Effect of Mixture Variables on Durability for Alkali-Activated Slag Cementitious
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Experiments Conducted
3. Results and Discussions
3.1. Water Absorption
3.2. Absorption Rate
3.3. Resistivity
3.4. Rapid Chloride Permeability Test
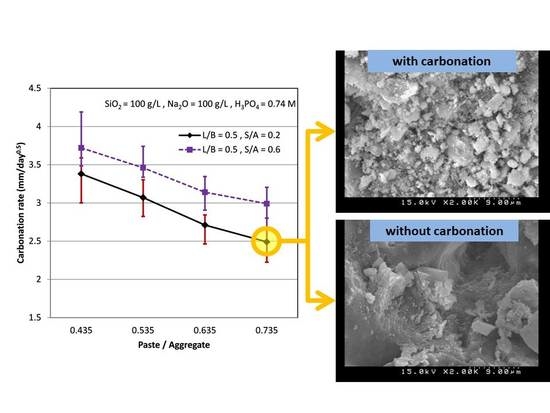
3.5. Carbonation Rate
3.6. SEM Observations
3.7. Performance Indicator
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ascione, F. Energy conservation and renewable technologies for buildings to face the impact of the climate change and minimize the use of cooling. Sol. Energy 2017, 154, 34–100. [Google Scholar] [CrossRef]
- Gavriletea, M.D. Environmental impacts of sand exploitation. Analysis of sand market. Sustainability 2017, 9, 1118. [Google Scholar] [CrossRef]
- Monkman, S.; MacDonald, M. Carbon dioxide upcycling into industrially produced concrete blocks. Constr. Build. Mater. 2016, 124, 127–132. [Google Scholar] [CrossRef]
- Omosebi, O.; Maheshwari, H.; Ahmed, R.; Shah, S.; Osisanya, S.; Hassani, S.; DeBruijn, G.; Cornell, W.; Simon, D. Degradation of well cement in HPHT acidic environment: Effects of CO2 concentration and pressure. Cem. Concr. Compos. 2016, 74, 54. [Google Scholar] [CrossRef]
- Golewski, G.L. Generalized fracture toughness and compressive strength of sustainable concrete including low calcium fly ash. Materials 2017, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.M. Alkali-activated cements: Opportunities and challenges. Cem. Concr. Res. 1999, 29, 249–254. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2009, 39, 1590–1597. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Rodríguez-Puertas, C.; del Mar Alonso, M.; Puertas, F. Alkali activated slag cements using waste glass as alternative activators. Rheological behavior. Bol. Soc. Esp. Ceram. Vidr. 2015, 54, 45–57. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Tognonvi, M.; Tagnit-Hamou, A.; Puertas, F. Durability of alkali-activated slag concretes prepared using waste glass as alternative activator. ACI Mater. J. 2015, 112, 791. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. The effect of ionic contaminants on the early-age properties of alkali-activated fly ash-based cements. Cem. Concr. Res. 2002, 32, 577–584. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Yan, F. Reduction in wear of metakaolinite-based geopolymer composite through filling of PTFE. Wear 2005, 258, 1562–1566. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Cheng, T.W.; Chiu, J.P. Fire-resistant geopolymer produce by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G. Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Palomo, A.; López dela Fuente, J.I. Alkali-activated cementitous materials: Alternative matrices for the immobilisation of hazardous wastes Part I. Stabilisation of boron. Cem. Concr. Res. 2003, 33, 281–288. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Effect of admixtures on properties of alkali-activated slag concrete. Cem. Concr. Res. 2001, 30, 1367–1374. [Google Scholar] [CrossRef]
- Fernandez-Jime´nez, A.; Puertas, F.; Palomo, J.G. Alkaliactivated slag mortars: mechanical strength behaviour. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar]
- Gong, C.; Yang, N. Effect of phosphate on the hydration of alkali-activated red mud–slag cementitious material. Cem. Concr. Res. 2000, 30, 1013–1016. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinkage properties of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Brough, A.R.; Holloway, M.; Sykes, J.; Atkinson, A. Sodium silicate-based alkali-activated slag mortars Part II. The retarding effect of additions of sodium chloride or malic acid. Cem. Concr. Res. 2000, 30, 1375–1379. [Google Scholar] [CrossRef]
- Chang, J.J. A study on the setting characteristics of sodium silicate-activated slag pastes. Cem. Concr. Res 2003, 33, 1005–1011. [Google Scholar] [CrossRef]
- Chang, J.J.; Yeih, W.; Hung, C.C. Effects of gypsum and phosphoric acid on the properties of sodium silicate-based alkali-activated slag pastes. Cem. Concr. Compos. 2005, 27, 85–91. [Google Scholar] [CrossRef]
- Berke, N.S.; Dallaire, M.P.; Hicks, M.C.; Kerkar, A. New developments in shrinkage-reducing admixtures. In Proceedings of the CANMET/ACI 5th International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Rome, Italy, 7–10 October 1997; pp. 971–998. [Google Scholar]
- Ai, H.; Young, J.F. Mechanisms of shrinkage reduction using a chemical admixture. In Proceedings of the 10th International Congress on the Chemistry of Cement, Gothenburg, Sweden, 2–6 June 1997. [Google Scholar]
- Shi, C.; Krivenko, P.V.; Roy, D. Alkali-Activated Cements and Concretes; Taylor and Francis: London, UK, 2006. [Google Scholar]
- Thunuguntla, C.S.; Rao, T.D.G. Mix design procedure for alkali-activated slag concrete using particle packing theory. J. Mater. Civ. Eng. 2018, 30, 04018113. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effects of ultra-fine materials on workability and strength of concrete containing alkali-activated slag as the binder. Cem. Concr. Res. 1999, 29, 459–462. [Google Scholar] [CrossRef]
- Collins, F.G.; Sanjayan, J.G. Workability and mechanical properties of alkali activated slag concrete. Cem. Concr. Res. 1999, 29, 455–458. [Google Scholar] [CrossRef]
- Golewski, G.L. Evaluation of morphology and size of cracks of the Interfacial Transition Zone (ITZ) in concrete containing fly ash (FA). J. Hazard. Mater. 2018, 357, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, R.S.; Provis, J.L. The interfacial transition zone in alkali-activated slag mortars. Front. Mater. 2015, 2, 70. [Google Scholar] [CrossRef]
- New Test Method for Measuring the Surface Resistivity of Hardened Concrete Using the Wenner Four-Electrode Method; ASTM WK37880; American Society for Testing and Materials: West Conshohocken, PA, USA, 2012.
- Ananthi, A.; Karthikeyan, J. Properties of industrial slag as fine aggregate in concrete. Int. J. Eng. Technol. Innov. 2015, 5, 132–140. [Google Scholar]
- Puertas, F.; Torres-Carrasco, M. Use of glass waste as an activator in the preparation of alkali-activated slag. Mechanical strength and paste characterization. Cem. Concr. Compos. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Bernal, S.A. Microstructural changes induced by CO2 exposure in alkali-activated slag/metakaolin pastes. Front. Mater. 2016, 3, 43. [Google Scholar] [CrossRef]
- Susan, A.B.; John, L.P.; David, G.B.; Adam, K.; Peter, D.; Jannie, S.J.D. Accelerated carbonation testing of alkali-activated binders significantly underestimates service life: The role of pore solution chemistry. Cem. Concr. Compos. 2012, 42, 1317–1326. [Google Scholar]
- Zhou, H.; Wu, X.; Xu, Z.; Tang, M. Kinetic study on hydration of alkali-activated slag. Cem. Concr. Res. 1993, 23, 1253–1258. [Google Scholar]
- Olofinnade, O.M.; Ede, A.N.; Ndambuki, J.M. Experimental investigation on the effect of elevated temperature on compressive strength of concrete containing waste glass powder. Int. J. Eng. Technol. Innov. 2017, 7, 280–291. [Google Scholar]















| Item | Composition | Value |
|---|---|---|
| The main chemical composition of slag (By weight percentage) | SiO2 (%) | 33.87 |
| Al2O3 (%) | 14.42 | |
| Fe2O3 (%) | 0.69 | |
| CaO (%) | 39.54 | |
| MgO (%) | 5.35 | |
| SO3 (%) | 2.47 | |
| Basicity coefficient Kb = (CaO + MgO)/(SiO2 + Al2O3) | 0.93 | |
| Physical properties | Specific weight | 2.90 |
| Ignition loss (%) | 0.28 | |
| Fineness (m2/kg) | 383 |
| Group | S/A | L/B | P/A | Air Content (%) | Liquid (kg/m3) | Binder (kg/m3) | Fine Aggregate (kg/m3) | Coarse Aggregate (kg/m3) | Slump (mm) |
|---|---|---|---|---|---|---|---|---|---|
| I | 0 | 0.5 | 0.635 | 1.8 | 285 | 570 | 0 | 1346 | 220 |
| 0.2 | 0.5 | 0.635 | 2.0 | 284 | 568 | 268 | 1073 | 190 | |
| 0.4 | 0.5 | 0.635 | 2.3 | 283 | 566 | 534 | 802 | 160 | |
| 0.6 | 0.5 | 0.635 | 2.4 | 282 | 564 | 799 | 533 | 130 | |
| 0.8 | 0.5 | 0.635 | 2.6 | 281 | 562 | 1062 | 265 | 110 | |
| II | 0.2 | 0.4 | 0.635 | 2.5 | 249 | 622 | 274 | 1098 | 70 |
| 0.2 | 0.5 | 0.635 | 2.0 | 284 | 568 | 268 | 1073 | 190 | |
| 0.2 | 0.6 | 0.635 | 1.5 | 314 | 523 | 263 | 1054 | 230 | |
| 0.2 | 0.7 | 0.635 | 1.2 | 339 | 484 | 259 | 1036 | 260 | |
| 0.6 | 0.4 | 0.635 | 2.8 | 247 | 619 | 818 | 546 | 50 | |
| 0.6 | 0.5 | 0.635 | 2.4 | 282 | 564 | 799 | 533 | 130 | |
| 0.6 | 0.6 | 0.635 | 1.9 | 311 | 519 | 784 | 523 | 170 | |
| 0.6 | 0.7 | 0.635 | 1.5 | 336 | 481 | 772 | 515 | 230 | |
| III | 0.2 | 0.5 | 0.435 | 2.1 | 230 | 459 | 317 | 1267 | 40 |
| 0.2 | 0.5 | 0.535 | 2.1 | 259 | 518 | 290 | 1162 | 80 | |
| 0.2 | 0.5 | 0.635 | 2.0 | 284 | 568 | 268 | 1073 | 190 | |
| 0.2 | 0.5 | 0.735 | 1.8 | 306 | 612 | 250 | 998 | 220 | |
| 0.6 | 0.5 | 0.435 | 2.7 | 227 | 455 | 941 | 627 | 20 | |
| 0.6 | 0.5 | 0.535 | 2.6 | 257 | 514 | 864 | 576 | 50 | |
| 0.6 | 0.5 | 0.635 | 2.4 | 282 | 564 | 799 | 533 | 130 | |
| 0.6 | 0.5 | 0.735 | 2.3 | 303 | 607 | 743 | 495 | 150 |
| Parameters | Fresh Properties | Durability | |||||
|---|---|---|---|---|---|---|---|
| Air Content | Slump | Absorption | Absorption Rate | Resistivity | Rapid Chloride Permeability Index | Carbonation Rate | |
| Increase S/A | + | − | + | + | − | + | + |
| Increase L/B | − | + | + | + | + | + | + |
| Increase P/A | − | + | − | − | + | − | − |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, C.-C.; Wu, Y.-C.; Lin, W.-T.; Chang, J.-J.; Yeih, W.-C. Effect of Mixture Variables on Durability for Alkali-Activated Slag Cementitious. Materials 2018, 11, 2252. https://0-doi-org.brum.beds.ac.uk/10.3390/ma11112252
Hung C-C, Wu Y-C, Lin W-T, Chang J-J, Yeih W-C. Effect of Mixture Variables on Durability for Alkali-Activated Slag Cementitious. Materials. 2018; 11(11):2252. https://0-doi-org.brum.beds.ac.uk/10.3390/ma11112252
Chicago/Turabian StyleHung, Chi-Che, Yuan-Chieh Wu, Wei-Ting Lin, Jiang-Jhy Chang, and Wei-Chung Yeih. 2018. "Effect of Mixture Variables on Durability for Alkali-Activated Slag Cementitious" Materials 11, no. 11: 2252. https://0-doi-org.brum.beds.ac.uk/10.3390/ma11112252