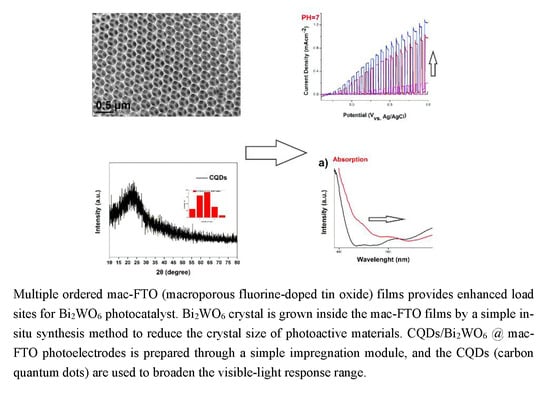
Carbon Dots-Decorated Bi2WO6 in an Inverse Opal Film as a Photoanode for Photoelectrochemical Solar Energy Conversion under Visible-Light Irradiation
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Synthesis of the Polystyrene Film Template
2.3. Fabrication of the Mac-FTO Electrode
2.4. Synthesis of the Bi2WO6@mac-FTO Photoelectrode
2.5. Synthesis of the Bi2WO6@mac-FTO Photoelectrode Decorated with CDs
3. Sample Characterization
4. Results and Discussion
4.1. Structural Characterization
4.2. Optical Properties
4.3. Photoelectrochemistry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, B.V.; Prasad, M.D.; Vithal, M. Enhanced visible light photocatalytic activity of Sn doped Bi2WO6 nanocrystals. Mater. Lett. 2015, 152, 200–202. [Google Scholar] [CrossRef]
- Yang, L.; Yao, Y.; Zhu, G.; Ma, M.; Wang, W.; Wang, L.; Zhang, H.; Zhang, Y.; Jiao, Z. Co doping of worm–like Cu2S: An efficient and durable heterogeneous electrocatalyst for alkaline water oxidation. J. Alloys Compd. 2018, 762, 637–642. [Google Scholar] [CrossRef]
- Cheng, W.; Pan, J.; Yang, J.; Zheng, Z.; Lu, F.; Chen, Y.; Gao, W. A photoelectrochemical aptasensor for thrombin based on the use of carbon quantum dot-sensitized TiO2 and visible-light photoelectrochemical activity. Mikrochim. Acta 2018, 185, 263. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.F.; Shi, R.; Yang, X.; Fu, W.F.; Chen, Y. P-doped ZnxCd1−xS solid solutions as photocatalysts for hydrogen evolution from water splitting coupled with photocatalytic oxidation of 5-hydroxymethylfurfural. Appl. Catal. B Environ. 2018, 233, 70–79. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Q.; Chen, J.; Huang, S.; Hu, L.; Zeng, Y.J.; Zhang, H.; Ruan, S.; Ohno, T. Hydrogen bonds in heterojunction photocatalysts for efficient charge transfer. Appl. Catal. B Environ. 2018, 234, 198–205. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splittin. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, M.; Zhang, M. Multiple layered macroporous SnO2 film for applications to photoelectrochemistry and morphology control of iron oxide nanocrystal. J. Power Sources 2018, 402, 62–67. [Google Scholar] [CrossRef]
- Yu, J.G.; Qi, L.F.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Atabaev, T.S.; Hossain, M.A.; Lee, D.; Kim, H.K.; Hwang, Y.H. Pt-coated TiO2 nanorods for photoelectrochemical water splitting applications. Results Phys. 2016, 6, 373–376. [Google Scholar] [CrossRef]
- Xia, J.; Di, J.; Yin, S.; Xu, H.; Zhang, J.; Xu, Y.; Xu, L.; Li, H.; Ji, M. Facile fabrication of the visible-light-driven Bi2WO6/BiOBr composite with enhanced photocatalytic activity. RSC Adv. 2014, 4, 82–90. [Google Scholar] [CrossRef]
- Yong, Z.; Ren, J.; Hu, H.; Li, P.; Ouyang, S.; Xu, H.; Wang, D. Synthesis, Characterization, and Photocatalytic Activity of g-C3N4/KTaO3Composites under Visible Light Irradiation. J. Nanomater. 2015, 2015, 821986. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, P.; Cai, W.; Yang, S.; Xu, X. Controllable Pt/ZnO Porous Nanocages with Improved Photocatalytic Activity. J. Phys. Chem. C 2008, 112, 19620–19624. [Google Scholar] [CrossRef]
- Liberato, M.; Scher, E.C.; Li, L.S.; Alivisatos, A.P. A Paul, Epitaxial growth and photochemical annealing of graded CdS/ZnS shells on colloidal CdSe nanorods. J. Am. Chem. Soc. 2002, 124, 7136–7145. [Google Scholar]
- Kumar, A.; Chaudhary, V. Optical and photophysical properties of Ag/CdS nanocomposites—An analysis of relaxation kinetics of the charge carriers. J. Photochem. Photobiol. A 2007, 189, 272–279. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, D.D.; Guo, P.; Leckie, J.O. An efficient bicomponent TiO2/SnO2 nanofiber photocatalyst fabricated by electrospinning with a side-by-side dual spinneret method. Nano Lett. 2007, 7, 1081–1085. [Google Scholar] [CrossRef]
- Wu, L.; Bi, J.; Li, Z.; Wang, X.; Fu, X. Rapid preparation of Bi2WO6 photocatalyst with nanosheet morphology via microwave-assisted solvothermal synthesis. Catal. Today 2008, 131, 15–20. [Google Scholar] [CrossRef]
- Albu, S.P.; Andrei, G.; Macak, J.M.; Robert, H.; Patrik, S. Self-organized, free-standing TiO2 nanotube membrane for flow-through photocatalytic applications. Nano Lett. 2007, 7, 1286–1289. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Huang, B. Fabrication and Characterization of Visible-Light-Driven Plasmonic Photocatalyst Ag/AgCl/TiO2 TiO2 Nanotube Arrays. J. Phys. Chem. C 2009, 113, 16394–16401. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, F.; Zhao, R.; Huai, X.; Han, J.; Wang, L. Aqueous synthesis of core/shell/shell CdSe/CdS/ZnS quantum dots for photocatalytic hydrogen generation. J. Mater. Sci. 2019, 54, 8571–8580. [Google Scholar] [CrossRef]
- Li, N.; Huang, H.; Bibi, R.; Shen, Q.; Ngulube, R.; Zhou, J.; Liu, M. Noble-metal-free MOF derived hollow CdS/TiO2 decorated with NiS cocatalyst for efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 476, 378–386. [Google Scholar] [CrossRef]
- Guo, F.; Cai, Y.; Guan, W.; Huang, H.; Liu, Y. Graphite carbon nitride/ZnIn2S4 heterojunction photocatalyst with enhanced photocatalytic performance for degradation of tetracycline under visible light irradiation. J. Phys. Chem. Solids 2017, 110, 370–378. [Google Scholar] [CrossRef]
- Yang, C.; Li, Q.; Xia, Y.; Lv, K.; Li, M. Enhanced visible-light photocatalytic CO2 reduction performance of Znln2S4 microspheres by using CeO2 as cocatalyst. Appl. Surf. Sci. 2019, 464, 388–395. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Torres-Martínez, L.M.; Moctezuma, E.; Singh, A.P.; Wickman, B. Green synthesis of earth-abundant metal sulfides (FeS2, CuS, and NiS2) and their use as visible-light active photocatalysts for H2 generation and dye removal. J. Mater. Sci. Mater. Electron. 2018, 29, 11613–11626. [Google Scholar] [CrossRef]
- Niu, Y.; Li, F.; Yang, K.; Wu, Q.; Xu, P.; Wang, R. Highly Efficient Photocatalytic Hydrogen on CoS/TiO2 Photocatalysts from Aqueous Methanol Solution. Int. J. Photoenergy 2018, 2018, 8143940. [Google Scholar] [CrossRef]
- Lu, L.; Xu, X.; An, K.; Wang, Y.; Shi, F.N. Coordination Polymer Derived NiS@g-C3N4 Composite Photocatalyst for Sulfur Vacancy and Photothermal Effect Synergistic Enhanced H2 Production. ACS Sustain. Chem. Eng. 2018, 6, 11869–11876. [Google Scholar] [CrossRef]
- Suroshe, J.S.; Mlowe, S.; Garje, S.S.; Revaprasadu, N. Preparation of Iron Sulfide Nanomaterials from Iron (II) Thiosemicarbazone Complexes and Their Application in Photodegradation of Methylene Blue. J. Inorg. Organomet. Polym. Mater. 2018, 28, 603–611. [Google Scholar] [CrossRef]
- Wang, B.; Pan, J.; Jiang, Z.; Dong, Z.; Zhao, C.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The bimetallic iron-nickel sulfide modified g-C3N4 nano-heterojunction and its photocatalytic hydrogen production enhancement. J. Alloys Compd. 2018, 766, 421–428. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Wang, H.; Dong, F.; Wu, Z. Fe-ions modified mesoporous Bi2WO6 nanosheets with high visible light photocatalytic activity. J. Colloid Interface Sci. 2012, 369, 373–380. [Google Scholar] [CrossRef]
- Liao, C.; Ma, Z.; Dong, G.; Qiu, J.; Xie, R.J. Flexible Porous SiO2-Bi2WO6 Nanofibers Film for Visible-Light Photocatalytic Water Purification. J. Am. Ceram. Soc. 2015, 98, 957–964. [Google Scholar] [CrossRef]
- Wang, Z.N.; Bai, F.Y.; Wang, X.; Shang, D.; Xing, Y.H. Photocatalytic activity of the modified composite photocatalyst by introducing the rich-nitrogen complex to the Bi2WO6. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 163, 73–78. [Google Scholar] [CrossRef]
- Zhu, Z.; Yan, Y.; Li, J. Preparation of flower-like BiOBr–WO3–Bi2WO6 ternary hybrid with enhanced visible-light photocatalytic activity. J. Alloys Compd. 2015, 651, 184–192. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.; Liu, Y.; Wu, S.; Tan, Y.Z.; Chew, J.W. Construction of hierarchical 2D-2D Zn3In2S6/fluorinated polymeric carbon nitride nanosheets photocatalyst for boosting photocatalytic degradation and hydrogen production performance. Appl. Catal. B Environ. 2018, 233, 58–69. [Google Scholar] [CrossRef]
- Yang, G.; Ding, H.; Chen, D.; Feng, J.; Hao, Q.; Zhu, Y. Construction of urchin-like ZnIn2S4-Au-TiO2 heterostructure with enhanced activity for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 234, 260–267. [Google Scholar] [CrossRef]
- Alagarasi, A.; Rajalakshmi, P.U.; Shanthi, K.; Selvam, P. Ordered mesoporous nanocrystalline titania: A promising new class of photocatalyic materials. Catal. Today 2018, 309, 202–211. [Google Scholar] [CrossRef]
- Qu, Z.; Na, W.; Liu, X.; Liu, H.; Su, X. A novel fluorescence biosensor for sensitivity detection of tyrosinase and acid phosphatase based on nitrogen-doped graphene quantum dots. Anal. Chim Acta 2018, 997, 52–59. [Google Scholar] [CrossRef]
- Rahbar, N.; Abbaszadegan, P.; Savarizadeh, A. A sensitive fluorescent sensing strategy for nanomolar levels of metformin using graphitic carbon nitride nanosheets as nanofluoroprobe. Anal. Chim. Acta 2018, 1026, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mo, G.; Feng, J.; He, X.; Tang, L.; Yu, C.; Deng, B. Based on ZnSe quantum dots labeling and single particle mode ICP-MS coupled with sandwich magnetic immunoassay for the detection of carcinoembryonic antigen in human serum. Anal. Chim. Acta 2018, 1028, 22–31. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, N.; Gui, W.; Ma, Q. Nitrogen-doped graphene quantum dots-based fluorescence molecularly imprinted sensor for thiacloprid detection. Talanta 2018, 183, 339–344. [Google Scholar] [CrossRef]
- Li, W.; Zhu, J.; Xie, G.; Ren, Y.; Zheng, Y.Q. Ratiometric system based on graphene quantum dots and Eu3+ for selective detection of tetracyclines. Anal. Chim. Acta 2018, 1022, 131–137. [Google Scholar] [CrossRef]
- Salvador, J.P.; Tassies, D.; Reverter, J.C.; Marco, M.P. Enzyme-linked immunosorbent assays for therapeutic drug monitoring coumarin oral anticoagulants in plasma. Anal. Chim. Acta 2018, 1028, 59–65. [Google Scholar] [CrossRef]
- Tang, D.; Liu, J.; Wu, X.; Liu, R.; Han, X.; Han, Y.; Huang, H.; Liu, Y.; Kang, Z. Carbon quantum dot/NiFe layered double-hydroxide composite as a highly efficient electrocatalyst for water oxidation. ACS Appl. Mater. Interfaces 2014, 6, 7918–7925. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef]
- Shi, R.; Wang, J.; Wang, Z.; Li, T.; Song, Y.F. Unique NiFe NiCoO2 hollow polyhedron as bifunctional electrocatalysts for water splitting. J. Energy Chem. 2019, 33, 74–80. [Google Scholar] [CrossRef]
- Chen, S.; Duan, J.; Jaroniec, M.; Qiao, S.Z. Three-dimensional N-doped graphene hydrogel/NiCo double hydroxide electrocatalysts for highly efficient oxygen evolution. Angew. Chem. Int. Ed. 2013, 52, 13567–13570. [Google Scholar] [CrossRef]
- Sharma, S.; Mehta, S.K.; Ibhadon, A.O.; Kansal, S.K. Fabrication of novel carbon quantum dots modified bismuth oxide (alpha-Bi2O3/C-dots): Material properties and catalytic applications. J. Colloid Interface Sci. 2019, 533, 227–237. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230. [Google Scholar] [CrossRef]
- Ye, K.H.; Wang, Z.; Gu, J.; Xiao, S.; Yuan, Y.; Zhu, Y.; Zhang, Y.; Mai, W.; Yang, S. Carbon quantum dots as a visible light sensitizer to significantly increase the solar water splitting performance of bismuth vanadate photoanodes. Energy Environ. Sci. 2017, 10, 772–779. [Google Scholar] [CrossRef]
- Yu, B.Y.; Kwak, S.Y. Carbon quantum dots embedded with mesoporous hematite nanospheres as efficient visible light-active photocatalysts. J. Mater. Chem. 2012, 22, 8345. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Lu, F.; Meziani, M.J.; Li, H.; Qi, G.; Zhou, B.; Harruff, B.A.; Kermarrec, F.; Sun, Y.P. Photoinduced electron transfers with carbon dots. Chem. Commun. 2009, 25, 3774–3776. [Google Scholar] [CrossRef]
- Zhang, M.; Robert Mitchell, W.; Huang, H.; Douthwaite, R.E. Ordered multilayer films of hollow sphere aluminium-doped zinc oxide for photoelectrochemical solar energy conversion. J. Mater. Chem. A 2017, 5, 22193–22198. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Chen, Z.; Ji, M.; Chen, H.; Liu, Y.; Xia, J.; Li, H. Enhanced reactive oxygen species activation for building carbon quantum dots modified Bi5O7I nanorod composites and optimized visible-light-response photocatalytic performance. J. Colloid Interface Sci. 2018, 532, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, C.; Lee, H.C.; Bard, A.J. Rapid Screening by Scanning Electrochemical Microscopy (SECM) of Dopants for Bi2WO6 Improved Photocatalytic Water Oxidation with Zn Doping. J. Phys. Chem. C 2015, 117, 9633–9640. [Google Scholar] [CrossRef]
- Que, Q.; Xing, Y.; He, Z.; Yang, Y.; Yin, X.; Que, W. Bi2O3/Carbon quantum dots heterostructured photocatalysts with enhanced photocatalytic activity. Mater. Lett. 2017, 209, 220–223. [Google Scholar] [CrossRef]
- Zhao, F.; Rong, Y.; Wan, J.; Hu, Z.; Peng, Z.; Wang, B. High photocatalytic performance of carbon quantum dots/TNTs composites for enhanced photogenerated charges separation under visible light. Catal. Today 2018, 315, 162–170. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Zhao, Q.; Liu, B.; Liu, S.; Wang, S. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci. 2017, 496, 425–433. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Chen, Q.; Qiu, Y.; Liu, B.; Zhang, M. Carbon Dots-Decorated Bi2WO6 in an Inverse Opal Film as a Photoanode for Photoelectrochemical Solar Energy Conversion under Visible-Light Irradiation. Materials 2019, 12, 1713. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12101713
Luo D, Chen Q, Qiu Y, Liu B, Zhang M. Carbon Dots-Decorated Bi2WO6 in an Inverse Opal Film as a Photoanode for Photoelectrochemical Solar Energy Conversion under Visible-Light Irradiation. Materials. 2019; 12(10):1713. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12101713
Chicago/Turabian StyleLuo, Dongxiang, Qizan Chen, Ying Qiu, Baiquan Liu, and Menglong Zhang. 2019. "Carbon Dots-Decorated Bi2WO6 in an Inverse Opal Film as a Photoanode for Photoelectrochemical Solar Energy Conversion under Visible-Light Irradiation" Materials 12, no. 10: 1713. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12101713