Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosan Inclusion Complex with Cinnamon Essential Oil
2.2. Coating Solutions and Preservation of Mangoes
2.2.1. Chitosan Coating with Chitosan-Cinnamon Essential Oil Microcapsules
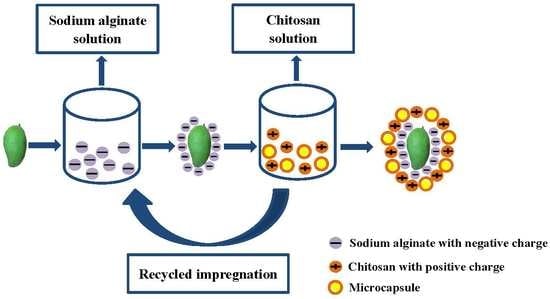
2.2.2. Alginate Coating
2.2.3. Alginate-Chitosan Layer-by-Layer Coating
2.3. Characterization of Microcapsules
2.3.1. Surface Morphology of Microcapsules
2.3.2. In Vitro Release of Chitosan-Cinnamon Essential Oil Microcapsules
2.4. Characterization of the Coatings
2.4.1. Zeta Potential Analysis of Sodium Alginate Solution, Chitosan Solution, and Chitosan Solution with Microcapsules
2.4.2. Surface Morphology of Coatings on the Mango Surface
2.4.3. Analysing the Contact Angles of the Mango Surface Coatings
2.5. Index Tests
2.5.1. Weight Loss Rate
2.5.2. pH
2.5.3. Firmness
2.5.4. Respiratory Rate
2.5.5. Titratable Acidity
2.5.6. Soluble Solids
2.5.7. Vitamin C
2.5.8. Chromatic Aberration
2.6. Statistical Analysis
3. Results and Discussion
3.1. Surface Morphology of Microcapsules
3.2. In Vitro Release Experiment on Chitosan-Cinnamon Essential Oil Microcapsules
3.3. Zeta Potential Analysis
3.4. Morphological Distribution of Coatings on the Mango Surface
3.5. Analysing the Contact Angle of the Mango Surface Coatings
3.6. Changes in the Weight Loss Rate
3.7. Changes in pH Value
3.8. Changes in Firmness
3.9. Changes in Respiration Rate
3.10. Changes in Titratable Acidity
3.11. Changes in Soluble Solid Content
3.12. Changes in Vitamin C
3.13. Changes in Colour
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Anusuya, P.; Nagaraj, R.; Janavi, G.J.; Subramanian, K.S.; Paliyath, G.; Subramanian, J. Pre-harvest sprays of hexanal formulation for extending retention and shelf-life of mango (Mangifera indica L.) fruits. Sci. Hortic. 2016, 211, 231–240. [Google Scholar] [CrossRef]
- Bang, D.Y.; Kyung, M.; Jung, B.Y.; Cho, M.C.; Choi, S.M.; Kim, Y.W.; Lim, S.K.; Lim, D.S.; Won, A.J.; Kwack, S.J.; et al. Human Risk Assessment of Endocrine-Disrupting Chemicals Derived from Plastic Food Containers. Compr. Rev. Food Sci. Food Saf. 2012, 11, 453–470. [Google Scholar] [CrossRef]
- Chen, J.; Shen, Y.; Chen, C.; Wan, C. Inhibition of Key Citrus Postharvest Fungal Strains by Plant Extracts In Vitro and In Vivo: A Review. Plants 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.C.; Embuscado, M.E. Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009. [Google Scholar]
- Dhital, R.; Joshi, P.; Becerra-Mora, N.; Umagiliyage, A.; Chai, T.; Kohli, P.; Choudhary, R. Integrity of edible nano-coatings and its effects on quality of strawberries subjected to simulated in-transit vibrations. LWT 2017, 80, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Mihai, A.L.; Popa, M.E. Chitosan coatings, a natural and sustainable food preservation method. J. Biotechnol. 2015, 208, S81. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Edible Coatings for Fresh-Cut Fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Elsabee, M.Z.; Abdou, E.S.; Nagy, K.S.; Eweis, M. Surface modification of polypropylene films by chitosan and chitosan/pectin multilayer. Carbohydr. Polym. 2008, 71, 187–195. [Google Scholar] [CrossRef]
- Pillai, C.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Marudova, M.; Lang, S.; Brownsey, G.J.; Ring, S.G. Pectin–chitosan multilayer formation. Carbohydr. Res. 2005, 340, 2144–2149. [Google Scholar] [CrossRef]
- Vargas, M.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. 5-Edible chitosan coatings for fresh and minimally processed foods. Emerg. Food Packag. Technol. 2012, 66–95. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; González-Martínez, C. Recent Advances in Edible Coatings for Fresh and Minimally Processed Fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511. [Google Scholar] [CrossRef]
- Alves, M.; Gonçalves, M.; Rocha, C.; Gonçalves, M.; Rocha, C. Effect of ferulic acid on the performance of soy protein isolate-based edible coatings applied to fresh-cut apples. LWT 2017, 80, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial Properties and Major Bioactive Components of Cinnamon Stick (Cinnamomum burmannii): Activity against Foodborne Pathogenic Bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef]
- Moreira, S.P.; De Carvalho, W.M.; Alexandrino, A.C.; De Paula, H.C.B.; Rodrigues, M.D.C.P.; De Figueiredo, R.W.; Maia, G.A.; De Figueiredo, E.M.A.T.; Brasil, I.M. Freshness retention of minimally processed melon using different packages and multilayered edible coating containing microencapsulated essential oil. Int. J. Food Sci. Technol. 2014, 49, 2192–2203. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Sapino, S.; Cavalli, R.; Trotta, M.; Trotta, F.; Martina, K. Inclusion of cinnamaldehyde in modified γ-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 445–450. [Google Scholar] [CrossRef]
- Valle, E.M.M.D. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Hill, J.P. Enzyme-Encapsulated Layer-by-Layer Assemblies: Current Status and Challenges toward Ultimate Nanodevices. In Modern Techniques for Nano- and Microreactors/-Reactions; Springer: Berlin, Germary, 2010; pp. 51–87. [Google Scholar]
- Gomes, C.; Moreira, R.G.; Castellperez, E. Poly (DL-lactide-co-glycolide) (PLGA) nanoparticles with entrapped trans-cinnamaldehyde and eugenol for antimicrobial delivery applications. J. Food Sci. 2015, 76, N16–N24. [Google Scholar] [CrossRef] [PubMed]
- Lins, A.C.D.A.; Cavalcanti, D.T.D.B.; Azoubel, P.M.; Melo, E.D.A.; Maciel, M.I.S. Effect of hydrocolloids on the physicochemical characteristics of yellow mombin structured fruit. Food Sci. Technol. 2014, 34, 456–463. [Google Scholar] [CrossRef] [Green Version]
- De Corato, U.; Salimbeni, R.; De Pretis, A.; Avella, N.; Patruno, G. Use of alginate for extending shelf life in a lyophilized yeast-based formulate in controlling green mould disease on citrus fruit under postharvest condition. Food Packag. Shelf Life 2018, 15, 76–86. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Gnoc, N.T.B.; Chin, K.B. Biosynthesis of Silver Nanoparticles from Persimmon Byproducts and Incorporation in Biodegradable Sodium Alginate Thin Film. J. Food Sci. 2017, 82, 2329–2336. [Google Scholar] [CrossRef]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-Layer Electrostatic Deposition of Edible Coating on Fresh Cut Melon Model: Anticipated and Unexpected Effects of Alginate–Chitosan Combination. Food Bioprocess Technol. 2013, 7, 1424–1432. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; Van Reenen, A.J.; Opara, U.L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Hu, S.-H.; Tsai, C.-H.; Liao, C.-F.; Liu, D.-M.; Chen, S.-Y. Controlled Rupture of Magnetic Polyelectrolyte Microcapsules for Drug Delivery. Langmuir 2008, 24, 11811–11818. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Kirsch, B.; Hauser, H.; Schneider, D.; Seuß-Baum, I.; Goycoolea, F.M. In Vitro and Sensory Evaluation of Capsaicin-Loaded Nanoformulations. PLoS ONE 2015, 10, e0141017. [Google Scholar] [CrossRef]
- Veronesi, F.; Boveri, G.; Raimondo, M. Amphiphobic Nanostructured Coatings for Industrial Applications. Materials 2019, 12, 787. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhang, L.; Wang, J.; Huang, X.; Zhao, Y.; Liu, Y.; Li, C. Application of chlorine dioxide microcapsule sustained-release antibacterial films for preservation of mangos. J. Food Sci. Technol. 2019, 56, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Hosseini, M.S.; Karimi, M.; Ebrahimzadeh, A. Effects of postharvest polyamine application and edible coating on maintaining quality of mango (Mangifera indica L.) cv. Langra during cold storage. Food Sci. Nutr. 2019, 7, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Jongsri, P.; Wangsomboondee, T.; Rojsitthisak, P.; Seraypheap, K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT 2016, 73, 28–36. [Google Scholar] [CrossRef]
- Gong, D.; Bi, Y.; Jiang, H.; Xue, S.; Wang, Z.; Li, Y.; Zong, Y.; Prusky, D. A comparison of postharvest physiology, quality and volatile compounds of “Fuji” and “Delicious” apples inoculated with Penicillium expansum. Postharvest Boil. Technol. 2019, 150, 95–104. [Google Scholar] [CrossRef]
- Yuan, H.; Tan, D.; Li, T. Changes of Sugar and Acids Contents in “Nanguo pear” from Different Areas. North Fruit 2017, 2, 14–16. (In Chinese) [Google Scholar]
- Cissé, M.; Polidori, J.; Montet, D.; Loiseau, G.; Ducamp-Collin, M.N. Preservation of mango quality by using functional chitosan-lactoperoxidase systems coatings. Postharvest Boil. Technol. 2015, 101, 10–14. [Google Scholar] [CrossRef]
- Khalili, S.T.; Mohsenifar, A.; Beyki, M.; Zhaveh, S.; Rahmani-Cherati, T.; Abdollahi, A.; Bayat, M.; Tabatabaei, M. Encapsulation of Thyme essential oils in chitosan-benzoic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. LWT 2015, 60, 502–508. [Google Scholar] [CrossRef]
- Wang, H.-J.; Li, X.-R.; Huang, Y.-Q.; Zhang, Y.-L.; Hu, X.; Liu, Y. In vitro and in vivo pharmaceutical behaviors of lycopene microcapsules. Yao Xue Xue Bao 2005, 40, 787–791. (In Chinese) [Google Scholar]
- Hu, M.; Mi, B. Layer-by-layer assembly of graphene oxide membranes via electrostatic interaction. J. Membr. Sci. 2014, 469, 80–87. [Google Scholar] [CrossRef]
- Bierhalz, A.C.; Da Silva, M.A.; De Sousa, H.C.; Braga, M.E.; Kieckbusch, T.G.; Braga, M.E.M. Influence of natamycin loading methods on the physical characteristics of alginate active films. J. Supercrit. Fluids 2013, 76, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Packaging strategies to prolong the shelf life of fresh carrots (Daucus carota L.). Innov. Food Sci. Emerg. Technol. 2012, 13, 215–220. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Q.; Cao, J.; Jiang, W. Effects of Chitosan Coating On Postharvest Quality of Mango (Mangifera Indica L. Cv. Tainong) Fruits. J. Food Proc. Preserv. 2008, 32, 770–784. [Google Scholar] [CrossRef]
- Koh, P.C.; Noranizan, M.A.; Hanani, Z.A.N.; Karim, R.; Rosli, S.Z. Application of edible coatings and repetitive pulsed light for shelf life extension of fresh-cut cantaloupe (Cucumis melo L. reticulatus cv. Glamour). Postharvest Boil. Technol. 2017, 129, 64–78. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T.V.R. Responses of fresh-cut products of four mango cultivars under two different storage conditions. J. Food Sci. Technol. 2017, 54, 1689–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caratan, A.G. Process for the Substantial Prolongation of the Storage Life of Grapes. U.S. Patent 9,295,266, 29 March 2016. [Google Scholar]
- Liu, K.; Xiang-Wei, L.I.; Wang, X.L. Effect of Bentonite Coating on Fresh-keeping of Mango Stored at Ambient Temperature. Food Sci. 2011, 32, 305–308. [Google Scholar]
- Parish, M.E.; Beuchat, L.R.; Suslow, T.V.; Harris, L.J.; Garrett, E.H.; Farber, J.N.; Busta, F.F. Methods to Reduce/Eliminate Pathogens from Fresh and Fresh-Cut Produce. Compr. Rev. Food Sci. Food Saf. 2010, 2, 161–173. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res. Int. 2011, 44, 2088–2099. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, L.; Wang, Y. Effect of alginate/nano-Ag coating on microbial and physicochemical characteristics of shiitake mushroom (Lentinus edodes) during cold storage. Food Chem. 2013, 141, 954–960. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Khaliq, G.; Mohamed, M.T.M.; Ding, P.; Ghazali, H.M.; Ali, A. Storage behaviour and quality responses of mango (Mangifera indica L.) fruit treated with chitosan and gum arabic coatings during cold storage conditions. Int. Food Res. J. 2016, 23, S141–S148. [Google Scholar]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A.; El-Shishtawy, R.M. Quality and biochemical changes of ‘Hindi-Besennara’ mangoes during shelf life as affected by chitosan, gallic acid and chitosan gallate. J. Food Sci. Technol. 2017, 54, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Brasil, I.; Gomes, C.; Puerta-Gomez, A.; Castell-Perez, M.; Moreira, R.G. Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT 2012, 47, 39–45. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J. Food Sci. Technol. 2016, 53, 4056–4066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Wu, Y.; Huang, W.; Yang, F.; Ren, X. Degradation of chitosan by hydrodynamic cavitation. Polym. Degrad. Stab. 2013, 98, 37–43. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes. Materials 2019, 12, 2039. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12132039
Yin C, Huang C, Wang J, Liu Y, Lu P, Huang L. Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes. Materials. 2019; 12(13):2039. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12132039
Chicago/Turabian StyleYin, Cheng, Chongxing Huang, Jun Wang, Ying Liu, Peng Lu, and Lijie Huang. 2019. "Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes" Materials 12, no. 13: 2039. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12132039