Matrix Nanocomposites via Lyotropic Liquid-Crystalline Monomers. J. Am. Chem. Soc. 1997, 119, 4092–4093. [Google Scholar] [CrossRef]
- Ishida, Y.; Amano, S.; Saigo, K. Template Polymerization of Columnar Architectures Based on the Salts of a Carboxylic Acid and 2-Amino Alcohols: Application to the Molecular Recognition of 2-Amino Alcohols. Chem. Commun. 2003, 18, 2338–2339. [Google Scholar] [CrossRef]
- Ishida, Y.; Amano, S.; Iwahashi, N.; Saigo, K. Switching of Structural Order in a Cross-Linked Polymer Triggered by the Desorption/Adsorption of Guest Molecules. J. Am. Chem. Soc. 2006, 128, 13068–13069. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Sakata, H.; Achalkumar, A.S.; Yamada, K.; Matsuoka, Y.; Iwahashi, N.; Amano, S.; Saigo, K. Guest-Responsive Covalent Frameworks by the Cross-Linking of Liquid-Crystalline Salts: Tuning of Lattice Flexibility by the Design of Polymerizable Units. Chem. Eur. J. 2011, 17, 14752–14762. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Kawabata, K.; Kaufman, G.; Elimelech, M.; Osuji, C.O. Highly Selective Vertically Aligned Nanopores in Sustainably Derived Polymer Membranes by Molecular Templating. ACS Nano 2017, 11, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
- Bögels, G.M.; van Kuringen, H.P.C.; Shishmanova, I.K.; Voets, I.K.; Schenning, A.P.H.J.; Sijbesma, R.P. Selective Absorption of Hydrophobic Cations in Nanostructured Porous Materials from Crosslinked Hydrogen-Bonded Columnar Liquid Crystals. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Pecinovsky, C.S.; Hatakeyama, E.S.; Gin, D.L. Polymerizable Photochromic Macrocyclic Metallomesogens: Design of Supramolecular Polymers with Responsive Nanopores. Adv. Mater. 2008, 20, 174–178. [Google Scholar] [CrossRef]
- Cattle, J.; Bao, P.; Bramble, J.P.; Bushby, R.J.; Evans, S.D.; Lydon, J.E.; Tate, D.J. Controlled Planar Alignment of Discotic Liquid Crystals in Microchannels Made Using SU8 Photoresist. Adv. Funct. Mater. 2013, 23, 5997–6006. [Google Scholar] [CrossRef]
- Choudhury, T.D.; Rao, N.V.S.; Tenent, R.; Blackburn, J.; Gregg, B.; Smalyukh, I.I. Homeotropic Alignment and Director Structures in Thin Films of Triphenylamine-Based Discotic Liquid Crystals Controlled by Supporting Nanostructured Substrates and Surface Confinement. J. Phys. Chem. B 2011, 115, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Monobe, H.; Awazu, K.; Shimizu, Y. Alignment Control of a Columnar Liquid Crystal for a Uniformly Homeotropic Domain Using Circularly Polarized Infrared Irradiation. Adv. Mater. 2006, 18, 607–610. [Google Scholar] [CrossRef]
- Nickmans, K.; Bögels, G.M.; Sánchez-Somolinos, C.; Murphy, J.N.; Leclère, P.; Voets, I.K.; Schenning, A.P.H.J. 3D Orientational Control in Self-Assembled Thin Films with Sub-5 Nm Features by Light. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Pouzet, E.; Cupere, V.D.; Heintz, C.; Andreasen, J.W.; Breiby, D.W.; Nielsen, M.M.; Viville, P.; Lazzaroni, R.; Gbabode, G.; Geerts, Y.H. Homeotropic Alignment of a Discotic Liquid Crystal Induced by a Sacrificial Layer. J. Phys. Chem. C 2009, 113, 14398–14406. [Google Scholar] [CrossRef]
- Tousley, M.E.; Feng, X.; Elimelech, M.; Osuji, C.O. Aligned Nanostructured Polymers by Magnetic-Field-Directed Self-Assembly of a Polymerizable Lyotropic Mesophase. ACS Appl. Mater. Interfaces 2014, 6, 19710–19717. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Takanishi, Y.; Ishikawa, K.; Takezoe, H. Magnetic Field Effect on the Alignment of a Discotic Liquid Crystal. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. Mol. Cryst. Liq. Cryst. 1999, 329, 589–595. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, S.-M.; Pate, B.D.; Chisholm, M.H.; Han, Y.-S. Magnetic Uniaxial Alignment of the Columnar Superstructure of Discotic Metallomesogens over the Centimetre Length Scale. J. Mater. Chem. 2006, 16, 2785–2791. [Google Scholar] [CrossRef]
- Geary, J.M.; Goodby, J.W.; Kmetz, A.R.; Patel, J.S. The Mechanism of Polymer Alignment of Liquid-crystal Materials. J. Appl. Phys. 1987, 62, 4100–4108. [Google Scholar] [CrossRef]
- Ohtake, T.; Ito, K.; Nishina, N.; Kihara, H.; Ohno, H.; Kato, T. Liquid-Crystalline Complexes of a Lithium Salt with Twin Oligomers Containing Oxyethylene Spacers. An Approach to Anisotropic Ion Conduction. Polym. J. 1999, 31, 1155–1158. [Google Scholar] [CrossRef]
- Ohtake, T.; Ogasawara, M.; Ito-Akita, K.; Nishina, N.; Ujiie, S.; Ohno, H.; Kato, T. Liquid-Crystalline Complexes of Mesogenic Dimers Containing Oxyethylene Moieties with LiCF3SO3: Self-Organized Ion Conductive Materials. Chem. Mater. 2000, 12, 782–789. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, K.S.; Lee, J.S.; Lee, A.S.; Park, S.K.; Hong, S.Y.; Lee, J.-C.; Mueller, K.T.; Hong, S.M.; Koo, C.M. Facilitated Ion Transport in Smectic Ordered Ionic Liquid Crystals. Adv. Mater. 2016, 28, 9301–9307. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Yoshio, M.; Kato, T.; Yoshizawa, M.; Ohno, H. Anisotropic Ion Conduction in a Unique Smectic Phase of Self-Assembled Amphiphilic Ionic Liquids. Chem. Commun. 2005, 10, 1333–1335. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wu, Y.; Tan, S.; Yang, X.; Wei, B. Enhancing Proton Conduction via Doping of Supramolecular Liquid Crystals (4-Alkoxybenzoic Acids) with Imidazole. Chem. Phys. Lett. 2015, 637, 22–25. [Google Scholar] [CrossRef]
- Kishimoto, K.; Yoshio, M.; Mukai, T.; Yoshizawa, M.; Ohno, H.; Kato, T. Nanostructured Anisotropic Ion-Conductive Films. J. Am. Chem. Soc. 2003, 125, 3196–3197. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.J.; Mather, P.T. Supramolecular Interactions in the Formation of Thermotropic Liquid Crystalline Polymers. In Liquid Crystalline Functional Assemblies and Their Supramolecular Structures; Kato, T., Ed.; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2007; pp. 119–149. [Google Scholar]
- Kishikawa, K.; Hirai, A.; Kohmoto, S. Fixation of Multilayered Structures of Liquid-Crystalline 2:1 Complexes of Benzoic Acid Derivatives and Dipyridyl Compounds and the Effect of Nanopillars on Removal of the Dipyridyl Molecules from the Polymers. Chem. Mater. 2008, 20, 1931–1935. [Google Scholar] [CrossRef]
- Shishmanova, I.K.; Bastiaansen, C.W.M.; Schenning, A.P.H.J.; Broer, D.J. Two-Dimensional PH-Responsive Printable Smectic Hydrogels. Chem. Commun. 2012, 48, 4555–4557. [Google Scholar] [CrossRef] [PubMed]
- Van Kuringen, H.P.C.; Eikelboom, G.M.; Shishmanova, I.K.; Broer, D.J.; Schenning, A.P.H.J. Responsive Nanoporous Smectic Liquid Crystal Polymer Networks as Efficient and Selective Adsorbents. Adv. Funct. Mater. 2014, 24, 5045–5051. [Google Scholar] [CrossRef]
- Van Kuringen, H.P.C.; Mulder, D.J.; Beltran, E.; Broer, D.J.; Schenning, A.P.H.J. Nanoporous Polymer Particles Made by Suspension Polymerization: Spontaneous Symmetry Breaking in Hydrogen Bonded Smectic Liquid Crystalline Droplets and High Adsorption Characteristics. Polym. Chem. 2016, 7, 4712–4716. [Google Scholar] [CrossRef]
- Van Kuringen, H.P.C.; Leijten, Z.J.W.A.; Gelebart, A.H.; Mulder, D.J.; Portale, G.; Broer, D.J.; Schenning, A.P.H.J. Photoresponsive Nanoporous Smectic Liquid Crystalline Polymer Networks: Changing the Number of Binding Sites and Pore Dimensions in Polymer Adsorbents by Light. Macromolecules 2015, 48, 4073–4080. [Google Scholar] [CrossRef]
- Saliba, S.; Mingotaud, C.; Kahn, M.L.; Marty, J.-D. Liquid Crystalline Thermotropic and Lyotropic Nanohybrids. Nanoscale 2013, 5, 6641–6661. [Google Scholar] [CrossRef] [PubMed]
- Shandryuk, G.A.; Matukhina, E.V.; Vasil’ev, R.B.; Rebrov, A.; Bondarenko, G.N.; Merekalov, A.S.; Gas’kov, A.M.; Talroze, R.V. Effect of H-Bonded Liquid Crystal Polymers on CdSe Quantum Dot Alignment within Nanocomposite. Macromolecules 2008, 41, 2178–2185. [Google Scholar] [CrossRef]
- Dasgupta, D.; Shishmanova, I.K.; Ruiz-Carretero, A.; Lu, K.; Verhoeven, M.; van Kuringen, H.P.C.; Portale, G.; Leclère, P.; Bastiaansen, C.W.M.; Broer, D.J.; et al. Patterned Silver Nanoparticles Embedded in a Nanoporous Smectic Liquid Crystalline Polymer Network. J. Am. Chem. Soc. 2013, 135, 10922–10925. [Google Scholar] [CrossRef] [PubMed]
- Longo, A.; Mulder, D.-J.; van Kuringen, H.P.C.; Hermida-Merino, D.; Banerjee, D.; Dasgupta, D.; Shishmanova, I.K.; Spoelstra, A.B.; Broer, D.J.; Schenning, A.P.H.J.; et al. On the Dimensional Control of 2D Hybrid Nanomaterials. Chem. Eur. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; van Kuringen, H.P.C.; Mulder, D.J.; Tan, S.; Wu, Y.; Borneman, Z.; Nijmeijer, K.; Schenning, A.P.H.J. Anisotropic Dye Adsorption and Anhydrous Proton Conductivity in Smectic Liquid Crystal Networks: The Role of Cross-Link Density, Order, and Orientation. ACS Appl. Mater. Interfaces 2017, 9, 35218–35225. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Kagimoto, J.; Ohno, H.; Kato, T. 3D Interconnected Ionic Nano-Channels Formed in Polymer Films: Self-Organization and Polymerization of Thermotropic Bicontinuous Cubic Liquid Crystals. J. Am. Chem. Soc. 2011, 133, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Soberats, B.; Yoshio, M.; Ichikawa, T.; Ohno, H.; Kato, T. Zwitterionic Liquid Crystals as 1D and 3D Lithium Ion Transport Media. J. Mater. Chem. A 2015, 3, 11232–11238. [Google Scholar] [CrossRef]
- Carter, B.M.; Wiesenauer, B.R.; Hatakeyama, E.S.; Barton, J.L.; Noble, R.D.; Gin, D.L. Glycerol-Based Bicontinuous Cubic Lyotropic Liquid Crystal Monomer System for the Fabrication of Thin-Film Membranes with Uniform Nanopores. Chem. Mater. 2012, 24, 4005–4007. [Google Scholar] [CrossRef]
- Zhou, M.; Nemade, P.R.; Lu, X.; Zeng, X.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. New Type of Membrane Material for Water Desalination Based on a Cross-Linked Bicontinuous Cubic Lyotropic Liquid Crystal Assembly. J. Am. Chem. Soc. 2007, 129, 9574–9575. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Nguyen, V.; Zhou, M.; Zeng, X.; Jin, J.; Elliott, B.J.; Gin, D.L. Crosslinked Bicontinuous Cubic Lyotropic Liquid-Crystal/Butyl-Rubber Composites: Highly Selective, Breathable Barrier Materials for Chemical Agent Protection. Adv. Mater. 2006, 18, 3294–3298. [Google Scholar] [CrossRef]
- Kerr, R.L.; Miller, S.A.; Shoemaker, R.K.; Elliott, B.J.; Gin, D.L. New Type of Li Ion Conductor with 3D Interconnected Nanopores via Polymerization of a Liquid Organic Electrolyte-Filled Lyotropic Liquid-Crystal Assembly. J. Am. Chem. Soc. 2009, 131, 15972–15973. [Google Scholar] [CrossRef] [PubMed]
- Henmi, M.; Nakatsuji, K.; Ichikawa, T.; Tomioka, H.; Sakamoto, T.; Yoshio, M.; Kato, T. Self-Organized Liquid-Crystalline Nanostructured Membranes for Water Treatment: Selective Permeation of Ions. Adv. Mater. 2012, 24, 2238–2241. [Google Scholar] [CrossRef] [PubMed]
- Marets, N.; Kuo, D.; Torrey, J.R.; Sakamoto, T.; Henmi, M.; Katayama, H.; Kato, T. Highly Efficient Virus Rejection with Self-Organized Membranes Based on a Crosslinked Bicontinuous Cubic Liquid Crystal. Adv. Healthc. Mater. 2017, 1700252. [Google Scholar] [CrossRef] [PubMed]
- Koçer, A.; Walko, M.; Meijberg, W.; Feringa, B.L. A Light-Actuated Nanovalve Derived from a Channel Protein. Science 2005, 309, 755–758. [Google Scholar]














© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugger, J.; Mulder, D.J.; Sijbesma, R.; Schenning, A. Nanoporous Polymers Based on Liquid Crystals. Materials 2018, 11, 104. https://0-doi-org.brum.beds.ac.uk/10.3390/ma11010104
Lugger J, Mulder DJ, Sijbesma R, Schenning A. Nanoporous Polymers Based on Liquid Crystals. Materials. 2018; 11(1):104. https://0-doi-org.brum.beds.ac.uk/10.3390/ma11010104
Chicago/Turabian StyleLugger, Jody, Dirk Jan Mulder, Rint Sijbesma, and Albert Schenning. 2018. "Nanoporous Polymers Based on Liquid Crystals" Materials 11, no. 1: 104. https://0-doi-org.brum.beds.ac.uk/10.3390/ma11010104